Abstract
Milk formation in the breast during breastfeeding is a complex hormonally regulated process, potentially sensitive to the effects of endocrine-disrupting chemical exposures. The environmental chemicals, per- and polyfluoroalkyl substances (PFAS) are known endocrine disruptors. PFAS exposure have been associated with insufficient mammary gland development in mice and reduced breastfeeding duration in humans. The aim of this review was to gather the epidemiological evidence on the association between PFAS exposure and breastfeeding duration. Using PubMed and Embase, we performed a systematic literature search (on 23 January 2023) to identify epidemiological studies examining the association between maternal PFAS exposure and breastfeeding duration. Animal studies, reviews, and non-English studies were excluded. The risk of bias was assessed using the risk of bias in non-randomized studies of exposures tool. Estimates describing the association between PFAS exposure and the duration of breastfeeding were identified, and the data were synthesized separately for each type of PFAS and for the duration of exclusive and total breastfeeding. Six studies with between 336 and 2374 participants each were identified. PFAS exposure was assessed in serum samples (five studies) or based on residential address (one study). Five out of six studies found shorter total duration of breastfeeding with higher PFAS exposure. The most consistent associations were seen for perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA). The finding of a potential causal association between PFAS exposure and breastfeeding duration is in agreement with findings from experimental studies.
1. Introduction
According to the World Health Organization, “breastfeeding is one of the most effective ways to ensure child health and survival” [1]. Breastmilk components include micronutrients and immunoglobulins, which shape the infant’s immune development [2,3], and breastfeeding has been associated with a reduced risk of respiratory infections [4], infection-related and all-cause mortality [5], attention deficit/hyperactivity disorder [6], and acute lymphoblastic leukemia [7], as well as higher intelligence [8] in the child. Maternal benefits associated with breastfeeding include reduced risk of breast cancer, ovarian cancer, and type 2 diabetes [9,10]. The World Health Organization and the American Academy of Pediatrics recommend exclusive breastfeeding for the first six months of infancy and continued breastfeeding for a year or more [11,12]. Yet, in low- and middle-income countries, less than 40% of infants younger than 6 months were breastfed [9]. In high-income countries breastfeeding duration is generally even shorter [9], and women often report experiences of undesired early weaning due to inadequate milk supply [13,14,15,16,17,18]. Sufficient milk formation depends on the development of the breast during early life, puberty, and pregnancy, a process which is hormonally regulated [19] and, subsequently, sensitive to effects of endocrine disrupting chemical exposures.
Per- and polyfluoroalkyl substances (PFAS) are a group of environmentally persistent oil and water-repellent chemicals widely used in consumer products such as food packaging, cosmetics, clothing, and furniture upholstery [20,21]. Human exposure to PFAS occurs through contaminated food and water, as well as inhaled exposures from treated materials or point sources [22]. For many years, the most widely produced and used types of PFAS were perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), but after they were listed under the Stockholm Convention on persistent organic pollutants in 2009 and 2019, respectively [23], they have been gradually replaced in global industrial products by other types of PFAS, including, but not limited to, perfluorohexane sulfonate (PFHxS), which was listed under the Stockholm Convention in 2022 [23], perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), perfluoroheptane sulfonate (PFHpS), and perfluorotridecanoic acid (PFTrDA), and others.
PFAS are known endocrine disruptors, with reported effects on female reproductive outcomes [24], and they have multiple other adverse health effects, including effects on the immune system, thyroid function, liver disease, kidney disease, cancer, lipid and insulin regulation, and developmental outcomes [25]. PFAS are transferred with breastmilk [26,27,28,29,30] and have been shown to have immunotoxic effects in children [31,32], especially during early infancy [33].
Early studies regarding the health effects of PFOA demonstrated a dose-dependent morbidity and mortality in mouse pups the first few days after birth [34]. A year later, those prenatal PFOA-induced effects were shown to be due to insufficient development of the dam mammary gland during pregnancy and deficient milk production during lactation [35]. In the last decade, several well-powered and well-designed cohort studies examining the association between PFAS and breastfeeding duration in women have been published. The aim of this systematic literature review was to summarize the effect measures describing the associations between maternal PFAS exposure and duration of breastfeeding across epidemiological studies, while taking differences in methodology and potential study-related bias into account.
2. Materials and Methods
On 19 January 2022, we performed a systematic literature search using PubMed and Embase (Ovid) to identify epidemiological studies examining the association between PFAS exposure in pregnant or breastfeeding women and the, subsequent, duration of breastfeeding. The search was repeated on 19 August 2022 and 23 January 2023. The search consisted of two facets, and the search terms within each facet were separated by an ‘OR’ operator, while the two facets were combined using an ‘AND’ operator (Table 1). Facet 1 consisted of different terms relevant to PFAS, including abbreviations of two of the most often detected/measured PFAS, namely PFOA and PFOS. Most studies on PFAS will include at least one of the most commonly reported PFAS, and by including PFOA and PFOS in the search we aimed to find studies that might not have used the collective term PFAS. Older studies often referred to PFAS as perfluorinated chemicals (PFC), but PFC is a more restrictive term and may not encompass all the PFAS reported. Facet 2 covered breastfeeding either as part of the title or as a key term. The search was restricted to records in English, and reviews were excluded (Table 1).

Table 1.
Search strategy.
The obtained records were imported into EndNote X9 (Clarivate, Chandler, AZ, USA), and duplicates were removed. One author (AT) performed the title and abstract screening and recorded the reasons for exclusion (biomonitoring reports, studies of PFAS transfer, studies of other outcomes than breastfeeding, reviews, and animal studies). All the records that were not excluded were read in full and assessed for eligibility, and those deemed eligible (epidemiological studies examining associations between maternal PFAS exposure and the duration of breastfeeding) were included in the review, in accordance with the PECO statement (Table 2), which describes the population, exposure, comparator, and outcomes [36].

Table 2.
PECO (population, exposure, comparator, and outcomes) statement.
Key information from the selected studies were gathered using Microsoft Excel version 16 (Microsoft Corporation, Redmond, WA, USA). The following key data were collected: first author, year of publication, study country, study design, number of participants (based on the largest number of participants in the main analyses), methods for assessing PFAS exposure and breastfeeding duration, median PFAS concentrations and duration of breastfeeding, statistical methods, adjustment set, approach to confounding from previous breastfeeding, effect estimates, and author conclusion.
The quality of the studies was assessed by two authors in collaboration (OA and AT) using the risk of bias in non-randomized studies of exposures (ROBINS-E) tool [37]. ROBINS-E was designed to assess the potential impact of an exposure on an outcome in non-randomized studies, according to seven domains: (1) risk of bias due to confounding, (2) risk of bias arising from measurement of the exposure, (3) risk of bias in the selection of participants in the study (or in the analysis), (4) risk of bias due to post-exposure interventions, (5) risk of bias due to missing data, (6) risk of bias arising from measurement of the outcome, and (7) risk of bias in the selection of the reported result. For each domain, the risk of bias was assessed as low risk, some concerns, high risk, or very high risk of bias [37]. In observational studies, the risk of residual confounding can seldom be excluded, and when assessing risk of bias due to confounding using ROBINS-E, low risk of bias is thus interpreted as a low risk of bias, except for concerns about residual confounding [37].
