Abstract
The liver is considered the major target organ affected by oral exposure to titanium dioxide nanoparticles (TiO2 NPs), but the mechanism of hepatotoxicity is not fully understood. This study investigated the effect of TiO2 NPs on the expression profile of long non-coding RNA (lncRNA) in hepatocytes and tried to understand the potential mechanism of hepatotoxicity through bioinformatics analysis. The human hepatocellular carcinoma cells (HepG2) were treated with TiO2 NPs at doses of 0–200 μg/mL for 48 h and then RNA sequencing was implemented. The differential lncRNAs between the control and TiO2 NPs-treated groups were screened, then the lncRNA–mRNA network and enrichment pathways were analyzed via multivariate statistics. As a result, 46,759 lncRNAs were identified and 129 differential lncRNAs were screened out. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that the targeted mRNAs of those differential lncRNAs were enriched in the Hedgehog signaling pathway, Vasopressin-regulated water reabsorption, and Glutamatergic synapse. Moreover, two lncRNA–mRNA networks, including lncRNA NONHSAT256380.1-JRK and lncRNA NONHSAT173563.1-SMIM22, were verified by mRNA detection. This study demonstrated that an alteration in the lncRNA expression profile could be induced by TiO2 NPs and epigenetics may play an important role in the mechanism of hepatotoxicity.
1. Introduction
Because of favorable mechanical properties and biocompatibility, nanomaterials have been applied to many aspects of our life and work, such as biomedicine [1], the food industry [2], and electronics [3]. The toxicity of nanomaterials has been increasingly studied [4,5]. Titanium dioxide nanoparticles (TiO2 NPs) are one of the most widely used nanomaterials. A study detected the titanium particles in a human post mortem liver and spleen and found that more than 24% of TiO2 was nanoscale [6]. More and more studies have found that TiO2 NPs can be cytotoxic [7,8] and genotoxic [9]. Oxidative stress was induced through the stimulating redox interactions, leading to DNA damage and genomic instability [10,11]. Moreover, there is a growing interest in the in vitro epigenetic changes induced by TiO2 NPs [12,13]. TiO2 NPs are one of the most commonly used nanomaterials in food additives, pharmaceuticals, and personal hygiene products, such as toothpaste [14], so oral exposure is more likely to happen. The liver is a multicellular organ that plays an important role in activating and eliminating many metabolites; therefore, the liver is the primary target organ of oral exposure to TiO2 NPs [15,16,17,18]. Many in vivo experiments found that oral exposure of TiO2 NPs can cause liver damage, hepatocyte necrosis, and liver function damage in mice [19,20]. Moreover, some studies concluded that acute toxicity of rats with TiO2 NPs induced adverse effects in the liver [21,22]. However, the key mechanism of hepatotoxicity induced by TiO2 NPs is not been fully understood and needs further study.
In addition to cytotoxic and genotoxic effects, nanoparticle-induced epigenetic changes and the epigenetic mechanisms behind observed toxicity have also attracted increasing attention. Some studies have found that exposure to nanomaterials can lead to epigenetic changes [23,24]. Pogribna et al. investigated the effect of TiO2 NP exposure on histone modifications, a major epigenetic mechanism in human colorectal (Caco-2) and lung (NL20) epithelial cell lines, and found changes in several histone modifications after exposure to TiO2 NPs [23]. Epigenetics is an important link between genotype and phenotype and plays a key role in the regulation of numerous cellular processes. The main mechanisms of epigenetics include DNA methylation, histone modification, and non-coding RNAs [25]. Non-coding RNAs (ncRNAs) refer to functional RNA molecules that cannot be translated into proteins, among which common regulatory non-coding RNAs include microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), and long non-coding RNAs (lncRNAs). Many ncRNAs can regulate gene expression through interactions with other epigenetic processes, such as histone modification, chromatin remodeling, and DNA methylation [26,27].
LncRNAs are non-coding RNAs whose transcript lengths range from 200 nt to 100 kb and are one of the key factors in gene transcriptional regulation, affecting all aspects of cellular homeostasis [28]. LncRNAs affect nearly all fundamental processes in living cells, including chromatin formation, replication, transcription, splicing, translation, and post-translational modification, and constitute the richest part of the transcriptional genome [29]. According to the position of lncRNA in the genome to nearby messenger RNAs (mRNAs), lncRNAs can be divided into the following five types [30]: long intergenic noncoding RNAs (lincRNAs), natural antisense transcripts (NATs), overlapping, bidirectional, and sense intronic. LincRNAs are open chromatin structures with transcripts less than 10 kb in length and do not appear at any protein-coding site [31]. LincRNAs are also the most numerous of the lncRNA types [32]. NATs are lncRNAs that block splice-site recognition and recruit epigenetic modifiers [33]. Overlapping transcripts are transcribed in the same direction as a protein-coding gene and contain one protein-coding gene [32]. Bidirectional transcripts compete for transcription initiation and promote chromatin modification of target genes [34]. Sense intronic transcripts are introns derived from a protein-coding gene and their transcription direction is the same as that of the neighboring protein-coding gene [30]. A significant amount of evidence suggests that lncRNAs regulate gene expression in multiple ways on the levels of epigenetic, chromatin remodeling, transcription, and translation [35] and they are potential biomarkers for diagnosing, prophesizing, and monitoring disease progression [36,37,38]. Because of the lower levels of splicing, polyadenylation, and nuclear localization, it is more complex to detect and quantify lncRNAs [35].
