Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum
Abstract
1. Introduction
2. Materials and Methods
2.1. Surfactants
2.2. Reagents
2.3. Active Matter Analysis
2.4. Critical Micelle Concentration
2.5. Zeta Potential (ζ-Potential) and Micelle Diameter (MD)
2.6. Pseudomonas Putida Toxicity Test
2.7. Marine Algae Toxicity Test
2.8. Model of Toxic Units
2.9. Statistical Analysis
3. Results
3.1. Critical Micelle Concentration (CMC)
3.2. Zeta Potential and Micelle Diameter
3.3. Toxicity of Individual Surfactants
3.4. Toxicity of Surfactants Mixtures
4. Discussion
4.1. Zeta Potential and Micelle Diameter of Individual and Mixtures of the Surfactant Solutions
4.2. Toxicity of Individual Surfactants
4.3. Toxicity of Surfactants Mixtures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Modor Intelligence. Surfactants Market, Growth, Trend, COVID-19 Impact and Forecast (2022–2027). 2022. Available online: https://www.Mordorintelligence.Com/Industry-Reports/Surfactants-Market (accessed on 21 January 2023).
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in Aquatic and Terrestrial Environment: Occurrence, Behavior, and Treatment Processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef] [PubMed]
- Aloui, F.; Kchaou, S.; Sayadi, S. Physicochemical Treatments of Anionic Surfactants Wastewater: Effect on Aerobic Biodegradability. J. Hazard. Mater. 2009, 164, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Kurdi, M.; Heerklotz, H. Classifying Surfactants with Respect to Their Effect on Lipid Membrane Order. Biophys. J. 2012, 102, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A Perspective on the Potential Risks of Emerging Contaminants to Human and Environmental Health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental Risks and Toxicity of Surfactants: Overview of Analysis, Assessment, and Remediation Techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Muherei, M.A.; Junin, R. Mixing Effect of Anionic and Nonionic Surfactants on Micellization, Adsorption and Partitioning of Nonionic Surfactant. Mod. Appl. Sci. 2008, 2, 3–12. [Google Scholar] [CrossRef]
- Salager, J.-L.; Marquez, R.; Bullon, J.; Forgiarini, A. Formulation in Surfactant Systems: From-Winsor-to-HLDN. Encyclopedia 2022, 2, 778–842. [Google Scholar] [CrossRef]
- Liang, L.X.; Dong, P.; Zhou, Y.; Zhang, L.; Qian, Z.; Geiger, S.D.; Bingheim, E.; Tang, X.; Wu, Y.; Lv, J.; et al. Joint Effects of Per- and Polyfluoroalkyl Substance Alternatives and Heavy Metals on Renal Health: A Community-Based Population Study in China. Environ. Res. 2023, 219, 115057. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Peijnenburg, W.J.G.M.; Vijver, M.G. Review and Prospects on the Ecotoxicity of Mixtures of Nanoparticles and Hybrid Nanomaterials. Environ. Sci. Technol. 2022, 56, 11238–11250. [Google Scholar] [CrossRef]
- Hu, G.; Wang, H.; Wan, Y.; Zhou, L.; Wang, Q.; Wang, M. Combined Toxicities of Cadmium and Five Agrochemicals to the Larval Zebrafish (Danio Rerio). Sci. Rep. 2022, 12, 16045. [Google Scholar] [CrossRef]
- Eom, H.; Kim, S.; Oh, S.E. Evaluation of Joint Toxicity of BTEX Mixtures Using Sulfur-Oxidizing Bacteria. J. Environ. Manag. 2023, 325, 116435. [Google Scholar] [CrossRef]