We assessed whether each study used appropriate methods to control for confounding, such as (but not exclusive to) adjustment, stratification, and restriction. Potential confounders relevant to most or all of the studies were reviewed. These factors included: prior history of breastfeeding, parity, maternal age, body mass index (BMI), gestational age, and education, but potential confounding factors will differ across studies, based on the study design, the PFAS exposure routes, and the reasons for breastfeeding termination. We considered potential confounding from previous breastfeeding particularly important, since PFAS are transferred to the infant in breastmilk [26,27,28,29,30], and thus, breastfeeding decreases maternal serum PFAS concentrations [38]. Consequently, women who have previously breastfed for a prolonged period will have lowered their circulating PFAS concentrations, and they may also breastfeed their next child and for a longer period [39]. Mothers that have not breastfed their previous children or only breastfed for a short period of time, will have relatively higher blood PFAS concentrations, and may be more likely to terminate breastfeeding of their next child early, resulting in a non-causal association between PFAS concentrations and the duration of breastfeeding.
When assessing bias due to exposure or outcome measurement errors we focused on the risk of differential misclassification. PFAS exposure can be measured using biological material or by using proxy measures. The precision of each method depends on the circumstances in the specific study, but some imprecision is inevitable. However, if the misclassification is non-differential, bias will most likely be towards null.
Bias due to missing data was assessed by identifying the extent of missing information about the exposure, outcomes, and potential confounders. If missingness was likely to affect the results, we further assessed the measures taken to address the bias.
The overall risk of bias was created using an algorithm based on all the domains of ROBINS-E. For a study to receive an overall low risk of bias assessment, the study would have to rank as low risk of bias in each query domain. This means that aside from the possibility of uncontrolled confounding, inherent to the study’s observational nature, there is little or no concern of bias in the study results.
The effect measures of interest were those describing the association between estimates of PFAS exposure and reported duration of breastfeeding, or the risk of breastfeeding termination at a given timepoint. The results were synthesized separately for each type of PFAS and for the duration of exclusive and total breastfeeding, with the duration of exclusive breastfeeding being the period from birth until introduction of formula or solid foods, and total duration of breastfeeding being the period from birth until breastfeeding is terminated completely. Two of the included studies reported results on breastfeeding initiation, and these endpoints were added to Section 3 after the initiation of the review. Due to the diversity of the included studies, it was not possible to identify a standardized metric to use across studies, and we, thus, reported the results based on the metrics used in each study.
3. Results
In the initial search in January 2022, 108 records were identified, 17 additional records were identified in August 2022, and 8 additional records were identified in January 2023. Among the 133 records identified in the most recent search, 31 were duplicates (Figure 1), and the majority of the remaining records did not examine the effects of PFAS on breastfeeding, but rather the PFAS concentrations in breastmilk, the transplacental transfer of PFAS from mother to child, the predictors of PFAS concentrations, or the PFAS associations with other outcomes. Thus, 96 records were removed through the initial screening, leaving 6 studies, which were all eligible for inclusion upon reading the full text (Figure 1). Three of the studies were conducted by the authors of this review.
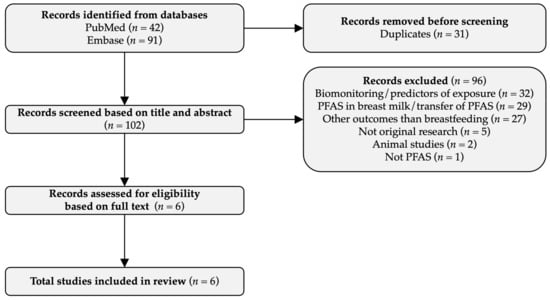
Figure 1.
Flow diagram.
3.1. Study Characteristics
The included studies were published between 2010 and 2022, and were based on data from women giving birth between 1996 and 2012 (Table 3). Five of the studies focused on women living in northern Europe [40,41,42,43,44] and one was from women in the United States [45]. The study size ranged from 336 [45] to 2374 participants [41]. Five studies were prospective cohort studies [40,42,43,44,45], and one was based on data from a natural experiment, in which contaminated drinking water caused serum PFHxS, PFOS, and PFOA concentrations to be 135, 35, and 5 times higher, respectively, in one Swedish municipality, Ronneby, compared to a nearby municipality [41]. The median duration of total breastfeeding ranged from 6 [45] to 9 months [44]. One study [42] did not consider exclusive breastfeeding, and one study [41] did not give the duration of exclusive breastfeeding. The median duration of exclusive breastfeeding in the other four studies is provided in Table 3, and ranged from 0.07 [45] to 5 months [44]. However, the definition of exclusive breastfeeding was not uniform across the studies. For example, one study relied on the American Academy of Pediatrics guidelines and equaled the duration of exclusive breastfeeding to the infant’s age at the first ever use of formula, water, juice or solid foods [45], while another study defined the duration of exclusive breastfeeding in accordance with the Danish health and food authorities, as the infant’s age when receiving more than one non-breastmilk meal per week [43].

Table 3.
Studies included in the review.
Information about breastfeeding was collected through questionnaires [42,43,44], standardized interviews [40,44,45], health charts [41,43], and text messages [43]. PFAS exposure was assessed based on residential address in one study (a proxy for drinking water exposure to PFAS) [41], while the other five studies relied on PFAS concentrations measured in maternal serum samples collected from gestational week four to two weeks after delivery. Serum PFAS concentrations varied greatly across the study population (Figure 2), but in all five studies, PFOS was found in higher concentrations than the other types of PFAS, with the median serum concentrations ranging from 7.56 to 33.4 ng/mL.
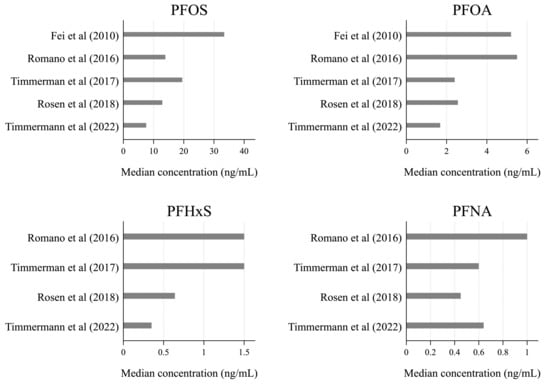
Figure 2.
Median serum concentrations of the four most frequently reported types of PFAS: PFOS, PFOA, PFHxS, and PFNA [40,42,43,44,45].
