Effect of Nanomaterials on Gut Microbiota
Abstract
1. Exposure of the Gut Microbiota to Nanomaterials
2. The Function of the Gut Microbiota
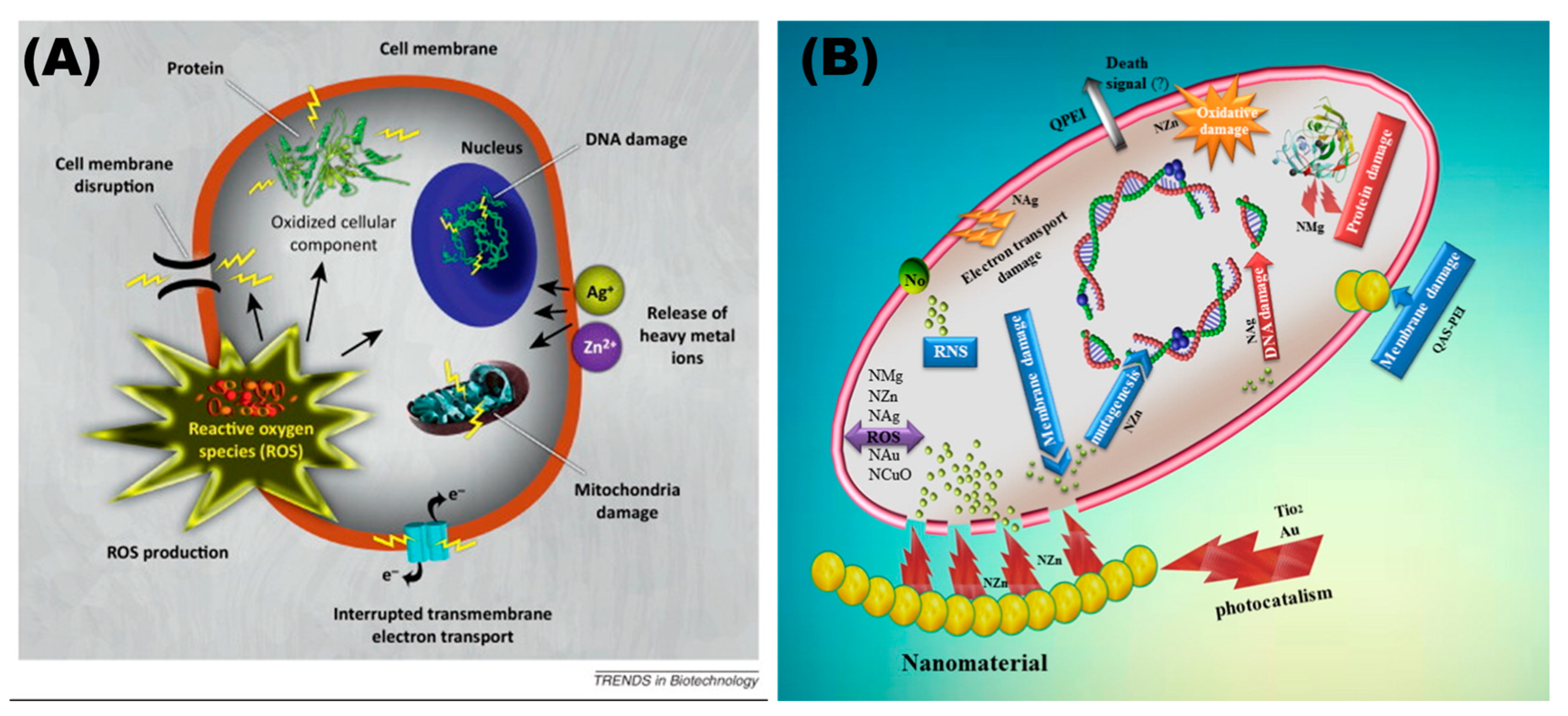
3. Antimicrobial Properties of Nanomaterials
4. Effects of Nanomaterials on Gut Microbiota
4.1. Titanium Dioxide Nanoparticles (TiO2 NPs)

| Animal | Physicochemical Properties | Exposure Dose | Exposure Time | Antibacterial Activity | Others |
|---|---|---|---|---|---|
| Albino mice [55] | Hexagonal (25.12 nm) | 50 μg, 100 μg | 18 d | Firmicutes ⬇ | |
| C57BL/6 [45] | Spherical E171 (28–1158 nm) | 2, 10, 50 mg/kg | 21 d | Levilactobacillus ⬆ Allobaculum ⬆ Adlercreutzia ⬇ Unclassified Clostridiaceae ⬇ | |
| C57BL/6 [56] | Anatase (25 nm) | 1 mg/kg | 7 d | Bifidobacterium ⬇ | |
| C57BL/6 [42] | Rutile | 100 mg/kg | 28 d | Proteobacteria ⬇ | The small intestine villi were long, and the villi epithelial cells were arranged irregularly. |
| C57BL/6J [43] | Anatase (10 nm, 50 nm) | diets containing 0.1% TiO2 NPs | 90 d | Bifidobacterium ⬇ Lactobacillus ⬇ | The body weight was lower than that of the control group, and it exacerbated the chronic colitis and immune response induced by Dextran Sulfate Sodium Salt (DSS). |
| Sprague–Dawley rats [44] | Anatase | 2, 10, 50 mg/kg | 28 d | L. gasseri ⬆ L.NK4A136_group ⬆ | Pathological inflammatory infiltrates and mitochondrial abnormalities cause significant alterations in the shape of the gut. |
| Sprague–Dawley rats [57] | Anatase (25.2 nm) | 100 mg/kg | 14 d | Anaerobium ⬆ Prevotella ⬆ Granulicatella ⬆ Lactobacillaceae ⬇ |
4.2. Silver Nanoparticles (Ag NPs)

| Animal | Physicochemical Properties | Exposure Dose | Exposure Time | Antibacterial Activity | Others |
|---|---|---|---|---|---|
| C57BL/6 [66] | 22.2 ± 6.1 nm | 0.1, 2, 40 μg | 120 d | Firmicutes ⬆ Bacteroidetes ⬇ | Changes in liver metabolism |
| C57BL/6 [67] | 55.17 ± 2.67 nm | 46, 460, 4600 μg/kg | 28 d | Firmicutes ⬆ Bacteroidetes ⬇ | |
| C57BL/6J [68] | 60–150 nm | 0.5, 2.5 mg/kg | 14 d 28 d | Lachnospiraceae ⬆ Bacteroidetes S24-7 ⬇ | Accumulates in the liver, spleen, and lungs. |
| Wistar rats [69] | 7 nm | 100 mg/kg | 28 d | Bacteroidota ⬆ Verrucomicrobia ⬇ Proteobacteria ⬇ Lactobacillaceae ⬇ | Minor inflammatory cell infiltration in the submucosa of the gastric mucosa; there are small yellowish to dark granules in the submucosa and macrophages at the tip of the duodenal villi. |
| Sprague–Dawley rats [70] | Spherical (50 nm) cube (45 nm) | 3.6 mg/kg | 14 d | Cube: Clostridium spp. ⬇ Bacteroides uniformis ⬇ Christensenellaceae ⬇ Coprococcus eutactus ⬇ Spherical: Coprococcus eutactus ⬇ Dehalobacterium spp. ⬇ Peptococcaeceae ⬇ Corynebacterium spp. ⬇ Aggregatibacter pneumotropica ⬇ | |
| Sprague–Dawley rats [58] | 10, 75, 110 nm | 18, 36 mg/kg | 91 d | Bifidobacterium ⬆ Firmicutes ⬇ | The expression level of MUC3, TLR2, TLR4, GPR43, FOXP3 were decreased. |
| Broiler chickens [71] | 50 nm | 25, 50, 75 ppm | 42 d | Total anaerobic bacteria ⬇ Escherichia coli ⬇ | It had side effects on the immune mechanism. |