To fully assess the toxicity of TiO2 NPs, it is critical to assess the epigenetic role of TiO2 NPs. However, there is no report yet to explore the function of lncRNAs in the process of TiO2 NP-induced toxicity. Therefore, this study treated HepG2 cells with 100 μg/mL TiO2 NPs for 48 h and investigated the changes in lncRNAs.
2. Materials and Methods
2.1. Characterization of Nanomaterials
The TiO2 NPs used in this study were obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). The detailed characterization methods and physicochemical properties of TiO2 NPs were described in our published paper [39]. JEM–1400 electron microscope (JEOL Company, Tokyo, Japan) was used to measure the equivalent diameter. X-ray powder diffractometry (XRD, PANalytical’s X’Pert PRO, X’Celerator, Almelo, The Netherlands) was used to test the crystal form. Dynamic light scattering instrument Zetasizer Nano ZS90 (Malvern Instruments Ltd., Malvern, UK) was used to measure the hydrated particle size and Zeta potential in the serum-free medium containing 1 mg/mL TiO2 NPs.
2.2. Cell Culture
Human hepatocellular carcinoma cells (HepG2), obtained from the National Biomedical Experimental Cell Resource Library of China, were routinely cultured in Minimum Essential Medium (MEM, HyClone, Thermo Scientific, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Thermo Scientific, Logan, UT, USA), 1% MEM Non-Essential Amino Acids Solution (100×) (NEAA, Gibco, Thermo Scientific, Logan, UT, USA), and 2% GlutaMAX-1 (Gibco, Thermo Scientific, Logan, UT, USA). For subculturing purposes, the cells were digested by 0.25% trypsin and seeded to 96-well plates at a density of 1 × 104 cells per well or 60 × 15 MM plates with 5 × 105 cells per well.
2.3. Cytotoxicity Assay Study
Cell Counting Kit-8 assay (CCK-8, Biotopped, Dojindo Laboratories, Kumamoto, Japan) was used to determine the cytotoxicity of TiO2 NPs, based on the measurement of the amount of methotrexate generated proportional to the number of living cells. After exposure to 0, 1.5625, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL TiO2 NPs for 48 h, the cells in the 96-well plate were incubated with CCK-8 solution for 2 h. After collecting the supernatants, a microplate reader was used to detect the value of absorbance at 450 nm, taking 600 nm as a parameter. The computation formula is as follows: cell viability = (E − B)/ (C − B). E refers to the experimental hole (containing cell, culture medium, CCK 8, and different concentrations of TiO2 NPs), C refers to the control hole (containing cell, culture medium, and CCK 8), and B refers to a blank hole without any cells and TiO2 NPs.
2.4. Construction of cDNA Libraries and RNA Sequencing
Every control and treatment group set up three repeat samples and then the samples were collected for RNA extraction. The extracted total RNA was qualified by Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and purified by RNAClean XP Kit (Cat A63987, Beckman Coulter, Inc., Kraemer Boulevard, Brea, CA, USA) and RNase-Free DNase Set (Cat#79254, QIAGEN, GmBH, Dusseldorf, Germany).
The purified total RNA was carried out with rRNA removal, fragmentation, first-strand cDNA synthesis, second-strand cDNA synthesis, end repair, 3′ end plus A, ligation joint, and enrichment. The cDNA was then sequenced with a high-throughput sequencer (Illumina Hiseq 2000/2500, San Diego, CA, USA).
2.5. Identification and Quantification of lncRNAs
Gffcompare (version 0.9.8) was applied to identify new transcripts that did not match known annotations and three types of transcripts were picked out with the conditions that transcription length was greater than or equal to 200 bp, the number of exons was greater than or equal to 2, and open reading frame (ORF) was less than 300 bp. Then, Contrastive Predictive Coding analysis (CPC), Coding-Non-Coding Index (CNCI) analysis, and Pfam protein domain analysis were performed to predict the lncRNAs. CPC used supervised machine learning to establish a classification model by learning peptide chain length, amino acid composition, protein homology, secondary structure, protein alignment, or expression [40]. Its classification model was mainly based on the characteristics of sequence ORF length and protein homology; Pfam was a large database of protein family collections, represented by multiple sequence alignments and hidden Markov models (HMMs) [41]. The assembled transcript sequence was annotated by the PfamScan tool. If the sequence matched the Pfam protein database, it was mRNA, and there was no comparison on lncRNA. CNCI identified coded and non-coding sequences by analyzing adjacent nucleotide triplets [42]. Then the transcript with CPC score < 0 and CNCI score < 0 and insignificant results of Pfam was picked out as potential lncRNAs. Finally, it was merged with the NONCODE data database (version: NONCODE 2016; http://www.noncode.org/, accessed on 15 November 2020) and the known lncRNAs in the Ensembl database to form the lncRNA sequence for subsequent analysis.
String tie (version: 1.3.0) was applied to quantify the expression of lncRNA sequences. Then edgeR was applied for differential lncRNA analysis between samples and the p-value was corrected through a multiple-hypothesis test, and the q-value was the corrected p-value by controlling FDR (False-Discovery Rate). The differential expression multiple fold change was calculated based on the FPKM value. The differential lncRNA filters were as follows: q-value ≤ 0.05 and fold change ≥ 2.
The structure of lncRNA and mRNA was compared and analyzed by comparing the differences in transcript length, exon number, and expression level of lncRNA and mRNA. The difference between lncRNA and mRNA molecules was obtained and the predicted lncRNA molecules were verified.
Trans regulation and Cis regulation were used for target gene prediction. Cis referred to how lncRNA regulated neighboring mRNAs (e.g., on the same chromosome) and trans referred to targets at the distal position of chromosomes after different chromosomes.