- Martin, O.; Scholze, M.; Ermler, S.; McPhie, J.; Bopp, S.K.; Kienzler, A.; Parissis, N.; Kortenkamp, A. Ten Years of Research on Synergisms and Antagonisms in Chemical Mixtures: A Systematic Review and Quantitative Reappraisal of Mixture Studies. Environ. Int. 2021, 146, 106206. [Google Scholar] [CrossRef]
- Sigurnjak Bureš, M.; Cvetnić, M.; Miloloža, M.; Kučić Grgić, D.; Markić, M.; Kušić, H.; Bolanča, T.; Rogošić, M.; Ukić, S. Modeling the Toxicity of Pollutants Mixtures for Risk Assessment: A Review. Environ. Chem. Lett. 2021, 19, 1629–1655. [Google Scholar] [CrossRef]
- Kar, S.; Leszczynski, J. Exploration of Computational Approaches to Predict the Toxicity of Chemical Mixtures. Toxics 2019, 7, 15. [Google Scholar] [CrossRef]
- Fernández-Serrano, M.; Jurado, E.; Fernández-Arteaga, A.; Ríos, F.; Lechuga, M. Ecotoxicological Assessment of Mixtures of Ether Carboxylic Derivative and Amine-Oxide-Based Non-Ionic Surfactants on the Aquatic Environment. J. Surfactants Deterg. 2014, 17, 1161–1168. [Google Scholar] [CrossRef]
- Ríos, F.; Fernández-Arteaga, A.; Lechuga, M.; Fernández-Serrano, M. Ecotoxicological Characterization of Surfactants and Mixtures of Them. In Toxicity and Biodegradation Testing, 1st ed.; Dino Bidoia, E., Nallin Montagnolli, R., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1, pp. 311–330. [Google Scholar] [CrossRef]
- García, M.T.; Campos, E.; Ribosa, I. Biodegradability and Ecotoxicity of Amine Oxide Based Surfactants. Chemosphere 2007, 69, 1574–1578. [Google Scholar] [CrossRef]
- Lechuga, M.; Fernández-Serrano, M.; Jurado, E.; Núñez-Olea, J.; Ríos, F. Acute Toxicity of Anionic and Non-Ionic Surfactants to Aquatic Organisms. Ecotoxicol. Environ. Saf. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- European Commission Scientific Committee on Health and Environmental Risk; Scientific Committee on Emerging and Newly Identified Health Risks; Scientific Committee on Consumer Safety. Toxicity and Assessment of Chemical Mixtures. 2012. [CrossRef]
- Altenburger, R.; Nendza, M.; Schüürmann, G. Mixture Toxicity and Its Modeling by Quantitative Structure-Activity Relationships. Environ. Toxicol. Chem. 2003, 22, 1900–1915. [Google Scholar] [CrossRef]
- Bragin, G.E.; Davis, C.W.; Kung, M.H.; Kelley, B.A.; Sutherland, C.A.; Lampi, M.A. Biodegradation and Ecotoxicity of Branched Alcohol Ethoxylates: Application of the Target Lipid Model and Implications for Environmental Classification. J. Surfactants Deterg. 2020, 23, 383–403. [Google Scholar] [CrossRef]
- Jurado, E.; Fernández-Serrano, M.; Lechuga, M.; Ríos, F. Environmental Impact of Ether Carboxylic Derivative Surfactants. J. Surfactants Deterg. 2012, 15, 1–7. [Google Scholar] [CrossRef]
- Clark, D.P.; Pazdernik, N.J. Basics of Biotechnology. In Biotechnology, 2nd ed.; Academic Cell: San Diego, CA, USA, 2016; pp. 1–31. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J. Pseudomonas Putida–Based Cell Factories. In Microbial Cell Factories Engineering for Production of Biomolecules, 1st ed.; Singh, V., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 165–181. [Google Scholar] [CrossRef]
- ISO 10712:1995; Water Quality—Pseudomonas Putida Growth Inhibition Test (Pseudomonas Cell Multiplication Inhibition Test). International Organization for Standardization: Geneva, Switzerland, 1995.