The choice of statistical analysis models differed across the studies. Five of the six studies used Cox proportional hazards models, two studies used logistic regression models, two studies used modified Poisson regression with robust standard errors, and one study used linear regression models (Table 4). Due to the differences in the data collection methods, statistical analyses, and reporting of the results across the studies, this review reports results using hazard ratios (HR), relative risks (RR), and odds ratios (OR), and for the exclusive and total duration of breastfeeding, as well as the initiation of breastfeeding, depending on the available information.

Table 4.
Analytical methods.
3.2. Study Risk of Bias Assessment
Using the ROBINS-E risk of bias analysis, all studies included in this review were considered to be of high quality and have only a low risk of bias, except for concerns about residual confounding (Table 5). Importantly, all six studies considered the risk of confounding from previous breastfeeding, though different approaches were used to control for such confounding. Three studies [42,43,45] obtained information about previous breastfeeding and took this into account in the analyses. Furthermore, all six studies either stratified the analyses by parity, or included an interaction term between PFAS and parity in the statistical models to identify the differential effects between women who had possibly previously breastfed and women who had not. Likewise, all studies adjusted for the variables known to impact breastfeeding duration, such as maternal age, BMI, and education/income.

Table 5.
Study risk of bias assessment.
One study relied on exposure data from a natural experiment [41], and the bias structure in this study was, therefore, different. Although previous breastfeeding is likely to have lowered the serum PFAS concentrations among exposed women, it would not have affected the residential address, which was used as a proxy for exposure in the study by Nielsen et al. Thus, including multiparous women will not overestimate the association in this study. However, some non-differential misclassification and, thus, bias towards the null is likely when exposure assessment is based on a proxy measure of exposure, such as residential address. Multiparous women will likely have lower serum PFAS concentrations than their nulliparous neighbors. Including multiparous women in the high-exposed group might, therefore, lower the median PFAS concentrations in this group and, thus, dilute the association between PFAS exposure and breastfeeding. Accordingly, Nielsen et al. found stronger associations between PFAS exposure and early breastfeeding termination when restricting the analyses to nulliparous women only [41].
Rosen et al. (2018) used combined data from two prior nested case-control studies, resulting in an over-representation of women with subfecundity and preeclampsia, which were both associated with serum PFAS concentrations and decreased breastfeeding duration in the study [42]. However, the authors adjusted for prior study status in their analyses, which should reduce the risk of selection bias based on over-representation [42].
Although PFAS are highly persistent, some imprecision is expected when measuring PFAS in serum at different timepoints during pregnancy and shortly after birth. However, the imprecision is expected to be unrelated to the duration of breastfeeding. Likewise, the women in most studies were unaware of their serum PFAS concentration when breastfeeding, and any misclassification of breastfeeding duration would, thus, be non-differential and most likely lead to bias towards the null.
3.3. Breastfeeding Initiation
Two studies examined the role of PFAS in breastfeeding initiation (Table 6). Nielsen et al. (2022) reported PFAS exposure to be associated with a 2.37 (95% confidence interval (CI): 0.84; 6.69) times higher risk of not initiating breastfeeding [41]. In addition, they found that the association between PFAS exposure and the risk of terminating breastfeeding was stronger in early lactation compared to when the child was older [41]. Rosen et al (2018) included only women who initiated breastfeeding in their study, but assessed initiation in a sensitivity analysis and found no statistically significant associations between serum PFAS concentrations and breastfeeding initiation [42].

Table 6.
Summary of study findings.
3.4. Exclusive Breastfeeding
Five studies examined the associations between PFAS and exclusive breastfeeding (Table 6), and the Swedish and American studies found no clear associations [41,45]; however, the American study had a very small window of exclusive breastfeeding in which to make comparisons (interquartile range 0.03–0.8 month) [45]. Timmermann et al. (2022) found an 8% (95% CI: 2; 14%) lower risk of breastfeeding termination with each doubling of serum PFHxS concentrations [43], while Fei et al. (2010) found a 37% increased hazard of terminating exclusive breastfeeding at any given time among women in the highest PFOS (95% CI: 14; 64%) and PFOA (95% CI: 12; 69%) quartile compared to those in the lowest quartile [40]. Timmerman et al. (2017) found that each doubling of PFOS, PFOA, and PFDA concentration was associated with a 0.3 (95% CI: 0.1; 0.6), 0.5 (95% CI: 0.3; 0.7), and 0.5 (95% CI: 0.0; 0.4) month shorter duration of exclusive breastfeeding, respectively (Table 6) [44]. When restricting the analyses to primiparous women, most of the mentioned associations were markedly weakened [40,43,44], except for PFDA, for which a doubling in serum concentrations was associated with a 0.5 (95% CI: 0.1; 0.9) month shorter duration of exclusive breastfeeding among primiparous Faroese women [44].
3.5. Total Breastfeeding
All six publications contributed data to our study through analyses of total breastfeeding and PFAS comparisons. Among Swedish women, higher exposure to a PFAS mixture in drinking water was associated with a 32% (95% CI: 6; 63%) higher hazard of terminating breastfeeding at 3 months [41], and among Danish women, each doubling in total serum PFAS concentration was associated with a 23% (95% CI: 8; 40%) higher hazard of terminating breastfeeding at any given time [43]. When restricting the analyses to primiparous women, the association in the Danish study was not substantially changed [43], while the association in the Swedish study was strengthened [41] (Table 6).
For specific types of PFAS, five studies examined the association between PFOS, PFOA, and breastfeeding, and four of these studies found decreased duration of breastfeeding with higher PFOS and PFOA concentrations (Figure 3). Fei et al. (2010) found a 40% increased hazard of terminating breastfeeding at any given time among Danish women in the highest PFOS (95% CI: 18; 65%) and PFOA (95% CI: 17; 68%) quartile compared to those in the lowest quartile, but the associations were weakened when restricting the analyses to primiparous women [40] (Table 6). Romano et al. (2016) found a 77% (95% CI: 23; 154%) increased risk of terminating breastfeeding before 3 months among American women in the highest compared to the lowest PFOA quartile, and the association persisted when restricting the analysis to primiparous women [45]. Slightly weaker associations were seen for PFOS [45]. Among Faroese women, each doubling in PFOS and PFOA concentration was associated with 1.4 (95% CI: 0.6; 2.1) and 1.3 (95% CI: 0.7; 1.9) months, respectively, shorter duration of breastfeeding, and the associations were not substantially weaker among primiparous women [44]. Finally, among Danish women, each doubling in serum PFOS and PFOA concentration during pregnancy was associated with an 18% (95% CI: 5; 33%) and 17% (95% CI: 5; 31%), respectively, higher hazard of terminating breastfeeding at any given time, with associations persisting among primiparous women [43].