Finally, KEGG enrichment (https://www.kegg.jp/, accessed on 15 November 2020) was used to analyze the target gene analysis of the differential lncRNA. The selected differentially expressed genes were mapped to each term of the KEGG database, the number of genes for each entry was calculated, and then a super geometric test was applied to a threshold of p-value ≤ 0.05 after correction by multiple-hypothesis tests, and the KEGG term that satisfied this condition was defined as the KEGG term that was significantly enriched in the differentially expressed genes.
2.6. Statistical Analysis
The numerical data were presented as mean ± standard deviation (m ± SD) of at least three determinations. The statistical analysis was performed by R 3.1.3. A p-value less than 0.05 was defined as statistical significance.
3. Results
3.1. Identification of TiO2 NPs
The TiO2 NPs used in the study were spherical and anatase type. Transmission electron microscopy (TEM) showed that the equivalent diameter of TiO2 NPs was 25.12 ± 5.64 nm. The hydrated particle size of TiO2 NPs (1 mg/mL) in a serum-free medium was 323.50 ± 85.44 nm and the zeta potential was −21.00 ± 0.72 mV (Figure 1).
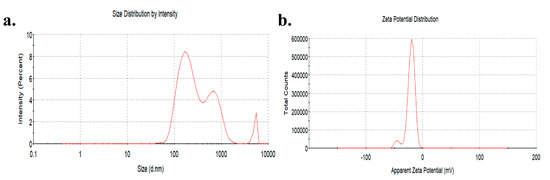
Figure 1.
Distribution of hydration particle size (a) and zeta potential (b) of TiO2 NPs in a serum-free medium.
3.2. Cytotoxicity of TiO2 NPs in HepG2 Cells
After 48 h exposure, the cell viability decreased gradually with an increase in the concentration of TiO2 NPs, and the cell viability of the 200 μg/mL group (65.25%) decreased significantly compared with the control groups, but because the cell viability of the 200 μg/mL group was too low, the final choice was 100 μg/mL (74.16%) as the concentration of the TiO2 NP treatment groups (Figure 2).
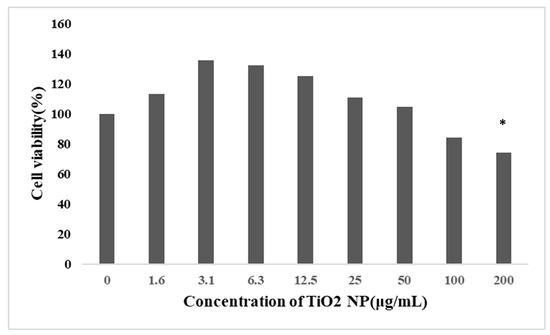
Figure 2.
Effect of TiO2 NPs on the viability of HepG2 cells (mean ± SD, n = 3). Each group set up three repeat biological samples. HepG2 cells were treated with TiO2 NPs at 0, 1.5625, 3.125, 6.25, 12.5, 25, 50, 100, and 200 μg/mL for 48 h. The cell viability was significantly decreased in the treatment groups at a concentration of 200 μg/mL. Cell viability did not decrease in a dose-dependent relationship. Significant difference from the control (* p < 0.05).
3.3. Predictions and Annotations of lncRNA-Seq Data
A total of 46,759 lncRNAs, including known and predicted lncRNAs, was identified. According to the position relationship of lncRNAs in the genome to nearby mRNAs, the number of intronic_sense, intronic_antisense, exonic_sense exonic_antisense, intergenic, and bidirectional RNA was 5089, 1621, 12,782, 9855, 12,829, and 4583, respectively. Further, 27.4% of the lncRNAs were lincRNAs, ranking first. Principal component analysis (PCA) scoring plots revealed that the control group and the treatment groups were separated, which represented the difference in lncRNA characteristics (Figure 3).
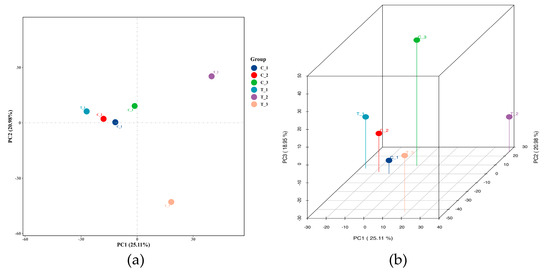
Figure 3.
lncRNA expression analysis of TiO2 NPs with a concentration of 0 and 100 μg/mL. Princi-pal component analysis (PCA) 2D (a) and 3D (b) plots were drawn based on the expression of trusted lncRNAs to compare the difference between the control and the treatment groups.
The length of the lncRNAs was between 32 and 674,512 bp and the median length was 821 bp. As shown in Figure 4a, lncRNA was slightly shorter than the mRNA (median length is 953 bp). Approximately 30.3% of lncRNAs contained two exons, while mRNAs contained several exons from 1 to 363 (Figure 4b). Expression-level analysis showed that the overall expression level of lncRNA was slightly lower than the expression level of mRNA (mean 0.31:0.52, Figure 4c).
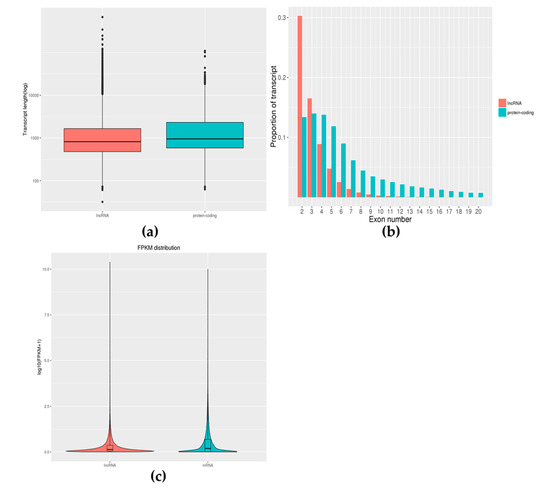
Figure 4.