- Feng, W.; Swift, S.; Singhal, N. Effects of Surfactants on Cell Surface Tension Parameters and Hydrophobicity of Pseudomonas Putida 852 and Rhodococcus Erythropolis 3586. Colloids Surf. B 2013, 105, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental Behaviour and Ecotoxicity of Cationic Surfactants towards Marine Organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.C.; Feijão, E.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; Fonseca, V.F.; et al. Glyphosate-Based Herbicide Toxicophenomics in Marine Diatoms: Impacts on Primary Production and Physiological Fitness. Appl. Sci. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Duarte, B.; Feijão, E.; de Carvalho, R.C.; Duarte, I.A.; Silva, M.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Marques, J.C.; et al. Effects of Propranolol on Growth, Lipids and Energy Metabolism and Oxidative Stress Response of Phaeodactylum Tricornutum. Biology 2020, 9, 478. [Google Scholar] [CrossRef]
- Feijão, E.; Cruz de Carvalho, R.; Duarte, I.A.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; et al. Fluoxetine Arrests Growth of the Model Diatom Phaeodactylum Tricornutum by Increasing Oxidative Stress and Altering Energetic and Lipid Metabolism. Front. Microbiol. 2020, 11, 1803. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Feijão, E.; Matos, A.R.; Cabrita, M.T.; Utkin, A.B.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; et al. Ecotoxicological Effects of the Anionic Surfactant Sodium Dodecyl Sulfate (SDS) in Two Marine Primary Producers: Phaeodactylum Tricornutum and Ulva Lactuca. Toxics 2022, 10, 780. [Google Scholar] [CrossRef]
- BS EN 14370:2004; Surface Active Agents. Determination of Surface Tension. AENOR: Madrid, Spain, 2004.
- ISO 10253:2006; Water Quality. Marine Algal Growth Inhibition Test with Skeletonema Costatum and Phaeodactylum Tricornutum. International Organization for Standardization: Geneva, Switzerland, 2006.
- Ríos, F.; Fernández-Arteaga, A.; Lechuga, M.; Fernández-Serrano, M. Ecotoxicological Characterization of Polyoxyethylene Glycerol Ester Non-Ionic Surfactants and Their Mixtures with Anionic and Non-Ionic Surfactants Environ. Sci. Pollut. Res. 2017, 24, 10121–10130. [Google Scholar] [CrossRef]