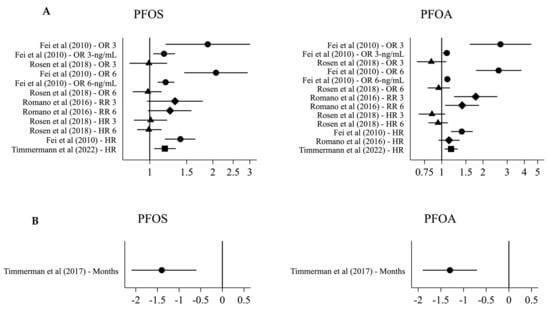
Figure 3.
Effect estimates for the association between serum PFOS/PFOA and breastfeeding. (A) OR 3/6: odds ratio for termination by 3/6 months for the highest compared to the lowest PFAS quartile Fei et al. (2010) [40], or with an interquartile range difference in PFAS Rosen et al. (2018) [42]. OR 3/6-ng/mL: odds ratio for termination by 3/6 months for each 1 ng/mL PFOS/10 ng/mL PFOA in-crease. RR 3/6: relative risk of termination by 3 months for the highest compared to the lowest PFAS quartile. HR 3/6: hazard ratio for termination at 3/6 months with an interquartile range dif-ference in PFAS. HR: HR for terminating breastfeeding for the highest compared to the lowest PFAS quartile Fei et al. (2010) [40], or with each doubling of PFAS Timmermann et al. (2022) [43] and Romano et al. (2016) [45]. (B) months difference in breastfeeding duration with each doubling of PFAS.
PFHxS was examined in four studies (Table 6). Three studies found reduced breastfeeding duration with increasing serum PFHxS concentrations [43,44,45], while one found longer breastfeeding duration with higher serum PFHxS concentrations among Norwegian women [42]. However, all confidence intervals were wide, and none of these findings reached statistical significance (Table 6).
PFNA was likewise examined in four studies, and while the hazard of terminating breastfeeding at 3 months was reduced by 23% (95% CI: 7; 37%) with an interquartile range increase in PFNA among Norwegian women [42], a doubling in PFNA was associated with 1.3 (95% CI: 0.7; 2.0) months shorter duration of breastfeed among Faroese women [44], a 17% (95% CI: 4; 31%) increased hazard of terminating breastfeeding at any given time among Danish women [43], and a 12% (95% CI: −19; 53%) increased risk of terminating breastfeeding before 3 months, among American women [45].
Three studies examined the association between PFDA and breastfeeding. One study found a 27% (95% CI: 14; 38%) lower hazard for breastfeeding cessation at 3 months with each interquartile range increase in PFDA [42], while another study found a 1.3 (95% CI: 0.7; 2.0) month shorter breastfeeding duration with each doubling of PFDA serum concentration [44], and one study found no association [43].
One study additionally examined PFHpS, and the longer carbon chain PFAS PFUnDA, PFDoDA, and PFTrDA. They found increased serum concentrations to be associated with a decreased risk of breastfeeding cessation, though confidence intervals were mostly wide [42].
4. Discussion
We systematically describe here, the evidence for associations of several legacy PFAS with the duration of breastfeeding in women from five countries. The majority of the reviewed studies found associations between higher maternal serum PFAS measures and the shorter total duration of breastfeeding, with PFOS, PFOA, and PFNA showing the most consistent associations. Associations between higher maternal serum PFAS measures and the reduced duration of exclusive breastfeeding were seen in a few studies, but the findings were not consistent across the studies. There are many consistent reports on the transfer of PFAS in milk to the infant [27,46,47,48] and the health effects thereof [25,49,50], but this review confirms that there are also effects on breastfeeding, potentially limiting the amount and quality of maternally-derived nutrition provided to the infant. Breast milk is a critical source of immunoglobulins and other proteins and fats needed to support the development of the infant’s nervous and immune system [2,3,51], and the infant may develop deficits in these systems because of PFAS exposure [52,53,54,55,56]. It has thus, become imperative to protect women of reproductive age and their infants from PFAS exposures.
Associations similar to those found in this review have been observed for other environmental chemicals, the pesticide metabolite dichlorodiphenyldichloroethylene (DDE) was linked to the shorter duration of breastfeeding in the 1980s and 90s [57,58], and, more recently, maternal Bisphenol A (BPA) exposure has also been linked to the reduced duration of breastfeeding, although the imprecision in the BPA studies did not allow for definitive conclusions [59,60].
Studies in mice also observe causal relationships between the exposure to some PFAS during pregnancy and adverse lactation outcomes. In pregnant mice, 5 mg/kg/d of PFOA exposure during pregnancy delayed differentiation of the lactating gland with subsequent growth restriction and increased neonatal mortality observed in pups [35,61], making them less likely to meet developmental milestones. Histopathological and molecular evaluations of the mammary gland, during the period of lactation, suggested that PFOA inhibited differentiation of the mammary epithelial cells and altered the expression of milk proteins in the gland, consistent with disrupted lactogenesis. Furthermore, studies in multiple strains of mice exposed during pregnancy to doses lower than 5 mg PFOA/kg/d revealed developmental abnormalities of the mammary epithelium in their female pups that persisted into adulthood [62,63,64] and were noted in multiple generations in CD-1 mice [61]. Together these studies suggest that the breast epithelium is a target for PFOA-induced toxicities. Recent studies on a PFOA replacement chemical, hexafluoropropylene oxide-dimer acid (HFPO-DA, commonly called GenX), similarly caused reduced mouse pup weight at postnatal day 5 [65] or postnatal days 1–21, following prenatal exposures [66]. Whether the differentiation of the mammary gland of their lactating dams were impacted by the exposure has yet to be reported for GenX.
There are potentially at least two important aspects of lactation that may be impacted by PFAS exposure, the proper differentiation of the epithelium (addressed above) and adequate endocrine function needed to support lactation. Despite consistencies between the experimental and epidemiological findings for a PFAS exposure-associated effect on lactation, relatively little is known about the potential mechanisms by which PFAS might affect breastfeeding. Prenatal PFAS exposure has been associated with lower prolactin concentrations in female infants [67], and in pregnant mice, exposure to PFOS significantly reduced the serum concentrations of prolactin-family hormones [68]. However, no such association was detected among pregnant women [43], though the timing of potential effects of PFAS on prolactin hormones during pregnancy remains unclear. Activation of the peroxisome proliferator-activated receptor (PPAR) α during pregnancy can impair mammary lobuloalveolar development in mice [69], and since the PPARα is activated by some PFAS [70], these chemicals could potentially affect mammary gland development and, thus, breastfeeding through activation of the PPARα There are numerous proteins known to be imperative to adequate milk production that have not been investigated as molecular targets of PFAS exposures (e.g., ErbB3, Akt, Stat5A [71]). Prolactin receptors, critical to proper lactation, are known to be regulated by numerous endocrine and mammary-made hormones shown to be altered by PFAS in other female reproductive tissues [24,72]. Finally, thyroid dysfunction is known to promote lactation deficits in rodent models [73], and thyroid hormones are consistently reported as altered by PFAS in women and rodents [74]. Recent work involving rats suggests that PFOS-induced decreases in systemic thyroid hormones may trigger expression of factors altering trafficking, metabolism, and excretion of the thyroid hormone, and that the pituitary and thyroid may not be the targets [75]. As the breast possesses these capabilities, they may be viable mechanisms to pursue in future work.
