White Stork Pellets: Non-Invasive Solution to Monitor Anthropogenic Particle Pollution
Abstract
1. Introduction
- (I)
- Investigate the application of white stork pellets for anthropogenic particle monitoring. Since the white stork is an undomesticated species that is ecologically associated with urban settlements, their habits (behavioural and dietary) could potentially make them effective indicators of micro-anthropogenic particle pollution caused by anthropogenic activities;
- (II)
- Perform polymer analysis on suspected anthropogenic and other non–biological particles;
- (III)
- Examine if there is a spatial variation in the number of micro-anthropogenic particles isolated, as the assumed polluted sampling site is an area surrounded by a major river, industry and agricultural land, and is adjacent to the urban centre;
- (IV)
- Investigate the prey composition of pellets to determine the prevalence of food sources and feeding habits of white storks in sampling locations.
2. Materials and Methods
2.1. Sampling Locations
2.2. Isolation and Analysis of Anthropogenic Particles
2.3. Spectroscopic Analysis
2.4. Prey Remains Isolation and Determination
2.5. Quality Control
2.6. Statistical Analysis
3. Results
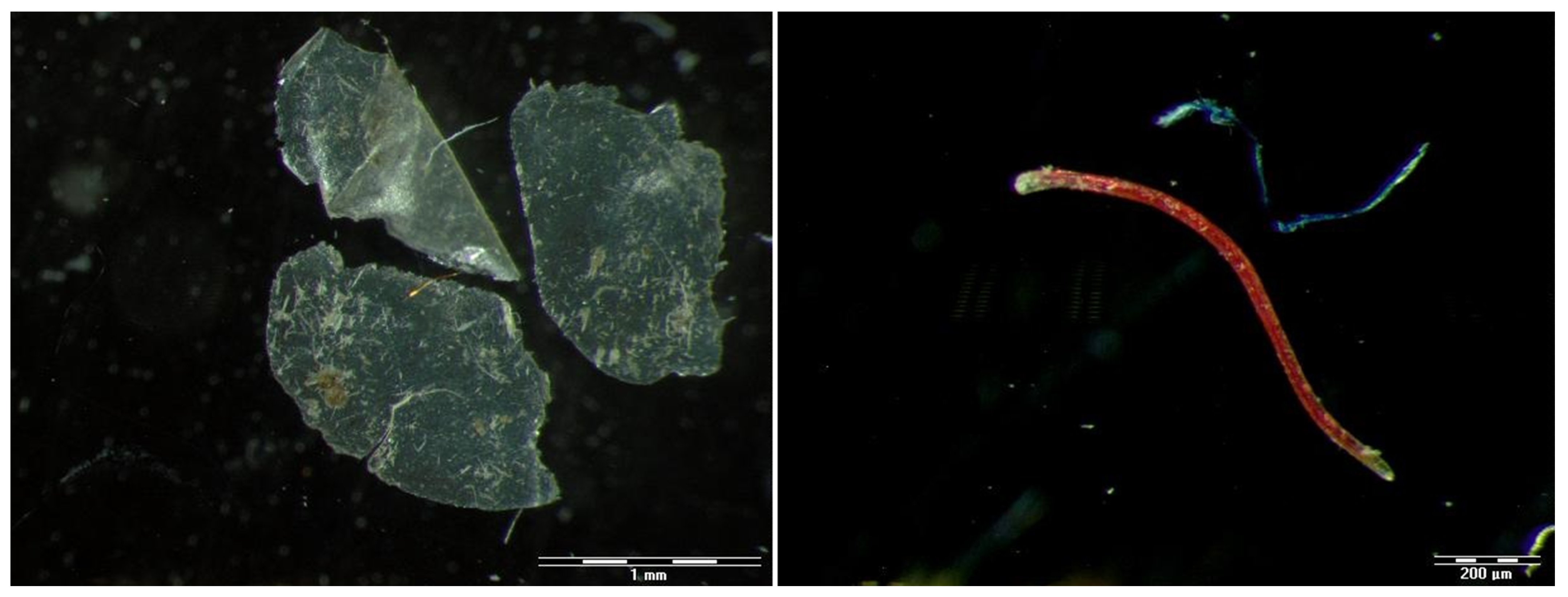
3.1. Isolated Anthropogenic Particles
3.2. ATR–FTIR Results of Analysed Particles
3.3. Spatial Variability
3.4. Dietary Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattsson, K.; da Silva, V.H.; Deonarine, A.; Louie, S.M.; Gondikas, A. Monitoring anthropogenic particles in the environment: Recent developments and remaining challenges at the forefront of analytical methods. Curr. Opin. Colloid. Interface Sci. 2021, 56, 101513. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, H.; Wu, W.; Wang, L.; Liu, J.; An, L.; Xu, Q. Microplastic characteristics in organisms of different trophic levels from Liaohe Estuary, China. Sci. Total Environ. 2021, 789, 148027. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, X.; Zheng, Q.; Mai, B.X.; Qiu, R. Transfer of Microplastics in Terrestrial and Aquatic Food Webs: The Impact of E-Waste Debris and Ecological Traits. Environ. Sci. Technol. 2022, 57, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, P.D.; Avery-Gomm, S.; Wood, J.; Bowes, V.; Wilson, L.; Morgan, K.H.; Boyd, W.S.; Hipfner, J.M.; Desforges, J.-P.; Bertram, D.F.; et al. Seasonal variability in vulnerability for Cassin’s auklets (Ptychoramphus aleuticus) exposed to microplastic pollution in the Canadian Pacific region. Sci. Total Environ. 2019, 649, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Amélineau, F.; Bonnet, D.; Heitz, O.; Mortreux, V.; Harding, A.; Karnovsky, N.; Walkusz, W.; Fort, J.; Grémillet, D. Microplastic pollution in the Greenland Sea: Background levels and selective contamination of planktivorous diving seabirds. Environ. Pollut. 2016, 219, 1131–1139. [Google Scholar] [CrossRef]