- Chen, F.; Wu, L.; Xiao, X.; Rong, L.; Li, M.; Zou, X. Mixture Toxicity of Zinc Oxide Nanoparticle and Chemicals with Different Mode of Action upon Vibrio Fischeri. Environ. Sci. Eur. 2020, 32, 41. [Google Scholar] [CrossRef]
- Broderius, S.J.; Kahl, M.D.; Hoglund, M.D. Use of Joint Toxic Response to Define the Primary Mode of Toxic Action for Diverse Industrial Organic Chemicals. Environ. Toxicol. Chem. 1995, 14, 1591–1605. [Google Scholar] [CrossRef]
- Hsu, J.C. Multiple Comparisons: Theory and Methods, 1st ed.; CRC Press: Boca Ratón, FL, USA, 1996. [Google Scholar] [CrossRef]
- Jurado, E.; Fernández-Serrano, M.; Núñez-Olea, J.; Luzón, G.; Lechuga, M. Acute Toxicity and Relationship between Metabolites and Ecotoxicity during the Biodegradation Process of Non-Ionic Surfactants: Fatty-Alcohol Ethoxylates, Nonylphenol Polyethoxylate and Alkylpolyglucosides. Water. Sci. Technol. 2009, 59, 2351–2358. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Casettari, L.; Vllasaliu, D.; Cangiotti, M.; Ottaviani, M.F.; Giorgioni, G.; Bonacucina, G.; Palmieri, G.F. Correlation among Chemical Structure, Surface Properties and Cytotoxicity of N-Acyl Alanine and Serine Surfactants. Eur. J. Pharm. Biopharm. 2016, 109, 93–102. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Xu, X.; Sun, C. Coupling Effects of Ionic Surfactants and Electrolytes on the Stability of Bulk Nanobubbles. Nanomaterials 2022, 12, 3450. [Google Scholar] [CrossRef]
- Ríos, F.; Fernández-Arteaga, A.; Fernández-Serrano, M.; Jurado, E.; Lechuga, M. Silica Micro- and Nanoparticles Reduce the Toxicity of Surfactant Solutions. J. Hazard. Mater. 2018, 353, 436–443. [Google Scholar] [CrossRef]
- Jurado, E.; Fernández-Serrano, M.; Núñez Olea, J.; Lechuga, M.; Jiménez, J.L.; Ríos, F. Acute Toxicity of Alkylpolyglucosides to Vibrio Fischeri, Daphnia Magna and Microalgae: A Comparative Study. Bull. Environ. Contam. Toxicol. 2012, 88, 290–295. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. Effects of Salt and Surfactant on Interfacial Characteristics of Water/Oil Systems: Molecular Dynamic Simulations and Dissipative Particle Dynamics. Ind. Eng. Chem. Res. 2019, 58, 8817–8834. [Google Scholar] [CrossRef]