| Zebrafish [72] | 10, 33, 100 μg/L | 45 d | Proteobacteria ⬆ | ||
| Drosophila melanogaster [59] | 7 μm 1.5 μm | 450 mg/mL | 7 d | Acetobacter ⬇ | |
| Weaned pigs [63] | 20, 40 mg/kg | 14 d | Coliforms ⬇ |
4.3. Zinc Oxide Nanoparticles (ZnO NPs)
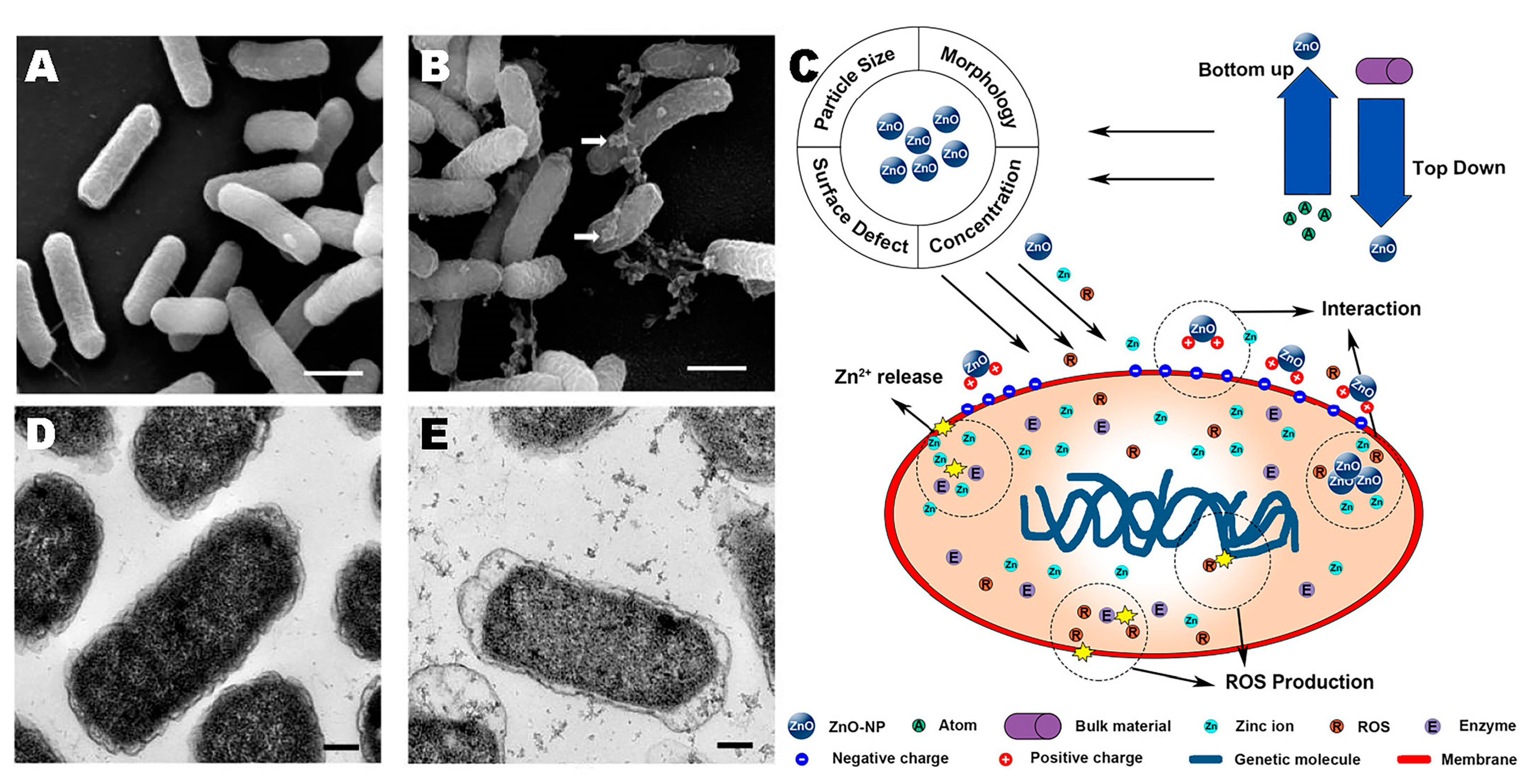
| Animal | Physicochemical Properties | Exposure Dose | Exposure Time | Antibacterial Activity | Others |
|---|---|---|---|---|---|
| Weaned piglets [81] | 23 nm | diets containing 0.3, 0.4, 0.5, 0.6 g/kg ZnO NPs | 14 d | Lactobacillaceae ⬆ Coliforms ⬇ | Improves growth performance, reduces the incidence of diarrhea, regulates immune status and antioxidant activity. |
| Weaned pigs [82] | 71.61 nm | 150, 300, 450, 3000 mg/kg | 21 d | Coliforms ⬇ | Reduces diarrhea and improves intestinal morphology. |
| Weaned piglets [83] | 23 nm | 600 mg/kg | 14 d | Ileum: Proteobacteria ⬆ Firmicutes ⬇ Cecum: Firmicutes ⬆ Colon: Firmicutes ⬆ Bacteroidetes ⬇ | Reduces diarrhea and improves intestinal morphology. |
| Wistar albino rats [73] | 1000 mg/kg | 28 d | Male: Firmicutes ⬆ Bacteroidetes ⬇ Female: Firmicutes ⬇ Verrucomicrobia ⬆ | ||
| C57BL/6 [84] | 50 nm | 26 mg/kg | 30 d | Actinobacteria ⬇ | |
| Hens [85] | 30 nm | 25, 50, 100 mg/kg | 63 d | SMB53 ⬆ Proteus ⬇ Lactobacillus ⬇ | |
| Cyprinus carpio [86] | diets containing 500 mg/kg ZnO NPs | 42 d | Flavobacteriumspecies ⬆ Aeromonasspp ⬆ |
4.4. Carbon-Based Nanomaterials (CNMs)

| Animal | Physicochemical Properties | Exposure Dose | Exposure Time | Antibacterial Activity | Others |
|---|---|---|---|---|---|
| CD-1 (ICR) mice [91] | SWCNT diameter: 1.04–1.17 nm, length: 1–5 μm | 0.05, 0.5, 2.5 mg/kg | 7 d | Bacteroidetes ⬆ Lachnospiraceae bacterium A4 ⬆ | Histological lesion scores increased, intestinal permeability increased, and the levels of pro-inflammatory cytokine (IL-1β, IL-6, and TNF-α) increased. |
| C57BL/6 [96] | MWCNT diameter: 10.7 ± 3.1 nm | 2.8 mg/kg | 28 d | Firmicutes ⬆ Tenericutes ⬆ Bacteroidetes ⬇ Proteobacteria ⬇ | Induced inflammation of the lungs. |
| C57BL/6 [97] | MWCNT diameter: 20–30 nm, length: 0.5–2 μm | 5 μg/kg | 15 d | Verrucomicrobia ⬆ Bacteroidetes ⬇ |
4.5. Effects of Other Nanomaterials on Gut Microbiota
| Animal | Nanomaterials | Physicochemical Properties | Exposure Dose | Exposure Time | Antibacterial Activity | Others |
|---|---|---|---|---|---|---|
| Weaned piglets [100] | Copper-loaded chitosan nanoparticles (CNP-Cu) | diameter: 121.9 nm, width: 23.1 nm | 100 mg/kg | 28 d | Levilactobacillus ⬆ Bifidobacterium ⬆ Escherichia coli ⬇ | The piglets’ average daily weight increased, feed intake increased, and the rate of diarrhea decreased; increased length of intestinal epithelial villi. |
| Broiler chickens [102] | nanoselenium | 0.075, 0.15, 0.3 mg/kg | 42 d | Lactobacilli ⬆ Coliforms ⬇ | Improves intestinal morphology and immune function. | |
| CD-1 (ICR) mice [98] | SiO2 NPs | 10.8 ± 1.7 nm | 2.5 mg/kg | 7 d | Firmicutes ⬆ Proteobacteria ⬆ Bacteroidetes ⬇ Lactobacillus ⬇ | Increased pro-inflammatory cytokines in the intestine. |