- Weitzel, S.L.; Feura, J.M.; Rush, S.A.; Iglay, R.B.; Woodrey, M.S. Availability and assessment of microplastic ingestion by marsh birds in Mississippi Gulf Coast tidal marshes. Mar. Pollut. Bull. 2021, 166, 112187. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Lee, K.K.; Tang, K.H.D.; Yap, P.S. Microplastics in the freshwater and terrestrial environments: Prevalence, fates, impacts and sustainable solutions. Sci. Total Environ. 2020, 719, 137512. [Google Scholar] [CrossRef]
- Campanale, C.; Galafassi, S.; Savino, I.; Massarelli, C.; Ancona, V.; Volta, P.; Uricchio, V.F. Microplastics pollution in the terrestrial environments: Poorly known diffuse sources and implications for plants. Sci. Total Environ. 2022, 805, 150431. [Google Scholar] [CrossRef]
- Nicastro, K.R.; Savio, R.L.; McQuaid, C.D.; Madeira, P.; Valbusa, U.; Azevedo, F.; Casero, M.; Lourenço, C.; Zardi, G.I. Plastic ingestion in aquatic-associated bird species in southern Portugal. Mar. Pollut. Bull. 2018, 126, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Henry, P.Y.; Wey, G.; Balança, G. Rubber Band Ingestion by a Rubbish Dump Dweller, the White Stork (Ciconia ciconia). Waterbirds 2011, 34, 504–508. [Google Scholar] [CrossRef]
- Peris, S. Feeding in urban refuse dumps: Ingestion of plastic objects by the White Stork (Ciconia ciconia). Ardeola 2003, 50, 81–84. [Google Scholar]
- Radhamany, D.; Das, K.S.; Azeez, P.A.; Wen, L.; Sreekala, L.K. Usage of nest materials by house sparrow (Passer domesticus) along an urban to rural gradient in Coimbatore, India. Trop. Life Sci. Res. 2016, 27, 127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Blair, R.B.; Jiang, P.; Ding, P. Nest composition adjustments by Chinese Bulbuls Pycnonotus sinensis in an urbanized landscape of Hangzhou (E China). Acta Ornithol. 2009, 44, 185–192. [Google Scholar] [CrossRef]
- Borgia, G. Bower quality, number of decorations and mating success of male satin bowerbirds (Ptilonorhynchus violaceus): An experimental analysis. Animal Behaviour 1985, 33, 266–271. [Google Scholar] [CrossRef]
- Jagiello, Z.A.; Dylewski, Ł.; Winiarska, D.; Zolnierowicz, K.M.; Tobolka, M. Factors determining the occurrence of anthropogenic materials in nests of the white stork Ciconia ciconia. Environ. Sci. Pollut. Res. Int. 2018, 25, 14726–14733. [Google Scholar] [CrossRef]
- de Souza, S.S.; Freitas, N.; Gonçalves, S.d.O.; da Luz, T.M.; Araújo, A.P.d.C.; Rajagopal, R.; Balasubramani, G.; Rahman, M.; Malafaia, G. Toxicity induced via ingestion of naturally-aged polystyrene microplastics by a small-sized terrestrial bird and its potential role as vectors for the dispersion of these pollutants. J. Hazard. Mater. 2022, 434, 128814. [Google Scholar] [CrossRef]
- Deoniziak, K.; Cichowska, A.; Niedźwiecki, S.; Pol, W. Thrushes (Aves: Passeriformes) as indicators of microplastic pollution in terrestrial environments. Sci. Total Environ. 2022, 853, 158621. [Google Scholar] [CrossRef]