- Qv, X.Y.; Jiang, J.G. Toxicity Evaluation of Two Typical Surfactants to Dunaliella Bardawil, an Environmentally Tolerant Alga. Environ. Toxicol. Chem. 2013, 32, 426–433. [Google Scholar] [CrossRef]
- Jurado, E.; Fernández-serrano, M.; Ríos, F.; Lechuga, M. Aerobic Biodegradation of Surfactants. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; In-Tech: Rijeka, Croatia, 2013; pp. 68–81. [Google Scholar] [CrossRef]
- Jackson, M.; Eadsforth, C.; Schowanek, D.; Delfosse, T.; Riddle, A.; Budgen, N. Comprehensive Review of Several Surfactants in Marine Environments: Fate and Ecotoxicity. Environ. Toxicol. Chem. 2016, 35, 1077–1086. [Google Scholar] [CrossRef]
- Mustapha, D.S.; Bawa-Allah, K.A. Differential Toxicities of Anionic and Nonionic Surfactants in Fish. Environ. Sci. Pollut. Res. 2020, 27, 16754–16762. [Google Scholar] [CrossRef]
- Rosen, M.J.; Li, F.; Morrall, S.W.; Versteeg, D.J. The Relationship between the Interfacial Properties of Surfactants and Their Toxicity to Aquatic Organisms. Environ. Sci. Technol. 2001, 35, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Romero, J.F.; Ríos, F.; Reyes-Requena, A.; Luzón-González, G.; García-López, A.I. Surface and Thermodynamics Properties of Commercial Fatty-Alcohol Ethoxylate Surfactants. J. Mol. Liq. 2023, 376, 121396. [Google Scholar] [CrossRef]
- Ríos, F.; Lechuga, M.; Fernández-Serrano, M.; Fernández-Arteaga, A. Aerobic Biodegradation of Amphoteric Amine-Oxide-Based Surfactants: Effect of Molecular Structure, Initial Surfactant Concentration and pH. Chemosphere 2017, 171, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Costello, J.F. Mechanisms of Action for General and Polar Narcosis: A Difference in Dimension. QSAR Comb. Sci. 2003, 22, 226–233. [Google Scholar] [CrossRef]
- Joshi, V.Y.; Kadam, M.M.; Sawant, M.R. Comparison of QSAR and QSPR Based Aquatic Toxicity for Mixed Surfactants. J. Surfactants Deterg. 2007, 10, 25–34. [Google Scholar] [CrossRef]
- Könnecker, G.; Regelmann, J.; Belanger, S.; Gamon, K.; Sedlak, R. Environmental Properties and Aquatic Hazard Assessment of Anionic Surfactants: Physico-Chemical, Environmental Fate and Ecotoxicity Properties. Ecotoxicol. Environ. Saf. 2011, 74, 1445–1460. [Google Scholar] [CrossRef]
