Gestational Exposure to Phthalates and Phthalate Replacements in Relation to Neurodevelopmental Delays in Early Childhood
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Phthalate Metabolite Measurements
2.3. Neurobehavioral Assessments
2.4. Statistical Analyses
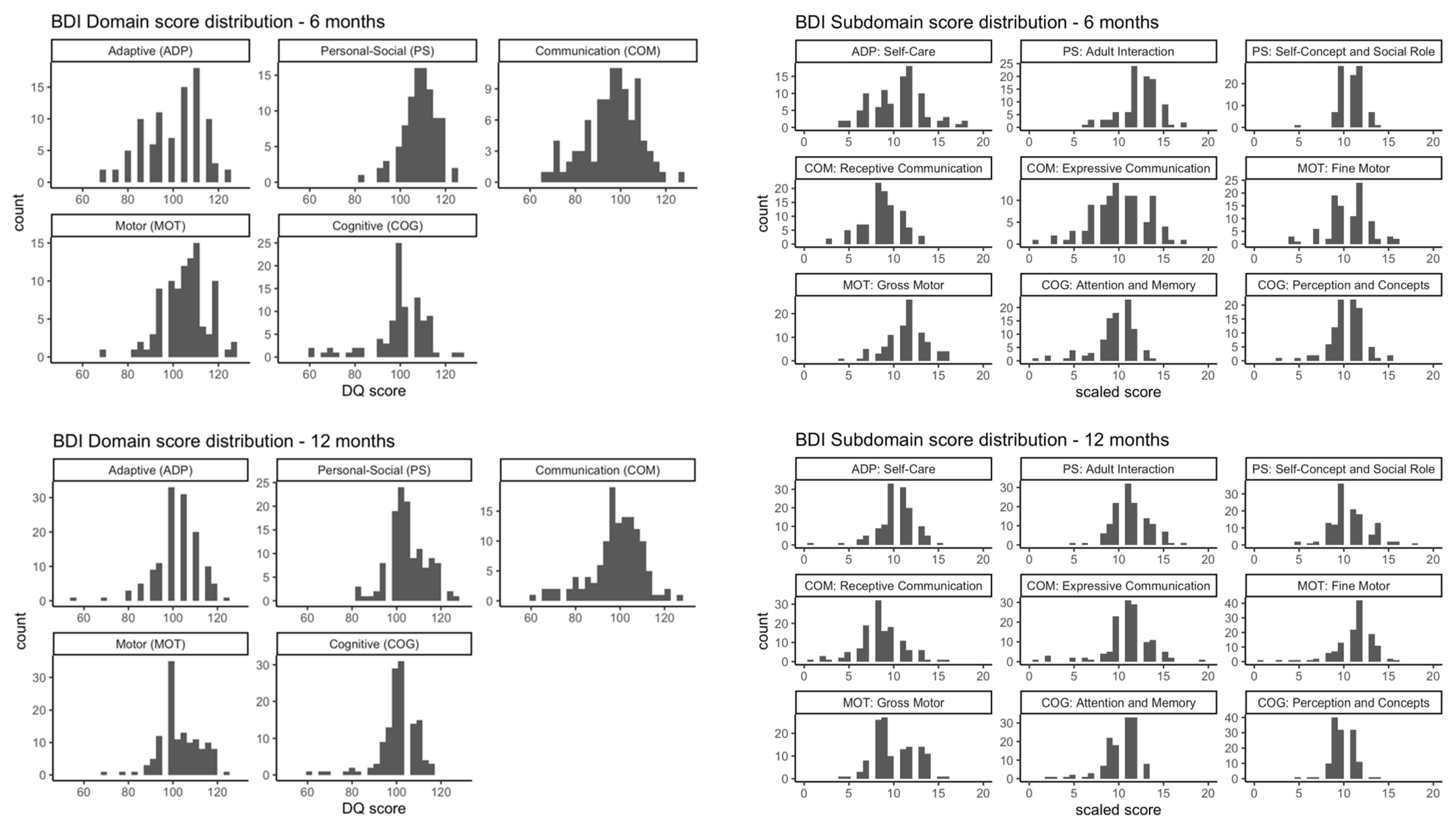
3. Results
3.1. Participant Characteristics