- Sherlock, C.; Fernie, K.J.; Munno, K.; Provencher, J.; Rochman, C. The potential of aerial insectivores for monitoring microplastics in terrestrial environments. Sci. Total Environ. 2022, 807 Pt 1, 150453. [Google Scholar] [CrossRef]
- Kralj, J.; Barišić, S.; Tutiš, V.; Ćiković, D. Croatian Bird Migration Atlas; Kralj, J., Barišić, S., Tutiš, V., Ćiković., D., Eds.; Atlas selidbe ptica Hrvatske; Hrvatska akademija znanosti i umjetnosti (HAZU): Zagreb, Croatia, 2013. [Google Scholar]
- Kosicki, J.Z.; Profus, P.; Dolata, P.T.; Tobółka, M. Food composition and energy demand of the White Stork Ciconia ciconia breeding population. Literature survey and preliminary results from Poland. In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006; pp. 169–183. [Google Scholar]
- Bjedov, D.; Velki, M.; Lackmann, C.; Begović, L.; Mikuška, T.; Jurinović, L.; Mikuška, A. Blood biomarkers in white stork (Ciconia ciconia) nestlings show different responses in several areas of Croatia. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 547–558. [Google Scholar] [CrossRef] [PubMed]
- de la Casa-Resino, I.; Hernández-Moreno, D.; Castellano, A.; Pérez-López, M.; Soler, F. Breeding near a landfill may influence blood metals (Cd, Pb, Hg, Fe, Zn) and metalloids (Se, As) in white stork (Ciconia ciconia) nestlings. Ecotoxicology 2014, 23, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- López-García, A.; Sanz-Aguilar, A.; Aguirre, J.I. The trade-offs of foraging at landfills: Landfill use enhances hatching success but decrease the juvenile survival of their offspring on white storks (Ciconia ciconia). Sci. Total Environ. 2021, 778, 146217. [Google Scholar] [CrossRef]
- Radović, A.; Tepić, N. Using Corine Land Cover habitat database for the analysis of breeding bird habitat: Case study of white storks (Ciconia ciconia) from northern Croatia. Biologia 2009, 64, 1212–1218. [Google Scholar] [CrossRef]
- Zurell, D.; von Wehrden, H.; Rotics, S.; Kaatz, M.; Groß, H.; Schlag, L.; Schäfer, M.; Sapir, N.; Turjeman, S.; Wikelski, M.; et al. Home Range Size and Resource Use of Breeding and Non-breeding White Storks Along a Land Use Gradient. Front. Ecol. Evol. 2018, 6, 79. [Google Scholar] [CrossRef]
- Ječmenica, B.; Kralj, J. Dispersal of the White Stork Ciconia ciconia in the Lonjsko polje Nature Park, Croatia. Larus Godišnjak Zavoda Za Ornitol. Hrvat. Akad. Znan. I Umjet. 2017, 52, 35–48. [Google Scholar] [CrossRef]
- Mikula, P.; Karg, J.; Jerzak, L.; Walasz, K.; Siekiera, J.; Czyż, S.; Mikicińska, K.; Pietkiewicz, M.; Sztwiertnia, H.; Wyka, J.; et al. Diet analysis and the assessment of plastic and other indigestible anthropogenic litter in the white stork pellets. Environ. Sci. Pollut. Res. 2023, 31, 6922–6928. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Beckingham, B.; Apintiloaiei, A.; Moore, C.; Brandes, J. Hot or not: Systematic review and laboratory evaluation of the hot needle test for microplastic identification. Microplastics Nanoplastics 2023, 3, 8. [Google Scholar] [CrossRef]