| Broiler chickens [103] | Iron nanoparticles | 50 ± 15 nm | 8 mg/kg | 42 d | Lachnospiraceae ⬆ Bacteroidaceae ⬆, Alistipes ⬆ Rikenellaceae ⬆ Lactobacillaceae ⬇ Anaerobes ⬇ | |
| Copper nanoparticles | 55 ± 15 nm | 1.7 mg/kg | 42 d | Rumen_occoccidae ⬆ genus Blautia ⬆ Bacteroides ⬆ Firmicutes ⬇ Lactobacillaceae ⬇ Rikenellaceae ⬇ | ||
| A mixture of Cu and Zn asparaginates | 65 ± 15 nm | 2.84 mg/kg | 42 d | Rumen occoccidae ⬆ Bacteroides ⬆ Firmicutes ⬇ Lactobacillaceae ⬇ Rikenellaceae ⬇ |
5. Summary and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, X.; Zhang, Y.; Zhang, C.; Lai, X.; Zhang, Y.; Wu, J.; Hu, C.; Shao, L. Nanomaterial-mediated autophagy: Coexisting hazard and health benefits in biomedicine. Part. Fibre Toxicol. 2020, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Mazari, S.A.; Ali, E.; Abro, R.; Khan, F.S.A.; Ahmed, I.; Ahmed, M.; Nizamuddin, S.; Siddiqui, T.H.; Hossain, N.; Mubarak, N.M.; et al. Nanomaterials: Applications, waste-handling, environmental toxicities, and future challenges—A review. J. Environ. Chem. Eng. 2021, 9, 105028. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yuan, Y.; Tan, Y.; Xia, C.; Li, F.; Ming, J. Research and Applications on Nanocapsule Technology in Functional Foods. Food Sci. 2013, 34, 359–368. [Google Scholar]
- Can, F.O.; Durak, M.Z. Encapsulation of Lemongrass Oil for Antimicrobial and Biodegradable Food Packaging Applications. Sci. Adv. Mater. 2021, 13, 803–811. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Lee, H.Y.; Nam, S.; Kim, M.J.; Kim, S.J.; Back, S.H.; Yoo, H.J. Butyrate Prevents TGF-beta 1-Induced Alveolar Myofibroblast Differentiation and Modulates Energy Metabolism. Metabolites 2021, 11, 258. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Boschiero, C.; Gao, Y.; Vi, R.L.B.; Ma, L.; Li, C.-j.; Liu, G.E. Butyrate Induces Modifications of the CTCF-Binding Landscape in Cattle Cells. Biomolecules 2022, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, Y.; Wang, J.; Wang, Z.; Li, J.; Yin, Y.; Yang, H. Dietary butyrate, lauric acid and stearic acid improve gut morphology and epithelial cell turnover in weaned piglets. Anim. Nutr. 2022, 11, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Bultman, S.J. Metaboloepigenetics: Interrelationships between energy metabolism and epigenetic control of gene expression. J. Cell. Physiol. 2012, 227, 3169–3177. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Velcich, A.; Wilson, A.J.; Augenlicht, L.H.; Gibson, P.R. Resistance to butyrate-induced cell differentiation and apoptosis during spontaneous Caco-2 cell differentiation. Gastroenterology 2001, 120, 889–899. [Google Scholar] [CrossRef]
- Jain, N.; Walker, W.A. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 14–25. [Google Scholar] [CrossRef]