In this study, we found the associations between the PFAS measures and the total duration of breastfeeding were stronger than those seen for PFAS and exclusive breastfeeding, and there are a couple of scenarios that might explain the difference. The assessment of total duration of breastfeeding through a questionnaire have been shown to be more reliable than assessment of exclusive breastfeeding by questionnaire [76], biasing estimates for exclusive breastfeeding more towards the null. The definition of exclusive breastfeeding is also not consistent across the studies, and studies with a strict definition had a very short median duration of exclusive breastfeeding making it harder to detect differences related to PFAS exposure. It is also possible that compared to early weaning, the decision to use nutritional supplementation is affected by factors unrelated to insufficient milk supply, such as maternal commitments outside the home. These factors are most likely unrelated to PFAS exposure, and the imprecision caused by not taking these factors into account will, thus, likely increase bias towards the null.
Among the six studies included in this review, five found associations between PFAS and reduced breastfeeding duration, while one study [42] found no such associations or even opposite effects. Based on the described methods, we cannot identify any obvious explanations for the differences in the results. Rosen et al. (2018) suggested that the different findings between theirs and other studies might be explained by differences in maternal serum PFAS concentrations [42]. However, when compared to the other studies, including those published after the Rosen et al. study, differences in the exposure concentrations do not seem to explain the differences.
The strengths of this review include that all six studies were prospective (ensuring temporality), of high quality, and had only a low risk of bias. Thus, all studies took previous breastfeeding into account, either directly or by stratifying by parity. Also, the majority of the studies adjusted for education, income, or other markers of socioeconomic status, which might be important, as socioeconomic status is associated with breastfeeding duration [77] and might also, in some settings, be associated with PFAS exposure [78].
An additional strength is that this review and the conclusions drawn from the six identified studies relied on studies with different designs. When assessing associations between PFAS exposure and breastfeeding duration, the risk of confounding from previous breastfeeding is likely the strongest cause of bias. Using a proxy measure for PFAS, as was conducted in the Swedish study [41], can eliminate this kind of bias, but at the cost of increasing the exposure measurement error [79]. Increased non-differential exposure measurement error will most likely lead to underestimation, while confounding from previous breastfeeding will lead to overestimation of the true association. Thus, when studies with different study designs and different bias structures find similar results, it is less likely that the findings are caused by bias [80].
A limitation of this review is that comparisons across the studies included in the review were impaired by the studies relying on different statistical methods, with some studies presenting ORs, while others presented RRs or HRs. As breastfeeding termination is not a rare outcome, the ORs will be higher than the RRs would have been using the same data, and the size of the effect estimates are not directly comparable across the studies. Despite these obvious differences, we included the estimates in joint figures allowing comparison of the direction of the effect estimates. Another limitation is that the six studies did not all examine the same set of PFAS. PFHpS, PFUnDA, PFDoDA, and PFTrDA were examined in only one study, whereas PFOS and PFOA were examined in five studies, making it easier to identify consistencies in PFOS and PFOA associations across the studies. An additional potential limitation is that we identified only six studies that examined the role of PFAS on breastfeeding, and that all studies were from either northern Europe or North America, and only one study included high-exposed women. Additional studies are needed from other parts of the world, including Asia, Africa, and South America, and studies from highly polluted areas would be of particular interest. Regardless, these data demonstrating consistent positive associations that are replicated across studies in different populations, with different study designs, reduce the likelihood that specific biases or potential confounders in individual studies explain the positive associations, especially when much of the bias is likely towards the null.
In the five studies relying on PFAS measurements in maternal blood samples, samples were obtained at various timepoints during pregnancy or in the early post-partum period. PFAS are highly persistent chemicals and, under normal circumstances, serum concentration will not change much over a year [81]. However, during pregnancy, maternal blood volume increases, and serum PFAS concentrations subsequently decrease [82]. The studies that collected blood samples at different timepoints did take the timing of blood sampling into account in their analyses; thus, we suspect that the timing of the exposure assessments did not severely affect the results.
As with all systematic reviews, we cannot dismiss the risk of publication bias, and it is possible that additional null-finding studies have not been published, which could have impacted our findings. PFAS have been getting more attention from research scientists, communities that have been shown to be highly exposed, public health professionals, and regulatory agencies [83,84]. By placing more focus on this topic of research, we hope to increase the body of literature examining the association between PFAS and breastfeeding duration. In fact, regulatory agencies examining experimental evidence are beginning to appreciate the effects of PFAS on the health and function of the breast, yet the National Academies of Sciences, Engineering, and Medicine did not consider duration of lactation in their 2022 “Guidance on PFAS Exposure, Testing, and Clinical Follow-Up”, and they chose adults as the population for proposed health guidance instead of infants [85]. At present, based on experimental studies in rodents, the development of the mammary gland is considered the most sensitive developmental outcome of PFOA exposure [22] and has been considered for regulatory action in the U.S. [86].
The benefits of breastfeeding are well known, and for decades, health authorities have worked to promote breastfeeding by providing education and support to new mothers and the health professionals around them [87]. However, little emphasis has been given to environmental exposures that might impair breastfeeding, the functional aspect of the breast. This review suggests that breastfeeding may be sensitive to PFAS exposures, thus, emphasizing that breastfeeding is vulnerable to these environmental pollutants. Thus, in addition to the direct negative effects of PFAS on child health [31,32], maternal PFAS exposure may also indirectly negatively impact child health through reduced breastfeeding. Given that other environmental and lifestyle factors also affect women’s ability to breastfeed [60,88,89], more research is needed to elucidate which exposures might impair breastfeeding and to better understand the mechanisms behind such effects.
Author Contributions
Conceptualization, A.T.; investigation, A.T.; RoB assessment, O.N.A.; quality control of RoB, S.E.F.; methodology, A.T., M.E.R., J.M.B., J.S.T., L.N.V., and S.E.F.; writing–original draft, A.T., L.N.V. and S.E.F.; writing–review and editing, O.N.A., M.E.R., J.M.B. and J.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
Joseph Braun and Suzanne Fenton were funded by the National Institute of Environmental Health Sciences; R01 ES032386 (JMB) and ES103375-01 (SEF). Megan Romano was funded by grant P20 GM104416 from the National Institute of General Medical Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available upon request.