- Horváth, G.; Jurčević Agić, I.; Merdić, E.; Torizs, I.; Purger, J. Monitoring sitnih sisavaca na temelju istraživanja sastava gvalica sova. In Priručnik za istraživanje bioraznolikosti duž rijeke Drave Sveučilište u Pečuhu; University of Pécs: Pécs, Hungary, 2007; pp. 203–217. [Google Scholar]
- Chinery, M. Insects of Britain and Northern Europe, 3rd ed.; HarperCollins: London, UK, 1993. [Google Scholar]
- Winkler, A.; Nessi, A.; Antonioli, D.; Laus, M.; Santo, N.; Parolini, M.; Tremolada, P. Occurrence of microplastics in pellets from the common kingfisher (Alcedo atthis) along the Ticino River, North Italy. Environ. Sci. Pollut. Res. Int. 2020, 27, 41731–41739. [Google Scholar] [CrossRef]
- Nessi, A.; Winkler, A.; Tremolada, P.; Saliu, F.; Lasagni, M.; Ghezzi, L.L.M.; Balestrieri, A. Microplastic contamination in terrestrial ecosystems: A study using barn owl (Tyto alba) pellets. Chemosphere 2022, 308 Pt 1, 136281. [Google Scholar] [CrossRef]
- Milchev, B.; Chobanov, D.; Simov, N. Diet and foraging habitats of non-breeding white storks (Ciconia ciconia) in Bulgaria. Arch. Biol. Sci. 2013, 65, 1007–1013. [Google Scholar] [CrossRef]
- Reynolds, C.; Ryan, P.G. Micro-plastic ingestion by waterbirds from contaminated wetlands in South Africa. Mar. Pollut. Bull. 2018, 126, 330–333. [Google Scholar] [CrossRef]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.; Barnes, P.; Bornman, J.; Gouin, T.; Madronich, S.; White, C.; Zepp, R.; Jansen, M. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci. Total Environ. 2022, 851, 158022. [Google Scholar] [CrossRef] [PubMed]
- Welsh, B.; Aherne, J.; Paterson, A.M.; Yao, H.; McConnell, C. Atmospheric deposition of anthropogenic particles and microplastics in south-central Ontario, Canada. Sci. Total Environ. 2022, 835, 155426. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, L.; Leterrier, Y.; Sunderland, P.; Månson, J. Life Cycle Engineering of Plastics: Technology, Economy and Environment; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Abraham, J.; Ghosh, E.; Mukherjee, P.; Gajendiran, A. Microbial degradation of low density polyethylene. Environ. Prog. Sustain. Energy 2017, 36, 147–154. [Google Scholar] [CrossRef]
- Sarker, M.; Mamunor Rashid, M.; Sadikur Rahman, M. Low Density Polyethylene (LDPE) Waste Plastic Transformation into Renewable Heavy Fuel Using Thermal Cracking. World Environ. 2012, 2, 140–147. [Google Scholar] [CrossRef][Green Version]
- Sudirman; Dharmayani, N.K.T.; Yuanita, E.; Ulfa, M.; Sudarma, I.M.; Ikhsan, A. Reprocessing plastic waste into petroleum fraction based on zero waste principle. AIP Conf. Proc. 2020, 2243, 020026. [Google Scholar]
- Habschied, K.; Mastanjević, K.; Šibalić, M.; Krstanović, V.; Galić, V. A Survey on Detection of Plastic-Related Chemicals in Beer Packaged in PET Using FT-IR Technology. Beverages 2022, 8, 73. [Google Scholar] [CrossRef]
- Bocqué, M.; Lapinte, V.; Courault, V.; Couve, J.; Cassagnau, P.; Robin, J. Phosphonated Lipids as Primary Plasticizers for PVC with Improved Flame Retardancy. Eur. J. Lipid Sci. Technol. 2018, 120, 1800062. [Google Scholar] [CrossRef]