- Cebula, A.; Seweryn, M.; Rempala, G.A.; Pabla, S.S.; McIndoe, R.A.; Denning, T.L.; Bry, L.; Kraj, P.; Kisielow, P.; Ignatowicz, L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 2013, 497, 258–262. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Lui, J.B.; Devarajan, P.; Teplicki, S.A.; Chen, Z. Cross-differentiation from the CD8 lineage to CD4 T cells in the gut-associated microenvironment with a nonessential role of microbiota. Cell Rep. 2015, 10, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Heimesaat, M.M.; Danker, K.; Struck, D.; Lohmann, U.; Plickert, R.; Bereswill, S.; Fischer, A.; Dunay, I.R.; Wolk, K.; et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J. Exp. Med. 2009, 206, 3047–3059. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Granlund, L.; Juvet, L.K.; Pedersen, J.I.; Nebb, H.I. Trans10, cis12-conjugated linoleic acid prevents triacylglycerol accumulation in adipocytes by acting as a PPARgamma modulator. J. Lipid Res. 2003, 44, 1441–1452. [Google Scholar] [CrossRef]
- Brown, J.M.; McIntosh, M.K. Conjugated linoleic acid in humans: Regulation of adiposity and insulin sensitivity. J. Nutr. 2003, 133, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Wargent, E.; Sennitt, M.V.; Stocker, C.; Mayes, A.E.; Brown, L.; O’Dowd, J.; Wang, S.; Einerhand, A.W.; Mohede, I.; Arch, J.R.; et al. Prolonged treatment of genetically obese mice with conjugated linoleic acid improves glucose tolerance and lowers plasma insulin concentration: Possible involvement of PPAR activation. Lipids Health Dis. 2005, 4, 3. [Google Scholar] [CrossRef]
- Goto, T.; Kim, Y.I.; Furuzono, T.; Takahashi, N.; Yamakuni, K.; Yang, H.E.; Li, Y.; Ohue, R.; Nomura, W.; Sugawara, T.; et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, potently activates PPARγ and stimulates adipogenesis. Biochem. Biophys. Res. Commun. 2015, 459, 597–603. [Google Scholar] [CrossRef]
- Velagapudi, V.R.; Hezaveh, R.; Reigstad, C.S.; Gopalacharyulu, P.; Yetukuri, L.; Islam, S.; Felin, J.; Perkins, R.; Borén, J.; Oresic, M.; et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 2010, 51, 1101–1112. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; Ruiz de Larramendi, I.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Daou, I.; Moukrad, N.; Zegaoui, O.; Rhazi Filali, F. Antimicrobial activity of ZnO-TiO2 nanomaterials synthesized from three different precursors of ZnO: Influence of ZnO/TiO2 weight ratio. Water Sci. Technol. 2018, 77, 1238–1249. [Google Scholar] [CrossRef]
- Sohm, B.; Immel, F.; Bauda, P.; Pagnout, C. Insight into the primary mode of action of TiO2 nanoparticles on Escherichia coli in the dark. Proteomics 2015, 15, 98–113. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 nanoparticles to Escherichia coli: Effects of particle size, crystal phase and water chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.J.; Pietrasiak, N.; Situ, S.F.; Abenojar, E.C.; Porche, M.; Kraj, P.; Lakliang, Y.; Samia, A.C. Iron Oxide and Titanium Dioxide Nanoparticle Effects on Plant Performance and Root Associated Microbes. Int. J. Mol. Sci. 2015, 16, 23630–23650. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Ashraf, A.; Zafar, S.; Zahid, K.; Shah, M.S.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboo, S. Synthesis, characterization, and antibacterial potential of silver nanoparticles synthesized from Coriandrum sativum L. J. Infect. Public Health 2019, 12, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Moriyama, A.; Yamada, I.; Takahashi, J.; Iwahashi, H. Oxidative stress caused by TiO(2) nanoparticles under UV irradiation is due to UV irradiation not through nanoparticles. Chem. Biol. Interact. 2018, 294, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Yousefi, M.; Dadashpour, M.; Hejazi, M.; Hasanzadeh, M.; Behnam, B.; de la Guardia, M.; Shadjou, N.; Mokhtarzadeh, A. Anti-bacterial activity of graphene oxide as a new weapon nanomaterial to combat multidrug-resistance bacteria. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 568–581. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S.; et al. Oral administration of rutile and anatase TiO(2) nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef]
- Rompelberg, C.; Heringa, M.B.; van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; van Bemmel, G.; Brand, W.; Oomen, A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO(2) particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Albukhaty, S.; Al-Bayati, L.; Al-Karagoly, H.; Al-Musawi, S. Preparation and characterization of titanium dioxide nanoparticles and in vitro investigation of their cytotoxicity and antibacterial activity against Staphylococcus aureus and Escherichia coli. Anim. Biotechnol. 2022, 33, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, G.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice than Non-Obese Mice: Gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small 2020, 16, e2001858. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Sulek, A.; Pucelik, B.; Kobielusz, M.; Labuz, P.; Dubin, G.; Dabrowski, J.M. Surface Modification of Nanocrystalline TiO2 Materials with Sulfonated Porphyrins for Visible Light Antimicrobial Therapy. Catalysts 2019, 9, 821. [Google Scholar] [CrossRef]
- Leung, Y.H.; Xu, X.; Ma, A.P.; Liu, F.; Ng, A.M.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurišić, A.B.; Lee, P.K.; et al. Toxicity of ZnO and TiO(2) to Escherichia coli cells. Sci. Rep. 2016, 6, 35243. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Saleem, S.; Ahamed, M.; Ahmad, J. Survival of probiotic bacteria in the presence of food grade nanoparticles from chocolates: An in vitro and in vivo study. Appl. Microbiol. Biotechnol. 2019, 103, 6689–6700. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, B.; Cui, J.; Gao, N.; Sun, H.; Meng, Q.; Wu, S.; Bo, J.; Yan, L.; et al. Prebiotic protects against anatase titanium dioxide nanoparticles-induced microbiota-mediated colonic barrier defects. Nanoimpact 2019, 14, 100164. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.Z.; Chen, L.; Lv, S.D.; Liu, S.J.; Nie, P.H.; Aguilar, Z.P.; Xu, H.Y. Restraining the TiO2 nanoparticles-induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicol. Environ. Saf. 2020, 206, 100164. [Google Scholar] [CrossRef]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Geller, B.; Moniz, K.; Das, P.; Chippindale, A.K.; Walker, V.K. Monitoring the developmental impact of copper and silver nanoparticle exposure in Drosophila and their microbiomes. Sci. Total. Environ. 2014, 487, 822–829. [Google Scholar] [CrossRef]
- Wilkinson, L.J.; White, R.J.; Chipman, J.K. Silver and nanoparticles of silver in wound dressings: A review of efficacy and safety. J. Wound Care 2011, 20, 543–549. [Google Scholar] [CrossRef]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Park, K.; Hong, Y.; Lee, E.S.; Kim, S.S.; Jung, Y.T.; Park, H.; Kwon, C.; Cho, Y.S.; Huh, Y.D. Reactive-oxygen-species-mediated mechanism for photoinduced antibacterial and antiviral activities of Ag(3)PO(4). J. Anal. Sci. Technol. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, M.; Herrer, R.; Casallas, M.C.; Abecia, L.; Ducha, J.J. Silver nanoparticles as a potential antimicrobial additive for weaned pigs. Anim. Feed. Sci. Technol. 2009, 150, 259–269. [Google Scholar] [CrossRef]
- Zhou, H.B.; Yang, D.T.; Ivleva, N.P.; Mircescu, N.E.; Schubert, S.; Niessner, R.; Wieser, A.; Haisch, C. Label-Free in Situ Discrimination of Live and Dead Bacteria by Surface-Enhanced Raman Scattering. Anal. Chem. 2015, 87, 6553–6561. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yu, N.; Wang, C.; Zhou, H.R.; Wu, C.; Yang, L.; Wei, S.; Miao, A.J. Changes in Gut Microbiota Structure: A Potential Pathway for Silver Nanoparticles to Affect the Host Metabolism. ACS Nano 2022, 16, 19002–19012. [Google Scholar] [CrossRef]
- van den Brule, S.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, X.; Wu, J.; Bao, L.; Chen, C. Oral administration of silver nanomaterials affects the gut microbiota and metabolic profile altering the secretion of 5-HT in mice. J. Mater. Chem. B 2023, 11, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Hahn, D.; Ossig, R.; Ritz, S.; Sauer, L.; Buesen, R.; Rehm, S.; Wohlleben, W.; Groeters, S.; Strauss, V.; et al. Gut microbiome and plasma metabolome changes in rats after oral gavage of nanoparticles: Sensitive indicators of possible adverse health effects. Part. Fibre Toxicol. 2022, 19, 21. [Google Scholar] [CrossRef]
- Javurek, A.B.; Suresh, D.; Spollen, W.G.; Hart, M.L.; Hansen, S.A.; Ellersieck, M.R.; Bivens, N.J.; Givan, S.A.; Upendran, A.; Kannan, R.; et al. Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles. Sci. Rep. 2017, 7, 2822. [Google Scholar] [CrossRef]
- Bolandi, N.; Hashemi, S.R.; Davoodi, D.; Dastar, B.; Hassani, S.; Ashayerizadeh, A. Performance, intestinal microbial population, immune and physiological responses of broiler chickens to diet with different levels of silver nanoparticles coated on zeolite. Ital. J. Anim. Sci. 2021, 20, 497–504. [Google Scholar] [CrossRef]