Acknowledgments
The authors would like to thank Christel Nielsen for help with interpretation of selected estimates from the publication, Nielsen, C.; Li, Y.; Lewandowski, M.; Fletcher, T.; Jakobsson, K. Breastfeeding initiation and duration after high exposure to perfluoroalkyl substances through contaminated drinking water: A cohort study from Ronneby, Sweden. Environmental research 2022;207:112206.
Conflicts of Interest
Joseph Braun and his institution were financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water. The authors have no other actual or potential competing financial interests.
References
- World Health Organisation. Breastfeeding. 2022. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 14 March 2022).
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front. Immunol. 2021, 12, 683022. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Victora, C.G. Short-Term Effects of Breastfeeding: A Systematic Review of the Benefits of Breastfeeding on Diarhoea and Pneumonia Mortality; World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Soled, D.; Keim, S.A.; Rapoport, E.; Rosen, L.; Adesman, A. Breastfeeding Is Associated with a Reduced Risk of Attention-Deficit/Hyperactivity Disorder Among Preschool Children. J. Dev. Behav. Pediatr. 2021, 42, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Saravia-Bartra, M.M.; Cazorla, P.; Ignacio-Cconchoy, F.L.; Cazorla-Saravia, P. Exclusive breastfeeding as a protective factor of acute lymphoblastic leukemia. Andes Pediatr. 2021, 92, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; de Sousa, B.A.; de Mola, C.L. Breastfeeding and neurodevelopmental outcomes. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 174–178. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef]
- World Health Organisation. Infant and Young Child Feeding. 9 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 11 March 2022).
- Meek, J.Y.; Noble, L.; Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Horton, B.J.; Chetwynd, E.; Watkins, S.; Grewen, K.; Meltzer-Brody, S. Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. J. Womens Health 2014, 23, 404–412. [Google Scholar] [CrossRef]
- Wambach, K.; Campbell, S.H.; Gill, S.L.; Dodgson, J.E.; Abiona, T.C.; Heinig, M.J. Clinical lactation practice: 20 years of evidence. J. Hum. Lact. 2005, 21, 245–258. [Google Scholar] [CrossRef]
- Ahluwalia, I.B.; Morrow, B.; Hsia, J. Why do women stop breastfeeding? Findings from the Pregnancy Risk Assessment and Monitoring System. Pediatrics 2005, 116, 1408–1412. [Google Scholar] [CrossRef]
- Li, R.; Fein, S.B.; Chen, J.; Grummer-Strawn, L.M. Why mothers stop breastfeeding: Mothers’ self-reported reasons for stopping during the first year. Pediatrics 2008, 122 (Suppl. 2), S69–S76. [Google Scholar] [CrossRef]
- Gianni, M.L.; Bettinelli, M.E.; Manfra, P.; Sorrentino, G.; Bezze, E.; Plevani, L.; Cavallaro, G.; Raffaeli, G.; Crippa, B.L.; Colombo, L.; et al. Breastfeeding Difficulties and Risk for Early Breastfeeding Cessation. Nutrients 2019, 11, 2266. [Google Scholar] [CrossRef]
- Lechosa-Muniz, C.; Paz-Zulueta, M.; Cayón-De las Cuevas, J.; Llorca, J.; Cabero-Pérez, M.J. Declared Reasons for Cessation of Breastfeeding during the First Year of Life: An Analysis Based on a Cohort Study in Northern Spain. Int. J. Environ. Res. Public Health 2021, 18, 8414. [Google Scholar] [CrossRef]
- Hannan, F.M.; Elajnaf, T.; Vandenberg, L.N.; Kennedy, S.H.; Thakker, R.V. Hormonal regulation of mammary gland development and lactation. Nat. Rev. Endocrinol. 2023, 19, 46–61. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls. Draft for Public Comment; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; et al. Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA J. 2020, 18, e06223. [Google Scholar]
- United Nations Environment Programme. PFASs Listed under the Stockholm Convention. 2022. Available online: http://chm.pops.int/Implementation/IndustrialPOPs/PFAS/Overview/tabid/5221/Default.aspx (accessed on 19 August 2022).
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, Y.; An, Q.; Yang, X.; Yin, S.; Ma, L.Q.; Liu, W. Prenatal and postnatal exposure to emerging and legacy per-/polyfluoroalkyl substances: Levels and transfer in maternal serum, cord serum, and breast milk. Sci. Total Environ. 2022, 812, 152446. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, U.B.; Grandjean, P.; Nielsen, F.; Weihe, P.; Budtz-Jørgensen, E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ. Sci. Technol. 2015, 49, 10466–10473. [Google Scholar] [CrossRef] [PubMed]
- Verner, M.A.; Ngueta, G.; Jensen, E.T.; Fromme, H.; Völkel, W.; Nygaard, U.C.; Granum, B.; Longnecker, M.P. A Simple Pharmacokinetic Model of Prenatal and Postnatal Exposure to Perfluoroalkyl Substances (PFASs). Environ. Sci. Technol. 2016, 50, 978–986. [Google Scholar] [CrossRef]
- Muller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Grønnestad, R.; Karimi, M.; Lie, E.; Manyilizu, W.B.; Mdegela, R.H.; Mokiti, F.; Murtadha, M.; et al. Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ. Res. 2019, 170, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Criswell, R.L.; Wang, Y.; Christensen, B.; Botelho, J.C.; Calafat, A.M.; Peterson, L.A.; Huset, C.A.; Karagas, M.R.; Romano, M.E. Concentrations of Per- and Polyfluoroalkyl Substances in Paired Maternal Plasma and Human Milk in the New Hampshire Birth Cohort. Environ. Sci. Technol. 2023, 57, 463–472. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Perfluoroalkyls; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, GA, USA, 2021.
- Zhang, X.; Xue, L.; Deji, Z.; Wang, X.; Liu, P.; Lu, J.; Zhou, R.; Huang, Z. Effects of exposure to per- and polyfluoroalkyl substances on vaccine antibodies: A systematic review and meta-analysis based on epidemiological studies. Environ. Pollut. 2022, 306, 119442. [Google Scholar] [CrossRef]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Timmermann, A.; Budtz-Jørgensen, E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017, 14, 188–195. [Google Scholar] [CrossRef]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518. [Google Scholar] [CrossRef]
- White, S.S.; Calafat, A.M.; Kuklenyik, Z.; Villanueva, L.; Zehr, R.D.; Helfant, L.; Strynar, M.J.; Lindstrom, A.B.; Thibodeaux, J.R.; Wood, C.; et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007, 96, 133–144. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121 Pt 1, 1027–1031. [Google Scholar] [CrossRef]
- ROBINS-E Development, Group; Higgins, J.; Morgan, R.; Rooney, A.; Taylor, K.; Thayer, K.; Silva, R.; Lemeris, C.; Akl, A.; Arroyave, W.; et al. Risk Of Bias In Non-randomized Studies-of Exposure (ROBINS-E). Launch Version. 2022. Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 21 March 2023).