- Barlow, C.Y.; Morgan, D.C. Polymer film packaging for food: An environmental assessment. Resour. Conserv. Recycl. 2013, 78, 74–80. [Google Scholar] [CrossRef]
- Katsura, T.; Sasaki, H. On-going solutions to environmental issues in plastic packaging. Packag. Technol. Sci. 2001, 14, 87–95. [Google Scholar] [CrossRef]
- Mattetti, M.; Beltramin, A.; Perez Estevez, M.A.; Varani, M.; Renzi, M.; Alberti, L. Start and stop systems on agricultural tractors as solution for saving fuel and emissions. Biosyst. Eng. 2022, 216, 108–120. [Google Scholar] [CrossRef]
- de Boer, C.; Paraffins, C.; Boer, J. (Eds.) The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 10. [Google Scholar]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Microplastics in Aquatic Environments and Their Toxicological Implications for Fish. In Toxicology—New Aspects to This Scientific Conundrum; Intechopen: London, UK, 2016. [Google Scholar]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New insights into the photo-degraded polystyrene microplastic: Effect on the release of volatile organic compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef]
- Liu, H.-D.; Gu, B.; Jia, F.; Li, Y.; Ying, Q.; Alamus; Yuan, W.F.; Chen, B.; He, Q. Facile fabrication of hydrophobic octadecylamine-functionalized polyurethane foam for oil spill cleanup. J. Macromol. Sci. Part A 2016, 53, 196–200. [Google Scholar] [CrossRef]
- Bohrer, D. Sources of Contamination in Medicinal Products and Medical Devices; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Narciso-Ortiz, L.; Coreño-Alonso, A.; Mendoza-Olivares, D.; Lucho-Constantino, C.A.; Lizardi-Jiménez, M.A. Baseline for plastic and hydrocarbon pollution of rivers, reefs, and sediment on beaches in Veracruz State, México, and a proposal for bioremediation. Environ. Sci. Pollut. Res. Int. 2020, 27, 23035–23047. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- García-Cruz, N.; Valdivia-Rivera, S.; Narciso-Ortiz, L.; García-Maldonado, J.; Uribe-Flores, M.; Aguirre-Macedo, M.; Lizardi-Jiménez, M. Diesel uptake by an indigenous microbial consortium isolated from sediments of the Southern Gulf of Mexico: Emulsion characterisation. Environ. Pollut. 2019, 250, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef] [PubMed]
- Yukioka, S.; Tanaka, S.; Nabetani, Y.; Suzuki, Y.; Ushijima, T.; Fujii, S.; Takada, H.; Van Tran, Q.; Singh, S. Occurrence and characteristics of microplastics in surface road dust in Kusatsu (Japan), Da Nang (Vietnam), and Kathmandu (Nepal). Environ. Pollut. 2020, 256, 113447. [Google Scholar] [CrossRef] [PubMed]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.; Kushlan, J.; Kahl, M. Storks, Ibises and Spoonbills of the World; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Alonso, J.C.; Alonso, J.A.; Carrascal, L.M. Habitat selection by foraging White Storks, Ciconia ciconia, during the breeding season. Can. J. Zool. 1991, 69, 1957–1962. [Google Scholar] [CrossRef]