- Martins, N.; Pereira, J.L.; Antunes, F.E.; Melro, E.; Duarte, C.M.G.; Dias, L.; Soares, A.M.V.M.; Lopes, I. Role of Surfactant Headgroups on the Toxicity of SLEnS-LAS Mixed Micelles: A Case Study Using Microtox Test. Sci. Total Environ. 2018, 643, 1366–1372. [Google Scholar] [CrossRef]

| Abbreviation | INCI Name 1 | CAS 2 | R 3 | E 4 | % Active Matter |
|---|---|---|---|---|---|
| EC-R8E8 | Capryleth-9 carboxylic acid | 53563-70-5 | 8 | 8 | 92.8 |
| EC-R12–14E3 | Laureth-4 carboxylic acid | 27306-90-7 | 12–14 | 3 | 92.5 |
| EC-R12–14E10 | Laureth-11 carboxylic acid | 27306-90-7 | 12–14 | 10 | 89.1 |
| AO-R12 | Lauryl dimethyl amine oxide | 1643-20-5 | 12 | - | 29.4 |
| AO-R14 | Myristyl dimethyl amine oxide | 3332-27-2 | 14 | - | 30.8 |
| AOP-Cocoamido | Cocoamidopropyl dimethyl amine oxide | 68155-09-9 | R’ = 12 | - | 34.3 |
| Surfactant | CMC (mgL−1) | CMCU |
|---|---|---|
| EC-R8E8 | 214.2 ± 5.2 | - |
| EC-R12–14E3 | 28.6 ± 4.8 | - |
| EC-R12–14E10 | 66.9 ± 7.2 | - |
| AO-R12 | 156.0 ± 10.5 | - |
| AO-R14 | 102.4 ± 3.2 | - |
| AOP-Cocoamido | 301.3 ± 5.1 | - |
| EC-R8E8 + EC-R12–14E10 | 100.2 ± 1.57 | 0.71 |
| EC-R8E8 + AO-Cocoamido | 442.7 ± 7.6 | 1.71 |
| AO-R12 + AO-Cocoamido | 403.8 ± 12.4 | 1.76 |
| AO-R14 + AO-Cocoamido | 157.9 ± 6.1 | 0.78 |
| Surfactant | ζ-Potential P. putida Medium, (±SD) | MD P. putida Medium, (nm ± SD) | ζ-Potential P. tricornutum Medium, (±SD) | MD P. tricornutum Medium, (nm ± SD) |
|---|---|---|---|---|
| EC-R8E8 | −13.6 ± 0.5 | 123.3 ± 19.2 | −3.5 ± 1.3 | 162.6 ± 22.0 |
| EC-R12–14E3 | −51.9 ± 2.2 | 19.9 ± 2.3 | −28.7 ± 1.1 | 18.1 ± 1.3 |
| EC-R12–14E10 | −9.0 ± 1.1 | 5.6 ± 0.4 | −2.4 ± 1.3 | 6.7 ± 0.1 |
| AO-R12 | −27.8 ± 1.5 | 52.4 ± 0.8 | −11.0 ± 0.6 | 195.0 ± 4.5 |
| AO-R14 | −14.7 ± 1.3 | 8.8 ± 0.1 | −6.9 ± 0.7 | 7.1 ± 1.1 |
| AOP-Cocoamido | −12.7 ± 0.2 | 54.1 ± 8.7 | −9.1 ± 0.4 | 326.9 ± 19.8 |
| EC-R8E8 + EC-R12–14E10 | −11.3 ± 1.1 | 7.0 ± 0.5 | −5.9 ± 0.6 | 6.4 ± 0.5 |
| EC-R8E8 + AO-Cocoamido | −12.8 ± 1.1 | 123.4 ± 12.0 | −7.1 ± 0.2 | 36.0 ± 1.1 |
| AO-R12 + AO-Cocoamido | −23.3 ± 3.4 | 48.2 ± 8.2 | −10.4 ± 2.1 | 68.6 ± 2.7 |
| AO-R14 + AO-Cocoamido | −12.0 ± 1.3 | 10.8 ± 2.6 | −10.0 ± 1.5 | 8.9 ± 1.6 |
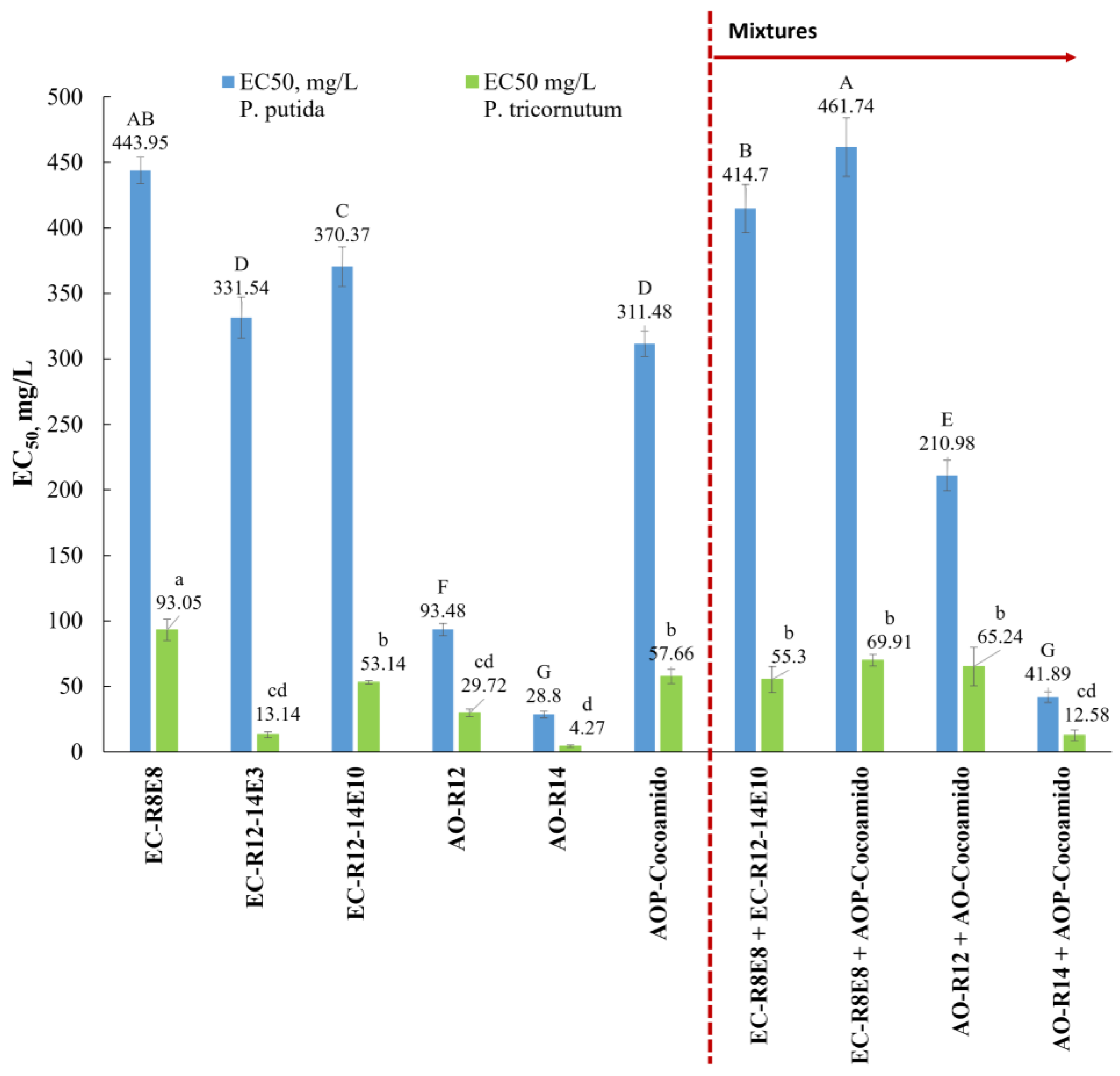
| Surfactant | EC50, P. putida mgL−1 | EC50, P. tricornutum mgL−1 |