- Chen, P.; Huang, J.; Rao, L.; Zhu, W.; Yu, Y.; Xiao, F.; Chen, X.; Yu, H.; Wu, Y.; Xu, K.; et al. Resistance and Resilience of Fish Gut Microbiota to Silver Nanoparticles. mSystems 2021, 6, e0063021. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, H.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Evaluation of the gut microbiome alterations in healthy rats after dietary exposure to different synthetic ZnO nanoparticles. Life Sci. 2023, 312, 121250. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Yoo, A.; Lin, M.; Mustapha, A. Zinc Oxide and Silver Nanoparticle Effects on Intestinal Bacteria. Materials 2021, 14, 2489. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Qing, Y.; Li, R.; Tang, X.; Guo, D.; Qin, Y. Enhancing ZnO-NP Antibacterial and Osteogenesis Properties in Orthopedic Applications: A Review. Int. J. Nanomed. 2020, 15, 6247–6262. [Google Scholar] [CrossRef]
- Sun, Y.B.; Xia, T.; Wu, H.; Zhang, W.J.; Zhu, Y.H.; Xue, J.X.; He, D.T.; Zhang, L.Y. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim. Feed. Sci. Technol. 2019, 258, 114312. [Google Scholar] [CrossRef]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878–64891. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Chen, C.; Jiang, X.; Qiu, J.; Qiu, Y.; Zhang, Y.; Wang, T.; Qin, X.; Zou, Z.; et al. Crosstalk of gut microbiota and serum/hippocampus metabolites in neurobehavioral impairments induced by zinc oxide nanoparticles. Nanoscale 2020, 12, 21429–21439. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Min, L.; Zhang, W.; Liu, J.; Hou, Z.; Chu, M.; Li, L.; Shen, W.; Zhao, Y.; Zhang, H. Zinc Oxide Nanoparticles Influence Microflora in Ileal Digesta and Correlate Well with Blood Metabolites. Front. Microbiol. 2017, 8, 992. [Google Scholar] [CrossRef]
- Chupani, L.; Barta, J.; Zuskova, E. Effects of food-borne ZnO nanoparticles on intestinal microbiota of common carp (Cyprinus carpio L.). Environ. Sci. Pollut. Res. Int. 2019, 26, 25869–25873. [Google Scholar] [CrossRef]
- Chatterjee, A.; Perevedentseva, E.; Jani, M.; Cheng, C.Y.; Ye, Y.S.; Chung, P.H.; Cheng, C.L. Antibacterial effect of ultrafine nanodiamond against gram-negative bacteria Escherichia coli. J. Biomed. Opt. 2015, 20, 051014. [Google Scholar] [CrossRef]
- Jian, H.J.; Wu, R.S.; Lin, T.Y.; Li, Y.J.; Lin, H.J.; Harroun, S.G.; Lai, J.Y.; Huang, C.C. Super-Cationic Carbon Quantum Dots Synthesized from Spermidine as an Eye Drop Formulation for Topical Treatment of Bacterial Keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Zheng, L.; Ouyang, H.; Wang, H.; Zhou, X.; Zhang, D.; Chai, Z.; Zhao, Y.; et al. Acute Oral Administration of Single-Walled Carbon Nanotubes Increases Intestinal Permeability and Inflammatory Responses: Association with the Changes in Gut Microbiota in Mice. Adv. Healthc Mater. 2018, 7, e1701313. [Google Scholar] [CrossRef] [PubMed]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic antibacterial effect of graphene quantum dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, S.; Wang, Z.; Xue, K.; Su, J.; Song, Y.; Chen, S.; Zhu, C.; Tang, B.Z.; Ye, R. Self-Reporting and Photothermally Enhanced Rapid Bacterial Killing on a Laser-Induced Graphene Mask. ACS Nano 2020, 14, 12045–12053. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Yadav, B.; Rosen, L.; Nagpal, R.; Yadav, H.; Yadav, J.S. Crosstalk between gut microbiota and lung inflammation in murine toxicity models of respiratory exposure or co-exposure to carbon nanotube particles and cigarette smoke extract. Toxicol. Appl. Pharmacol. 2022, 447, 116066. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Chen, X.; Wang, C.; Chen, X.; Liu, W.; Huang, K.; Chen, H.; Yang, J. Multi-walled carbon nanotubes exacerbate doxorubicin-induced cardiotoxicity by altering gut microbiota and pulmonary and colonic macrophage phenotype in mice. Toxicology 2020, 435, 152410. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. Nanoimpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Han, X.Y.; Du, W.L.; Fan, C.L.; Xu, Z.R. Changes in composition a metabolism of caecal microbiota in rats fed diets supplemented with copper-loaded chitosan nanoparticles. J. Anim. Physiol. Anim. Nutr. 2010, 94, e138–e144. [Google Scholar] [CrossRef]
- Wang, M.Q.; Du, Y.J.; Wang, C.; Tao, W.J.; He, Y.D.; Li, H. Effects of copper-loaded chitosan nanoparticles on intestinal microflora and morphology in weaned piglets. Biol. Trace Elem. Res. 2012, 149, 184–189. [Google Scholar] [CrossRef]
- Zhang, T.; Li, D.; Zhu, X.; Zhang, M.; Guo, J.; Chen, J. Nano-Al(2)O(3) particles affect gut microbiome and resistome in an in vitro simulator of the human colon microbial ecosystem. J. Hazard. Mater. 2022, 439, 129513. [Google Scholar] [CrossRef] [PubMed]
- Khajeh Bami, M.; Afsharmanesh, M.; Espahbodi, M.; Esmaeilzadeh, E. Effects of dietary nano-selenium supplementation on broiler chicken performance, meat selenium content, intestinal microflora, intestinal morphology, and immune response. J. Trace Elem. Med. Biol. 2022, 69, 126897. [Google Scholar] [CrossRef] [PubMed]
- Yausheva, E.; Miroshnikov, S.; Sizova, E. Intestinal microbiome of broiler chickens after use of nanoparticles and metal salts. Environ. Sci. Pollut. Res. Int. 2018, 25, 18109–18120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, J.; Yu, N.; Shi, J.; Zhang, Y.; Chen, Z.; Jia, G. Effect of Nanomaterials on Gut Microbiota. Toxics 2023, 11, 384. https://doi.org/10.3390/toxics11040384
Ma Y, Zhang J, Yu N, Shi J, Zhang Y, Chen Z, Jia G. Effect of Nanomaterials on Gut Microbiota. Toxics. 2023; 11(4):384. https://doi.org/10.3390/toxics11040384
Chicago/Turabian StyleMa, Ying, Jiahe Zhang, Nairui Yu, Jiaqi Shi, Yi Zhang, Zhangjian Chen, and Guang Jia. 2023. "Effect of Nanomaterials on Gut Microbiota" Toxics 11, no. 4: 384. https://doi.org/10.3390/toxics11040384
APA StyleMa, Y., Zhang, J., Yu, N., Shi, J., Zhang, Y., Chen, Z., & Jia, G. (2023). Effect of Nanomaterials on Gut Microbiota. Toxics, 11(4), 384. https://doi.org/10.3390/toxics11040384