- Vrezec, A. Insects in the White Stork Ciconia ciconia diet as indicators of its feeding conditions: The first diet study in Slovenia. Acrocephalus 2009, 30, 25–29. [Google Scholar] [CrossRef]
- Johst, K.; Brandl, R.; Pfeifer, R. Foraging in a Patchy and Dynamic Landscape: Human Land Use and the White Stork. Ecol. Appl. 2001, 11, 60. [Google Scholar] [CrossRef]
- Kwieciński, Z.; Kwiecińska, H.; Ratajszczak, R.; Ćwiertnia, P.; Tryjanowski, P. Food selection of the white storks Ciconia ciconia under captive conditions. In White Stork Study in Poland: Biology, Ecology and Conservation; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2015. [Google Scholar]
- Mužinić, J.; Rašajski, J. On food and feeding habits of the White Stork, Ciconia cciconia, in Central Balkans. Okol. Der Vögel 1992, 14, 211–223. [Google Scholar]
- Rékási, J. A study of the White Stork population of North Bácska in 1999. Ornis Hungarica 2000, 10, 225–229. [Google Scholar]
- Surdo, S.; Zapparrata, C.; Ientile, R.; Massa, B. Evidence suggests an opportunistic entomophagous diet of the White Stork Ciconia ciconia in Sicily during breeding and post-breeding periods. Avocetta 2022, 46, 49–56. [Google Scholar]


| Polymer | Uses | Associated with Plastic Masses |
|---|---|---|
| (3-aminopropyl)triethoxysilane | thermoplastic polymer | yes |
| 1,2-octadecanediol | personal care products | no |
| 1,3,5-trimethylcyclohexane | by-product of PE | yes |
| 1-chlorohexadecane | additive used in plastic production | yes |
| 3-(2-imidazolin-1-YL)propyltriethoxysilane | resin and plastic production | yes |
| 3-methylheptane | product of PS degradation | yes |
| Butyl stearate | additive used in plastic production | yes |
| Dioctyl sebacate | additive used in plastic production | yes |
| Dotriacontane | by-product of PE | yes |
| Enzacryl polyacetal | thermoplastic polymer | yes |
| Ethyl palmitate | product of PU degradation | yes |
| Hexacosanol | plastic production | yes |
| Hexatriacontane | petroleum product | no |
| L(-)-glyceraldehyde unnatural forms | naturally occurring | no |
| Methyl linoleate | PVC plasticiser | yes |
| Octacosane | by-product of PE | yes |
| Octadecylamine | product of PU degradation | yes |
| Paraffin oil | plastic production | yes |
| Polystyrene | plastic polymer | yes |
| Tetradodecylammonium bromide | surfactant and catalyst | no |
| Toluene-4-sulfonic acid | surfactant and catalyst | no |
| Vinylidene chloride | plastic production | yes |
| nparticle | Mass (g) | nparticle gpellet–1 | Min | Max | Mean ± SD | |
|---|---|---|---|---|---|---|
| Study Site 1 (npellet = 10) | 284 | 13.23 | 21.47 | <0.50 | 20.00 | 2.54 ± 1.68 |
| 239 | 6.25 | 38.22 | 1.00 | 40.00 | 2.27 ± 3.10 | |
| 33 | 11.26 | 2.93 | 1.00 | 10.00 | 3.12 ± 1.68 | |
| 105 | 12.28 | 8.55 | 1.00 | 10.00 | 2.10 ± 1.21 | |
| 86 | 12.28 | 7.00 | <0.50 | 10.00 | 2.37 ± 1.30 | |
| 660 | 9.00 | 73.37 | 1.00 | 22.00 | 2.39 ± 0.93 | |