Feature comparison of lncRNA with mRNA. Comparative analysis of the length distribution of lncRNA and mRNA was performed and we found that lncRNA was slightly shorter than the mRNA (a). Comparative analysis of exon numbers of lncRNA and mRNA was performed and we found that mRNAs contained a larger range of exons (b). The expression values of lncRNAs and mRNAs were averaged separately and the box pattern was plotted with the values of both log10 (FPKM + 1) (c). Comparing the expression levels of lncRNAs and mRNAs, it was found that there was a difference in the expression levels of the two.
3.4. Analysis of Differential Expression of lncRNA
Finally, 129 differential lncRNAs were screened, of which 65 were up-regulated and 64 were down-regulated (Figure 5a). Among the differential lncRNAs, there was 1 belonging to intronic sense,1 intronic antisense, 51 exonic sense, 31 exonic antisense, 33 intergenic, and 12 bidirectional, respectively (Figure 5b). The cluster heat maps of the differential lncRNAs in the TiO2 NP treatment groups compared with the control group are shown in Figure 5c, suggesting that the effect on lncRNA was different between the treatment and control groups. According to the expression profile of lncRNAs in human tissues in the NONCODE database, the up-regulated lncRNAs were mainly expressed in the testes and placenta and the down-regulated lncRNAs were mainly expressed in the adrenal, kidney, and brain.
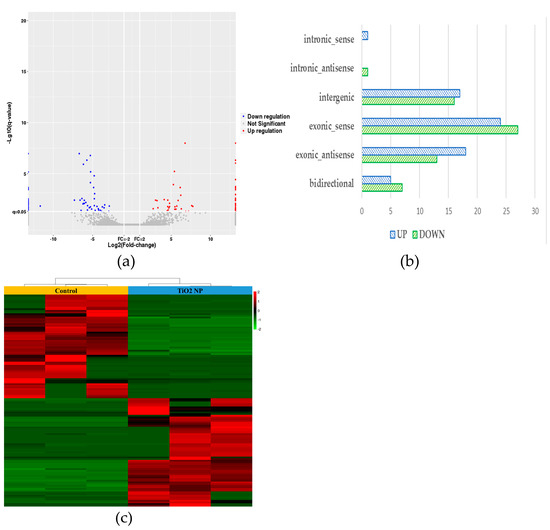
Figure 5.
lncRNA differential expression analysis of TiO2 NPs with concentrations of 0 and 100 μg/mL. A volcanic map of differentially expressed genes in the treatment group showed the number of up-regulated and down-regulated genes (a). The histogram of the relative expression of differential lncRNAs was drawn and showed that the lncRNAs belonging to the exonic-sense class counts had the highest proportion (b). A heat map of cluster analysis between the treatment group and control group demonstrated their characteristic difference (c).
3.5. Enrichment Analysis of Differential lncRNA Target Genes
The target gene of differential lncRNA was intersected with mRNA. As a result, the lncRNA NONHSAT173563.1 was down-regulated and the matching mRNA SMIM22 was up-regulated (p < 0.05). The lncRNA NONHSAT256380.1 was down-regulated and the matching mRNA JRK was down-regulated (p < 0.05) (Figure 6a). The changes in the two matching mRNAs were statistically significant.
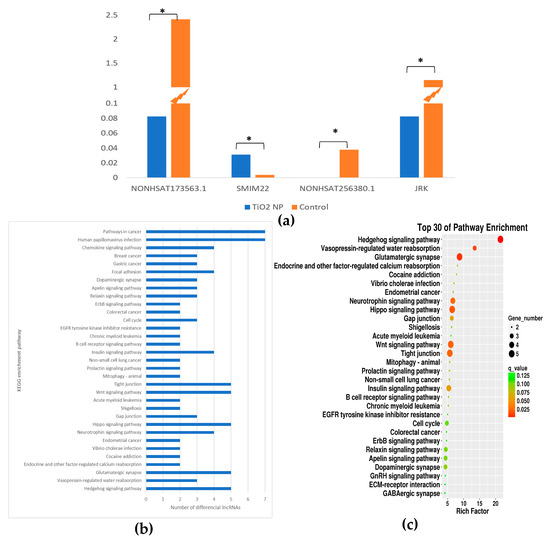
Figure 6.
Pathway analysis of TiO2 NPs with concentrations of 0 and 100 μg/mL. A bar graph of differential lncRNAs numbers in different pathways of KEGG was drawn (a). A KEGG enrichment analysis bubble plot was drawn in the descending order of q value corresponding to each entry in the TiO2 NP treatment group (b). The relative content of the two lncRNAs and corresponding mRNAs in the TiO2 NP treatment and the control group was drawn in a histogram (* p < 0.05) (c).
KEGG enrichment analysis was performed on the intersecting gene. The results showed that the Hedgehog signaling pathway, Vasopressin-regulated water reabsorption, and Glutamatergic synapse were the three most significant pathways of enrichment (q < 0.05) (Figure 6b,c). In the Hedgehog signaling pathway, NONHSAT041057.2, NONHSAT091417.2, NONHSAT250525.1, NONHSAT056661.2, and MSTRG.32312.1 changed.