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Odland, J.Ø.; Van de Bor, M.; Jacobsen, G.W.; Sandanger, T.M. Factors Associated with Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines: A Descriptive Study of Parous Women in Norway and Sweden. PLoS ONE 2016, 11, e0166127. [Google Scholar] [CrossRef]
- Kronborg, H.; Foverskov, E.; Væth, M.; Maimburg, R.D. The role of intention and self-efficacy on the association between breastfeeding of first and second child, a Danish cohort study. BMC Pregnancy Childbirth 2018, 18, 454. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand. J. Work Environ. Health 2010, 36, 413–421. [Google Scholar] [CrossRef]
- Nielsen, C.; Li, Y.; Lewandowski, M.; Fletcher, T.; Jakobsson, K. Breastfeeding initiation and duration after high exposure to perfluoroalkyl substances through contaminated drinking water: A cohort study from Ronneby, Sweden. Environ. Res. 2022, 207, 112206. [Google Scholar] [CrossRef]
- Rosen, E.M.; Brantsæter, A.L.; Carroll, R.; Haug, L.S.; Singer, A.B.; Zhao, S.; Ferguson, K.K. Maternal Plasma Concentrations of Per- and polyfluoroalkyl Substances and Breastfeeding Duration in the Norwegian Mother and Child Cohort. Environ. Epidemiol. 2018, 2, e027. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Andersen, M.S.; Budtz-Jørgensen, E.; Boye, H.; Nielsen, F.; Jensen, R.C.; Bruun, S.; Husby, S.; Grandjean, P.; Jensen, T.K. Pregnancy Exposure to Perfluoroalkyl Substances and Associations With Prolactin Concentrations and Breastfeeding in the Odense Child Cohort. J. Clin. Endocrinol. Metab. 2022, 107, e631–e642. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Budtz-Jørgensen, E.; Petersen, M.S.; Weihe, P.; Steuerwald, U.; Nielsen, F.; Jensen, T.K.; Grandjean, P. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod. Toxicol. 2017, 68, 164–170. [Google Scholar] [CrossRef]
- Romano, M.E.; Xu, Y.; Calafat, A.M.; Yolton, K.; Chen, A.; Webster, G.M.; Eliot, M.N.; Howard, C.R.; Lanphear, B.P.; Braun, J.M. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ. Res. 2016, 149, 239–246. [Google Scholar] [CrossRef]
- Mondal, D.; Weldon, R.H.; Armstrong, B.G.; Gibson, L.J.; Lopez-Espinosa, M.J.; Shin, H.M.; Fletcher, T. Breastfeeding: A potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ. Health Perspect. 2014, 122, 187–192. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Sabaredzovic, A.; Namork, E.; Nygaard, U.C.; Granum, B.; Haug, L.S. Exposure of Norwegian toddlers to perfluoroalkyl substances (PFAS): The association with breastfeeding and maternal PFAS concentrations. Environ. Int. 2016, 94, 687–694. [Google Scholar] [CrossRef]
- Kärrman, A.; Ericson, I.; van Bavel, B.; Darnerud, P.O.; Aune, M.; Glynn, A.; Lignell, S.; Lindström, G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007, 115, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Von Holst, H.; Nayak, P.; Dembek, Z.; Buehler, S.; Echeverria, D.; Fallacara, D.; John, L. Perfluoroalkyl substances exposure and immunity, allergic response, infection, and asthma in children: Review of epidemiologic studies. Heliyon 2021, 7, e08160. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Goudarzi, H.; Oulhote, Y. Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Curr. Environ. Health Rep. 2018, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rodriquez-Palmero, M.; Koletzko, B.; Jensen, R. Nutritional and biochemical properties of human milk, Part I: General aspects, proteins, and carbohydrates. Clin. Perinatol. 1999, 26, 307–333. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Mielke, H.; Fromme, H.; Völkel, W.; Menzel, J.; Peiser, M.; Zepp, F.; Willich, S.N.; Weikert, C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. [Google Scholar] [CrossRef]
- Forns, J.; Verner, M.A.; Iszatt, N.; Nowack, N.; Bach, C.C.; Vrijheid, M.; Costa, O.; Andiarena, A.; Sovcikova, E.; Høyer, B.B.; et al. Early Life Exposure to Perfluoroalkyl Substances (PFAS) and ADHD: A Meta-Analysis of Nine European Population-Based Studies. Environ. Health Perspect. 2020, 128, 57002. [Google Scholar] [CrossRef]
- Varsi, K.; Torsvik, I.K.; Huber, S.; Averina, M.; Brox, J.; Bjørke-Monsen, A.L. Impaired gross motor development in infants with higher PFAS concentrations. Environ. Res. 2022, 204 Pt D, 112392. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Jensen, K.J.; Nielsen, F.; Budtz-Jørgensen, E.; van der Klis, F.; Benn, C.S.; Grandjean, P.; Fisker, A.B. Serum Perfluoroalkyl Substances, Vaccine Responses, and Morbidity in a Cohort of Guinea-Bissau Children. Environ. Health Perspect. 2020, 128, 87002. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Pedersen, H.S.; Weihe, P.; Bjerregaard, P.; Nielsen, F.; Heilmann, C.; Grandjean, P. Concentrations of tetanus and diphtheria antibodies in vaccinated Greenlandic children aged 7–12 years exposed to marine pollutants, a cross sectional study. Environ. Res. 2022, 203, 111712. [Google Scholar] [CrossRef]
- Gladen, B.C.; Rogan, W.J. DDE and shortened duration of lactation in a northern Mexican town. Am. J. Public Health 1995, 85, 504–508. [Google Scholar] [CrossRef]
- Rogan, W.J.; Gladen, B.C.; McKinney, J.D.; Carreras, N.; Hardy, P.; Thullen, J.; Tingelstad, J.; Tully, M.A. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: Effects on growth, morbidity, and duration of lactation. Am. J. Public Health 1987, 77, 1294–1297. [Google Scholar] [CrossRef]
- Kasper, N.; Peterson, K.E.; Zhang, Z.; Ferguson, K.K.; Sánchez, B.N.; Cantoral, A.; Meeker, J.D.; Téllez-Rojo, M.M.; Pawlowski, C.M.; Ettinger, A.S. Association of Bisphenol A Exposure with Breastfeeding and Perceived Insufficient Milk Supply in Mexican Women. Matern. Child Health J. 2016, 20, 1713–1719. [Google Scholar] [CrossRef]
- Mehlsen, A.; Høllund, L.; Boye, H.; Frederiksen, H.; Andersson, A.M.; Bruun, S.; Husby, S.; Jensen, T.K.; Timmermann, C.A. Pregnancy exposure to bisphenol A and duration of breastfeeding. Environ. Res. 2022, 206, 112471. [Google Scholar] [CrossRef]