|---|---|---|
| EC-R8E8 | 443.95 ± 10.24 | 93.05 ± 8.21 |
| EC-R12–14E3 | 331.54 ± 15.77 | 13.14 ± 2.25 |
| EC-R12–14E10 | 370.37 ± 15.02 | 53.14 ± 1.22 |
| AO-R12 | 93.48 ± 4.76 | 29.72 ± 2.91 |
| AO-R14 | 28.8 ± 2.48 | 4.27 ± 1.03 |
| AOP-Cocoamido | 311.48 ± 9.54 | 57.66 ± 5.48 |
| Surfactant A | Surfactant B | EC50, P. putida mgL−1 (95% CI) | TUA | TUB | TUmix | TUr | Type of Action |
|---|---|---|---|---|---|---|---|
| EC-R8E8 | EC-R12–14E10 | 414.71 ± 18.33 | 0.47 | 0.56 | 1.03 | 1.83 | Concentration addition |
| EC-R8E8 | AO-Cocoamido | 461.74 ± 22.15 | 0.52 | 0.74 | 1.26 | 1.70 | Less than additive (antagonism) |
| AO-R12 | AO-Cocoamido | 210.98 ± 11.49 | 1.13 | 0.34 | 1.47 | 1.30 | Less than additive (antagonism) |
| AO-R14 | AO-Cocoamido | 41.89 ± 3.87 | 0.73 | 0.07 | 0.79 | 1.09 | Response addition |
| Surfactant A | Surfactant B | EC50, P. tricornutum mgL−1 (95% CI) | TUA | TUB | TUmix | TUr | Type of Action |
|---|---|---|---|---|---|---|---|
| EC-R8E8 | EC-R12–14E10 | 55.3 ± 10.10 | 0.30 | 0.52 | 0.82 | 1.57 | Concentration addition |
| EC-R8E8 | AO-Cocoamido | 69.91 ± 4.45 | 0.38 | 0.61 | 0.98 | 1.62 | Concentration addition |
| AO-R12 | AO-Cocoamido | 65.24 ± 14.70 | 1.10 | 0.57 | 1.66 | 1.52 | Less than additive (antagonism) |
| AO-R14 | AO-Cocoamido | 12.58 ± 4.18 | 1.47 | 0.11 | 1.58 | 1.07 | Response addition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos, F.; Lechuga, M.; Lobato-Guarnido, I.; Fernández-Serrano, M. Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum. Toxics 2023, 11, 344. https://doi.org/10.3390/toxics11040344
Ríos F, Lechuga M, Lobato-Guarnido I, Fernández-Serrano M. Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum. Toxics. 2023; 11(4):344. https://doi.org/10.3390/toxics11040344
Chicago/Turabian StyleRíos, Francisco, Manuela Lechuga, Ismael Lobato-Guarnido, and Mercedes Fernández-Serrano. 2023. "Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum" Toxics 11, no. 4: 344. https://doi.org/10.3390/toxics11040344
APA StyleRíos, F., Lechuga, M., Lobato-Guarnido, I., & Fernández-Serrano, M. (2023). Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum. Toxics, 11(4), 344. https://doi.org/10.3390/toxics11040344