4. Discussion
TiO2 NPs are exposed to the human body through many pathways and have adverse effects on human health. Moreover, the liver is the target organ of oral exposure to TiO2 NPs. The objective of this study was to analyze the effects of TiO2 NP exposure on the expression profile of lncRNAs and we tried to understand the potential mechanism of hepatotoxicity through bioinformatics analysis. Through the differential lncRNA analysis and lncRNA–mRNA network, we found that TiO2 NPs could induce a change in the expression profile of lncRNAs and may interfere with the Hedgehog signaling pathway and Glutamatergic synapse, eventually leading to hepatotoxicity.
From the CCK-8 assay, TiO2 NPs can be slightly toxic to human liver cells. However, many researchers have found that hepatotoxicity is one of the target organ effects of oral exposure to TiO2 NPs [17,43]. Geraets et al. [43] investigated the tissue distribution and blood kinetics of various TiO2 NPs in rats and found that the liver was identified as the main target tissue, followed by the spleen and lung. Another study found that the liver was the tissue most sensitive to TiO2 NP-induced oxidative stress [44]. Many in vivo studies have found that TiO2 NPs may produce ROS and promote oxidative stress and liver inflammation [44,45,46]. Sprague-Dawley rats were orally exposed to 0, 2, 10, and 50 mg/kg TiO2 NPs for 90 days and were found to induce tissue-specific oxidative stress and elemental imbalance in the liver [44]. In addition, many in vitro studies have found that TiO2 NPs can induce damage to hepatocyte line cells [47]. Current major toxicity mechanisms may exert cytotoxic effects on the structure and function of the liver by inducing oxidative stress, inflammation, and apoptosis [16,48,49]. Oxidative stress, considered a common mechanism of the toxicity in NPs, can damage lipids, carbohydrates, proteins, and DNA, ultimately leading to hepatotoxicity [50]. Azim et al. treated mice with anatase TiO2 NPs (21 nm, 150 mg/kg/day) for 2 weeks and then added three kinds of antioxidants (idebenone, carnosine, and vitamin E) for 1 month. They finally found that TiO2 NPs significantly injured liver function and can be alleviated after the use of antioxidants [49]. This study attempted to further understand the new mechanism of hepatotoxicity from the perspective of epigenetics and found that lncRNAs may play an important role.
In the study, some changes in lncRNAs and changes in the mRNAs matched with differential lncRNAs occurred, with statistical significance, implying that epigenetics may play a role in hepatotoxicity. Epigenetics is an important link in the regulation of genotype and phenotype. The regulation and dysregulation of genotype and phenotype often lead to the occurrence of diseases and have long-term negative effects. According to the 3R principle, epigenetics is also gradually being used in the toxicity study of nanomaterials. Some studies have also found that, in addition to genetic and cytotoxic effects, they can also affect the epigenome of target cells [23,51]. Lu et al. exposed human and murine macrophages (THP-1 and RAW264.7, respectively) and human small-airway epithelial cells (SAECs) to environmentally relevant concentrations of TiO2 NPs, resulting in modest alterations in DNA methylation [51]. Another study also found that low concentrations of TiO2 NPs can alter the enzymes responsible for epigenetic modifications [52]. Because their concentrations are well below sublethal levels, changes in DNA methylation can serve as good biomarkers of early exposure to TiO2 NPs. Therefore, epigenetic studies are critical for a complete assessment of potential risks from nanoparticle exposure.
In recent years, lncRNAs have become an important class of regulators of gene expression and epigenetic regulation [53]. Some reports found that lncRNAs play a role in cell-cycle regulation, apoptosis, and the establishment of cellular identity [54,55]. Changes in the expression of lncRNAs have been proven to be linked with cancer (e.g., prostate cancer) and several neurological disorders [31,56]. One study proposed that the use of electrochemical nucleic acid sensors is very sensitive to lncRNA HULC detection, providing a new alternative for clinical HCC diagnosis [57]. The study did find that certain lncRNAs (such as NONHSAT256380.1 and NONHSAT173563.1) showed remarkable changes, which may be prevalent to the hepatotoxicity of TiO2 NPs. Therefore, lncRNAs can help to study the mechanism of hepatotoxicity in more depth and explore the role of epigenetic regulation in hepatotoxicity.
In addition, small integral membrane protein 22 (SMIM22, CASIMO1), matched with the up-regulated lncRNA (NONHSAT173563.1), has been shown to play a key role in carcinogenesis, cell proliferation, and cell lipid homeostasis [58]. The depletion of Jrk helix-turn-helix protein (JRK, JH8, jerky), matched with the down-regulated lncRNA (NONHSAT256380.1), inhibits the transcriptional activity of β-catenin and reduces cell proliferation, and it has been validated for carcinogenic effects in primary tumors [59]. From the result of KEGG enrichment analysis, TiO2 NPs could interfere with the Hedgehog signaling pathway, which played a key role in tissue development and dryness. The imbalance in the Hedgehog signaling pathway was present in many different tumors, such as skin, brain, liver, and gallbladder [60]. There are three homology genes for Hedgehogs in mammals: Sonic Hedgehog (SHH), Indian Hedgehog (IHH), and Desert Hedgehog (DHH) [61]. Hedgehog signaling is controlled by two receptors, Patched (Ptc) and Smoothened (Smo), on the membrane of the target cell [62]. These unique signaling molecules are highly expressed in most malignant tissues and have been considered biomarkers for progression and prognosis [63,64]. Additionally, many in vitro studies have found that chronic liver damage or liver cancer may activate the sonic hedgehog (SHH) pathway [65,66].