- White, S.S.; Stanko, J.P.; Kato, K.; Calafat, A.M.; Hines, E.P.; Fenton, S.E. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ. Health Perspect. 2011, 119, 1070–1076. [Google Scholar] [CrossRef]
- Macon, M.B.; Villanueva, L.R.; Tatum-Gibbs, K.; Zehr, R.D.; Strynar, M.J.; Stanko, J.P.; White, S.S.; Helfant, L.; Fenton, S.E. Prenatal perfluorooctanoic acid exposure in CD-1 mice: Low-dose developmental effects and internal dosimetry. Toxicol. Sci. 2011, 122, 134–145. [Google Scholar] [CrossRef]
- Tucker, D.K.; Macon, M.B.; Strynar, M.J.; Dagnino, S.; Andersen, E.; Fenton, S.E. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod. Toxicol. 2015, 54, 26–36. [Google Scholar] [CrossRef]
- White, S.S.; Kato, K.; Jia, L.T.; Basden, B.J.; Calafat, A.M.; Hines, E.P.; Stanko, J.P.; Wolf, C.J.; Abbott, B.D.; Fenton, S.E. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod. Toxicol. 2009, 27, 289–298. [Google Scholar] [CrossRef]
- Cope, H.A.; Blake, B.E.; Love, C.; McCord, J.; Elmore, S.A.; Harvey, J.B.; Chappell, V.A.; Fenton, S.E. Latent, sex-specific metabolic health effects in CD-1 mouse offspring exposed to PFOA or HFPO-DA (GenX) during gestation. Emerg. Contam. 2021, 7, 219–235. [Google Scholar] [CrossRef]
- Edwards, T. An Oral (Gavage) Reproduction/Developmental Toxicity Screening Study of H-28548 in Mice (DuPont); WIL Research Laboratories, LLC: Ashland, OH, USA, 2010. [Google Scholar]
- Itoh, S.; Araki, A.; Mitsui, T.; Miyashita, C.; Goudarzi, H.; Sasaki, S.; Cho, K.; Nakazawa, H.; Iwasaki, Y.; Shinohara, N.; et al. Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environ. Int. 2016, 94, 51–59. [Google Scholar] [CrossRef]
- Lee, C.K.; Kang, S.G.; Lee, J.T.; Lee, S.W.; Kim, J.H.; Kim, D.H.; Son, B.C.; Kim, K.H.; Suh, C.H.; Kim, S.Y.; et al. Effects of perfluorooctane sulfuric acid on placental PRL-family hormone production and fetal growth retardation in mice. Mol. Cell. Endocrinol. 2015, 401, 165–172. [Google Scholar] [CrossRef]
- Yang, Q.; Kurotani, R.; Yamada, A.; Kimura, S.; Gonzalez, F.J. Peroxisome proliferator-activated receptor alpha activation during pregnancy severely impairs mammary lobuloalveolar development in mice. Endocrinology 2006, 147, 4772–4780. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Q. Mediating Roles of PPARs in the Effects of Environmental Chemicals on Sex Steroids. PPAR Res. 2017, 2017, 3203161. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.M.; Vaught, D.B.; Joly, M.M.; Hicks, D.J.; Sanchez, V.; Owens, P.; Rahman, B.; Elion, D.L.; Balko, J.M.; Cook, R.S. ErbB3 drives mammary epithelial survival and differentiation during pregnancy and lactation. Breast Cancer Res. 2017, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Hapon, M.B.; Simoncini, M.; Via, G.; Jahn, G.A. Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction 2003, 126, 371–382. [Google Scholar] [CrossRef]
- Boesen, S.A.H.; Long, M.; Wielsøe, M.; Mustieles, V.; Fernandez, M.F.; Bonefeld-Jørgensen, E.C. Exposure to Perflouroalkyl acids and foetal and maternal thyroid status: A review. Environ. Health 2020, 19, 107. [Google Scholar] [CrossRef]
- Davidsen, N.; Ramhøj, L.; Lykkebo, C.A.; Kugathas, I.; Poulsen, R.; Rosenmai, A.K.; Evrard, B.; Darde, T.A.; Axelstad, M.; Bahl, M.I.; et al. PFOS-induced thyroid hormone system disrupted rats display organ-specific changes in their transcriptomes. Environ. Pollut. 2022, 305, 119340. [Google Scholar] [CrossRef]
- Bruun, S.; Buhl, S.; Husby, S.; Jacobsen, L.N.; Michaelsen, K.F.; Sørensen, J.; Zachariassen, G. Breastfeeding, Infant Formula, and Introduction to Complementary Foods-Comparing Data Obtained by Questionnaires and Health Visitors’ Reports to Weekly Short Message Service Text Messages. Breastfeed. Med. 2017, 12, 554–560. [Google Scholar] [CrossRef]
- Newhook, J.T.; Newhook, L.A.; Midodzi, W.K.; Murphy Goodridge, J.; Burrage, L.; Gill, N.; Halfyard, B.; Twells, L. Poverty and Breastfeeding: Comparing Determinants of Early Breastfeeding Cessation Incidence in Socioeconomically Marginalized and Privileged Populations in the FiNaL Study. Health Equity 2017, 1, 96–102. [Google Scholar] [CrossRef]
- Buekers, J.; Colles, A.; Cornelis, C.; Morrens, B.; Govarts, E.; Schoeters, G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int. J. Environ. Res. Public Health 2018, 15, 2818. [Google Scholar] [CrossRef]
- Weisskopf, M.G.; Webster, T.F. Trade-offs of Personal Versus More Proxy Exposure Measures in Environmental Epidemiology. Epidemiology 2017, 28, 635–643. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Chen, L.; Tong, C.; Huo, X.; Zhang, J.; Tian, Y.; Cohort, S.B. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: A longitudinal cohort with repeated measurements. Chemosphere 2021, 267, 128899. [Google Scholar] [CrossRef]
- Banwell, C.; Housen, T.; Smurthwaite, K.; Trevenar, S.; Walker, L.; Todd, K.; Rosas, M.; Kirk, M. Health and social concerns about living in three communities affected by per- and polyfluoroalkyl substances (PFAS): A qualitative study in Australia. PLoS ONE 2021, 16, e0245141. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up; National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- Post, G.B. Recent US State and Federal Drinking Water Guidelines for Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem. 2020, 40, 550–563. [Google Scholar] [CrossRef]
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Richter, L.M.; Victora, C.G. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef]
- Criswell, R.; Crawford, K.A.; Bucinca, H.; Romano, M.E. Endocrine-disrupting chemicals and breastfeeding duration: A review. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 388–395. [Google Scholar] [CrossRef]
- Lee, S.; Kelleher, S.L. Biological underpinnings of breastfeeding challenges: The role of genetics, diet, and environment on lactation physiology. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E405–E422. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).