| 1411 | 27.93 | 50.51 | <0.50 | 13.00 | 2.33 ± 0.93 | |
| 796 | 7.20 | 110.49 | <0.50 | 7.00 | 2.02 ± 0.93 | |
| 1996 | 22.08 | 90.39 | <0.50 | 20.00 | 1.80 ± 1.29 | |
| 1858 | 13.51 | 137.51 | <0.50 | 12.00 | 2.32 ± 1.26 | |
| Study Site 2 (npellet = 10) | 51 | 9.63 | 5.30 | <0.50 | 5.00 | 1.37 ± 0.91 |
| 27 | 10.73 | 2.52 | <0.50 | 3.00 | 1.28 ± 0.71 | |
| 35 | 17.16 | 2.04 | <0.50 | 1.00 | 0.73 ± 0.24 | |
| 33 | 7.53 | 4.38 | <0.50 | 2.25 | 0.93 ± 0.46 | |
| 125 | 11.51 | 10.86 | <0.50 | 4.25 | 1.32 ± 0.83 | |
| 12 | 8.24 | 1.46 | <0.50 | 1.20 | 0.76 ± 0.22 | |
| 12 | 4.80 | 2.50 | <0.50 | 2.50 | 1.25 ± 0.58 | |
| 4 | 11.87 | 0.34 | <0.50 | 1.20 | 0.85 ± 0.31 | |
| 9 | 8.95 | 1.01 | <0.50 | 35.00 | 5.83 ± 11.23 | |
| 93 | 7.23 | 12.87 | <0.50 | 9.00 | 1.84 ± 1.46 |
| Class | Order | Family | Species | Study Site 1 | Study Site 2 |
|---|---|---|---|---|---|
| Mammalia | Rodentia | x | x | ||
| Arachnida | Araneae | x | |||
| Clitellata | Opisthopora | Lumbricidae | x | x | |
| Mollusca | Gastropoda | Gastropoda terrestria sp. | x | ||
| Insecta | Diptera | x | |||
| Hymenoptera | Formicidae | x | |||
| Orthoptera | Gryllidae | x | x | ||
| Tettigoniidae | x | ||||
| Acrididae | x | ||||
| Gryllotalpidae | Gryllotalpa gryllotalpa | x | x | ||
| Coleoptera | Chrysomelidae | x | |||
| Silphidae/ | x | x | |||
| Lucanidae | Dorcus parallelipipedus | x | x | ||
| Cerambycidae | x | ||||
| Tenebrionidae | Blaps mortisaga | x | |||
| Scarabaeidae | Melolontha sp. | x | |||
| Melolontha melolontha | x | ||||
| Oryctes nasicornis | x | ||||
| Cetonia aurata | x | ||||
| Carabidae | Carabus sp. | x | x | ||
| Abax sp. | x | x | |||
| Calosoma sp. | x | ||||
| Harpalus sp. | x | ||||
| Abax sp. | x | x | |||
| Carabus ullrichi Germar | x | x | |||
| Carabus granulatus | x | ||||
| Carabus violaceus | x | ||||
| Carabus coriaceus | x | ||||
| Carabus intricatus | x | ||||
| Calosoma auropunctatum | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjedov, D.; Mikuška, A.; Gvozdić, V.; Glavaš, P.; Gradečak, D.; Sudarić Bogojević, M. White Stork Pellets: Non-Invasive Solution to Monitor Anthropogenic Particle Pollution. Toxics 2024, 12, 236. https://doi.org/10.3390/toxics12040236
Bjedov D, Mikuška A, Gvozdić V, Glavaš P, Gradečak D, Sudarić Bogojević M. White Stork Pellets: Non-Invasive Solution to Monitor Anthropogenic Particle Pollution. Toxics. 2024; 12(4):236. https://doi.org/10.3390/toxics12040236
Chicago/Turabian StyleBjedov, Dora, Alma Mikuška, Vlatka Gvozdić, Petar Glavaš, Dora Gradečak, and Mirta Sudarić Bogojević. 2024. "White Stork Pellets: Non-Invasive Solution to Monitor Anthropogenic Particle Pollution" Toxics 12, no. 4: 236. https://doi.org/10.3390/toxics12040236
APA StyleBjedov, D., Mikuška, A., Gvozdić, V., Glavaš, P., Gradečak, D., & Sudarić Bogojević, M. (2024). White Stork Pellets: Non-Invasive Solution to Monitor Anthropogenic Particle Pollution. Toxics, 12(4), 236. https://doi.org/10.3390/toxics12040236