The main advantage of this article is the use of epigenetics to study the alterations in the lncRNA expression profile induced by TiO2 NPs in hepatotoxicity. In the future, the influence of oral exposure to nano-titanium dioxide on epigenetics and related mechanisms can be further studied. However, this study also has some drawbacks. Firstly, this study lacks more in-depth studies on screened lncRNAs and, secondly, the verification of this study is at the mRNA level, so there is a lack of PCR verification at the lncRNA level. Therefore, we will next conduct more in-depth studies on differential lncRNAs, such as knocking out relevant genes to study their impact on subsequent functions. We will also further focus on the effects of apoptosis or genetic damage of TiO2 NPs.
5. Conclusions
The present study focused on alterations in the lncRNA expression profile in HepG2 cells after exposure to TiO2 NPs and its potential role in the mechanism of hepatotoxicity. It was demonstrated that exposure to TiO2 NPs could induce a series of differential lncRNAs, represented by lncRNA NONHSAT256380.1 and lncRNA NONHSAT173563.1. Meanwhile, the target gene analysis indicated that these differential lncRNAs may be involved in hepatotoxicity by interfering with the Hedgehog signaling pathway. Two lncRNA–mRNA networks, including lncRNA NONHSAT256380.1-JRK and lncRNA NONHSAT173563.1-SMIM22, were verified. It was suggested that epigenetics may play an important role in the mechanism of hepatotoxicity induced by TiO2 NPs.
Author Contributions
Conceptualization, Z.C.; methodology, J.S.; software, J.S.; writing—original draft preparation, J.S.; data curation, Y.Z. and Y.M.; writing—review and editing, J.S., Y.Z. and Y.M.; visualization, J.S.; supervision, Z.C. and G.J.; project administration, Z.C. and G.J.; funding acquisition, Z.C. and G.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (81703257) and National Key R&D Program of the Ministry of Science and Technology of China (2017YFC1600200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Garoli, D.; Lovato, L.; Della Giustina, G.; Oliverio, M.; Francardi, M.; Zanchetta, E.; Brusatin, G.; De Angelis, F. Directly nanopatternable nanoporous titania—Application to cell growth engineering. Microelectron. Eng. 2016, 155, 102–106. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Lah, N.A.C.; Zubir, M.N.M.; Samykano, M.A.L. Chapter 20—Engineered Nanomaterial in Electronics and Electrical Industries. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 324–364. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Hamdy, N.M.; Boseila, A.A.; Ramadan, A.; Basalious, E.B. Iron Oxide Nanoparticles-Plant Insignia Synthesis with Favorable Biomedical Activities and Less Toxicity, in the “Era of the-Green”: A Systematic Review. Pharmaceutics 2022, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Heringa, M.B.; Peters, R.J.B.; Bleys, R.; van der Lee, M.K.; Tromp, P.C.; van Kesteren, P.C.E.; van Eijkeren, J.C.H.; Undas, A.K.; Oomen, A.G.; Bouwmeester, H. Detection of titanium particles in human liver and spleen and possible health implications. Part Fibre Toxicol. 2018, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Sarikhani, M.; Vaghefi Moghaddam, S.; Firouzamandi, M.; Hejazy, M.; Rahimi, B.; Moeini, H.; Alizadeh, E. Harnessing rat derived model cells to assess the toxicity of TiO(2) nanoparticles. J. Mater. Sci. Mater. Med. 2022, 33, 41. [Google Scholar] [CrossRef] [PubMed]
- Elje, E.; Mariussen, E.; Moriones, O.H.; Bastús, N.G.; Puntes, V.; Kohl, Y.; Dusinska, M.; Rundén-Pran, E. Hepato(Geno)Toxicity Assessment of Nanoparticles in a HepG2 Liver Spheroid Model. Nanomaterials 2020, 10, 545. [Google Scholar] [CrossRef]
- Kirkland, D.; Aardema, M.J.; Battersby, R.V.; Beevers, C.; Burnett, K.; Burzlaff, A.; Czich, A.; Donner, E.M.; Fowler, P.; Johnston, H.J.; et al. A weight of evidence review of the genotoxicity of titanium dioxide (TiO(2)). Regul. Toxicol. Pharmacol. RTP 2022, 136, 105263. [Google Scholar] [CrossRef]
- Safwat, G.; Mohamed, A.A.; Mohamed, H.R.H. Estimation of genotoxicity, apoptosis and oxidative stress induction by TiO(2) nanoparticles and acrylamide subacute oral coadministration in mice. Sci. Rep. 2022, 12, 18648. [Google Scholar] [CrossRef]
- Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Kumari, P.; Singh, S.; Sinha, A.; Saha, A.K.; Kaushik, N.K.; Raina, V.; et al. In vivo intrinsic atomic interaction infer molecular eco-toxicity of industrial TiO(2) nanoparticles via oxidative stress channelized steatosis and apoptosis in Paramecium caudatum. Ecotoxicol. Environ. Saf. 2022, 241, 113708. [Google Scholar] [CrossRef]
- Pogribna, M.; Word, B.; Lyn-Cook, B.; Hammons, G. Effect of titanium dioxide nanoparticles on histone modifications and histone modifying enzymes expression in human cell lines. Nanotoxicology 2022, 16, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Costa, C.; Pires, J.; Teixeira, J.P.; Fraga, S. How can exposure to engineered nanomaterials influence our epigenetic code? A review of the mechanisms and molecular targets. Mutat. Res. Rev. Mutat. Res. 2021, 788, 108385. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Gramacho, A.; Rolo, D.; Vital, N.; Silva, M.J.; Louro, H. Cellular and Molecular Mechanisms of Toxicity of Ingested Titanium Dioxide Nanomaterials. Adv. Exp. Med. Biol. 2022, 1357, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Paulraj, R. Oxidative stress mediated cytotoxicity of TiO2 nano anatase in liver and kidney of Wistar rat. Toxicol. Environ. Chem. 2012, 94, 146–163. [Google Scholar] [CrossRef]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: In vivo and in vitro study. Int. J. Nanomed. 2019, 14, 1919–1936. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Kumar, A.; Vallabani, N.V.; Pandey, A.K.; Dhawan, A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine 2014, 9, 1423–1434. [Google Scholar] [CrossRef]
- Ali, S.A.; Rizk, M.Z.; Hamed, M.A.; Aboul-Ela, E.I.; El-Rigal, N.S.; Aly, H.F.; Abdel-Hamid, A.Z. Assessment of titanium dioxide nanoparticles toxicity via oral exposure in mice: Effect of dose and particle size. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2019, 24, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Shirdare, M.; Jabbari, F.; Salehzadeh, M.; Ziamajidi, N.; Nourian, A.; Heidarisasan, S.; Ghavimishamekh, A.; Taheri Azandariani, M.; Abbasalipourkabir, R. Curcuma reduces kidney and liver damage induced by titanium dioxide nanoparticles in male Wistar rats. Avicenna J. Phytomedicine 2022, 12, 537–547. [Google Scholar] [CrossRef]
- Sallam, M.F.; Ahmed, H.M.S.; El-Nekeety, A.A.; Diab, K.A.; Abdel-Aziem, S.H.; Sharaf, H.A.; Abdel-Wahhab, M.A. Assessment of the Oxidative Damage and Genotoxicity of Titanium Dioxide Nanoparticles and Exploring the Protective Role of Holy Basil Oil Nanoemulsions in Rats. Biol. Trace Elem. Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Hassanein, K.M.A.; El-Amir, Y.O. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int. J. Vet. Sci. Med. 2018, 6, 16–21. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, Y.; Li, Y.; Zhao, C.; Song, B.; Li, Y.; Zhou, Y. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ. Toxicol. Pharmacol. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Pogribna, M.; Koonce, N.A.; Mathew, A.; Word, B.; Patri, A.K.; Lyn-Cook, B.; Hammons, G. Effect of titanium dioxide nanoparticles on DNA methylation in multiple human cell lines. Nanotoxicology 2020, 14, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Y.; Ye, H.; Huang, K.; Lv, Z.; Ke, Y. Different effects of titanium dioxide nanoparticles instillation in young and adult mice on DNA methylation related with lung inflammation and fibrosis. Ecotoxicol. Environ. Saf. 2019, 176, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.I.; Valdés, A.; Fernández, A.F.; Torrecillas, R.; Fraga, M.F. The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome. Int. J. Nanomed. 2016, 11, 6297–6306. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Wang, H. Long noncoding RNAs in DNA methylation: New players stepping into the old game. Cell Biosci. 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Peschansky, V.J.; Wahlestedt, C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Liu, L.; Deng, H.; Zhang, J.; Xu, Q.; Cen, B.; Ji, A. Regulation of lncRNA expression. Cell. Mol. Biol. Lett. 2014, 19, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, R.X. Regulatory non-coding RNAs: Revolutionizing the RNA world. Mol. Biol. Rep. 2014, 41, 3915–3923. [Google Scholar] [CrossRef]
- Knauss, J.L.; Sun, T. Regulatory mechanisms of long noncoding RNAs in vertebrate central nervous system development and function. Neuroscience 2013, 235, 200–214. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Knee, R.; Murphy, P.R. Regulation of gene expression by natural antisense RNA transcripts. Neurochem. Int. 1997, 31, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Pelechano, V.; Järvelin, A.I.; Steinmetz, L.M. Functional consequences of bidirectional promoters. Trends Genet. TIG 2011, 27, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wahlestedt, C.; Kapranov, P. Strategies to Annotate and Characterize Long Noncoding RNAs: Advantages and Pitfalls. Trends Genet. TIG 2018, 34, 704–721. [Google Scholar] [CrossRef]
- Mutzel, V.; Schulz, E.G. Dosage Sensing, Threshold Responses, and Epigenetic Memory: A Systems Biology Perspective on Random X-Chromosome Inactivation. BioEssays News Rev. Mol. Cell. Dev. Biol. 2020, 42, e1900163. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, L.; Wan, F. Molecular mechanisms of TUG1 in the proliferation, apoptosis, migration and invasion of cancer cells. Oncol. Lett. 2019, 18, 4393–4402. [Google Scholar] [CrossRef]
- St Laurent, G.; Vyatkin, Y.; Antonets, D.; Ri, M.; Qi, Y.; Saik, O.; Shtokalo, D.; de Hoon, M.J.; Kawaji, H.; Itoh, M.; et al. Functional annotation of the vlinc class of non-coding RNAs using systems biology approach. Nucleic Acids Res. 2016, 44, 3233–3252. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Han, S.; Zheng, P.; Chen, Z.; Jia, G. Titanium dioxide nanoparticles induced reactive oxygen species (ROS) related changes of metabolomics signatures in human normal bronchial epithelial (BEAS-2B) cells. Toxicol. Appl. Pharmacol. 2022, 444, 116020. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Bailey, T.L.; Perkins, A.C.; Tallack, M.R.; Xu, Z.; Liu, H. Prediction of novel long non-coding RNAs based on RNA-Seq data of mouse Klf1 knockout study. BMC Bioinform. 2012, 13, 331. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, P.; Han, S.; Zhang, J.; Li, Z.; Zhou, S.; Jia, G. Tissue-specific oxidative stress and element distribution after oral exposure to titanium dioxide nanoparticles in rats. Nanoscale 2020, 12, 20033–20046. [Google Scholar] [CrossRef] [PubMed]
- Attia, H.F.; Soliman, M.M.; Abdel-Rahman, G.H.; Nassan, M.A.; Ismail, S.A.; Farouk, M.; Solcan, C. Hepatoprotective Effect of N-Acetylcystiene on the Toxic Hazards of titanium Dioxide Nanoparticles. Am. J. Pharmacol. Toxicol. 2013, 8, 141. [Google Scholar] [CrossRef]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The Potential Liver, Brain, and Embryo Toxicity of Titanium Dioxide Nanoparticles on Mice. Nanoscale Res. Lett. 2017, 12, 478. [Google Scholar] [CrossRef]
- Wang, Y.; Aker, W.G.; Hwang, H.M.; Yedjou, C.G.; Yu, H.; Tchounwou, P.B. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci. Total Environ. 2011, 409, 4753–4762. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fan, X.; Yin, Y.; Guo, Q.; Yang, D.; Wei, X.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; et al. Mechanisms of titanium dioxide nanoparticle-induced oxidative stress and modulation of plasma glucose in mice. Environ. Toxicol. 2019, 34, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Azim, S.A.; Darwish, H.A.; Rizk, M.Z.; Ali, S.A.; Kadry, M.O. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: Possible role of some antioxidants. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2015, 67, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Havrilla, C.M.; Brady, T.C.; Abramo, K.H.; Levin, E.D. Oxidative stress in toxicology: Established mammalian and emerging piscine model systems. Environ. Health Perspect. 1998, 106, 375–384. [Google Scholar] [CrossRef]
- Lu, X.; Miousse, I.R.; Pirela, S.V.; Melnyk, S.; Koturbash, I.; Demokritou, P. Short-term exposure to engineered nanomaterials affects cellular epigenome. Nanotoxicology 2016, 10, 140–150. [Google Scholar] [CrossRef]
- Jayaram, D.T.; Payne, C.K. Intracellular Generation of Superoxide by TiO(2) Nanoparticles Decreases Histone Deacetylase 9 (HDAC9), an Epigenetic Modifier. Bioconjugate Chem. 2020, 31, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 2011, 12, 136–149. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Prensner, J.R.; Iyer, M.K.; Balbin, O.A.; Dhanasekaran, S.M.; Cao, Q.; Brenner, J.C.; Laxman, B.; Asangani, I.A.; Grasso, C.S.; Kominsky, H.D.; et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat. Biotechnol. 2011, 29, 742–749. [Google Scholar] [CrossRef]
- Liu, F.; Xiang, G.; Jiang, D.; Zhang, L.; Chen, X.; Liu, L.; Luo, F.; Li, Y.; Liu, C.; Pu, X. Ultrasensitive strategy based on PtPd nanodendrite/nano-flower-like@GO signal amplification for the detection of long non-coding RNA. Biosens. Bioelectron. 2015, 74, 214–221. [Google Scholar] [CrossRef]
- Polycarpou-Schwarz, M.; Gross, M.; Mestdagh, P.; Schott, J.; Grund, S.E.; Hildenbrand, C.; Rom, J.; Aulmann, S.; Sinn, H.P.; Vandesompele, J.; et al. The cancer-associated microprotein CASIMO1 controls cell proliferation and interacts with squalene epoxidase modulating lipid droplet formation. Oncogene 2018, 37, 4750–4768. [Google Scholar] [CrossRef] [PubMed]
- Pangon, L.; Ng, I.; Giry-Laterriere, M.; Currey, N.; Morgan, A.; Benthani, F.; Tran, P.N.; Al-Sohaily, S.; Segelov, E.; Parker, B.L.; et al. JRK is a positive regulator of beta-catenin transcriptional activity commonly overexpressed in colon, breast and ovarian cancer. Oncogene 2016, 35, 2834–2841. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, V.; Stecca, B. Role of Protein Kinases in Hedgehog Pathway Control and Implications for Cancer Therapy. Cancers 2019, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, H.Y.; Zhang, L.; Zhou, Y.; Wu, J. Hedgehog Signaling, a Critical Pathway Governing the Development and Progression of Hepatocellular Carcinoma. Cells 2021, 10, 123. [Google Scholar] [CrossRef]
- Jeng, K.S.; Chang, C.F.; Lin, S.S. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int. J. Mol. Sci. 2020, 21, 758. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, K.; Wang, Y.; Yao, B.; Li, Q.; Wang, L.; Dou, C.; Liu, Q.; Zheng, X. Hypoxia Accelerates Aggressiveness of Hepatocellular Carcinoma Cells Involving Oxidative Stress, Epithelial-Mesenchymal Transition and Non-Canonical Hedgehog Signaling. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- de Reyniès, A.; Javelaud, D.; Elarouci, N.; Marsaud, V.; Gilbert, C.; Mauviel, A. Large-scale pan-cancer analysis reveals broad prognostic association between TGF-β ligands, not Hedgehog, and GLI1/2 expression in tumors. Sci. Rep. 2020, 10, 14491. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, H.X.; Li, J.M.; Li, X.Y.; Li, Y.N.; Shi, Y.; Wang, D. Sonic hedgehog signaling pathway mediates development of hepatocellular carcinoma. Tumor. Biol. 2016, 37, 16199–16205. [Google Scholar] [CrossRef] [PubMed]
- Swiderska-Syn, M.; Xie, G.; Michelotti, G.A.; Jewell, M.L.; Premont, R.T.; Syn, W.K.; Diehl, A.M. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology 2016, 64, 232–244. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).