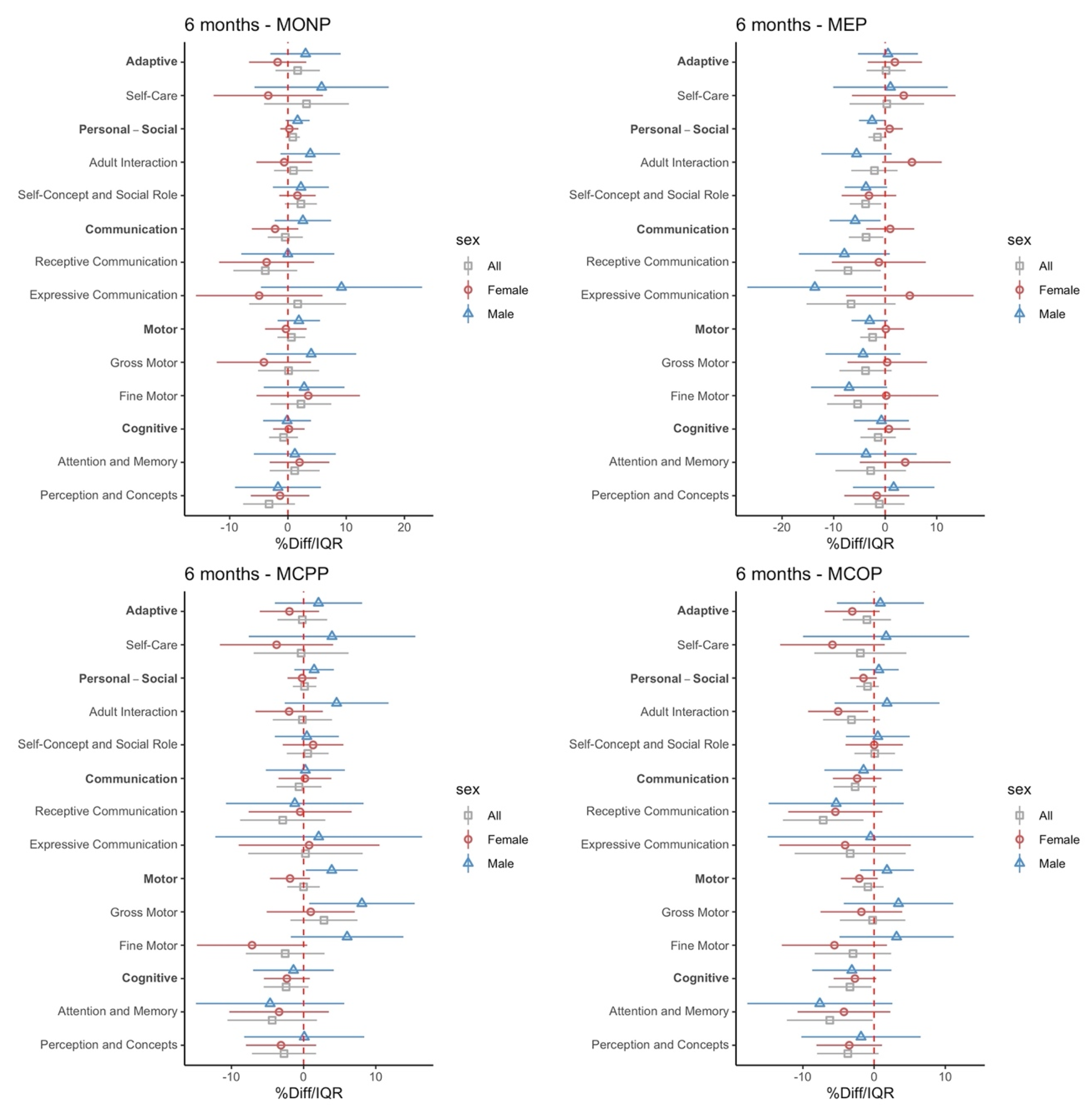
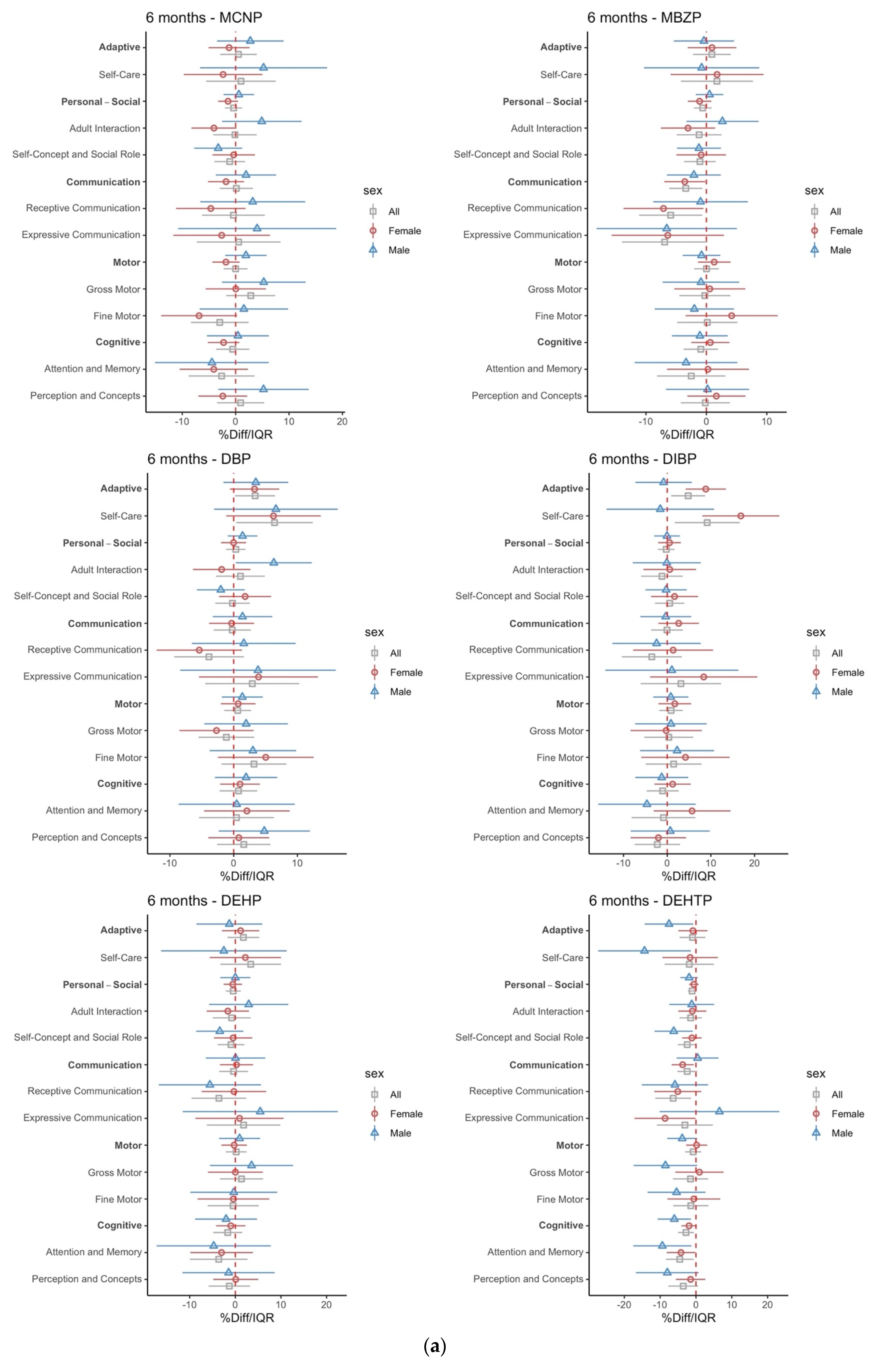
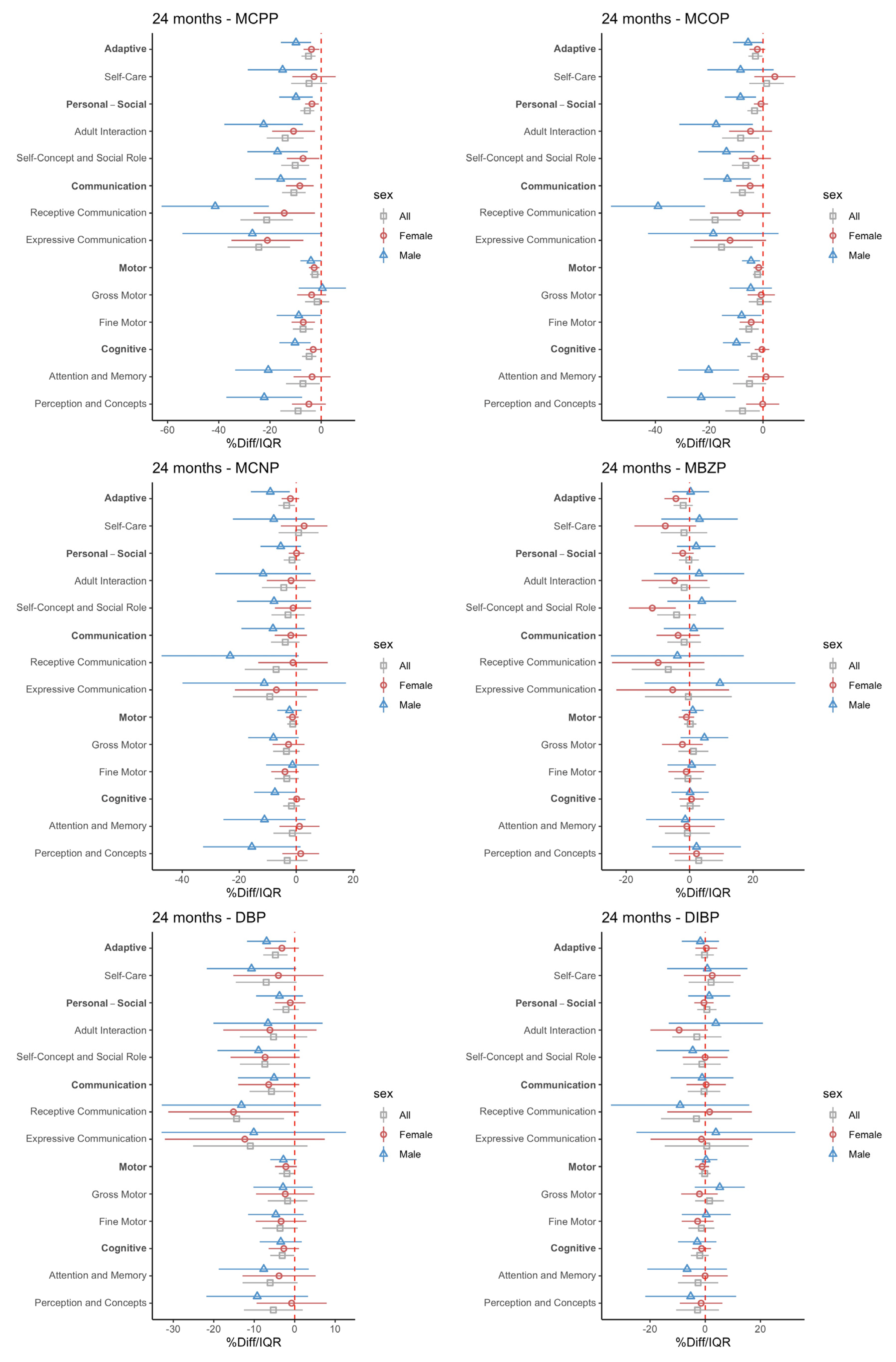
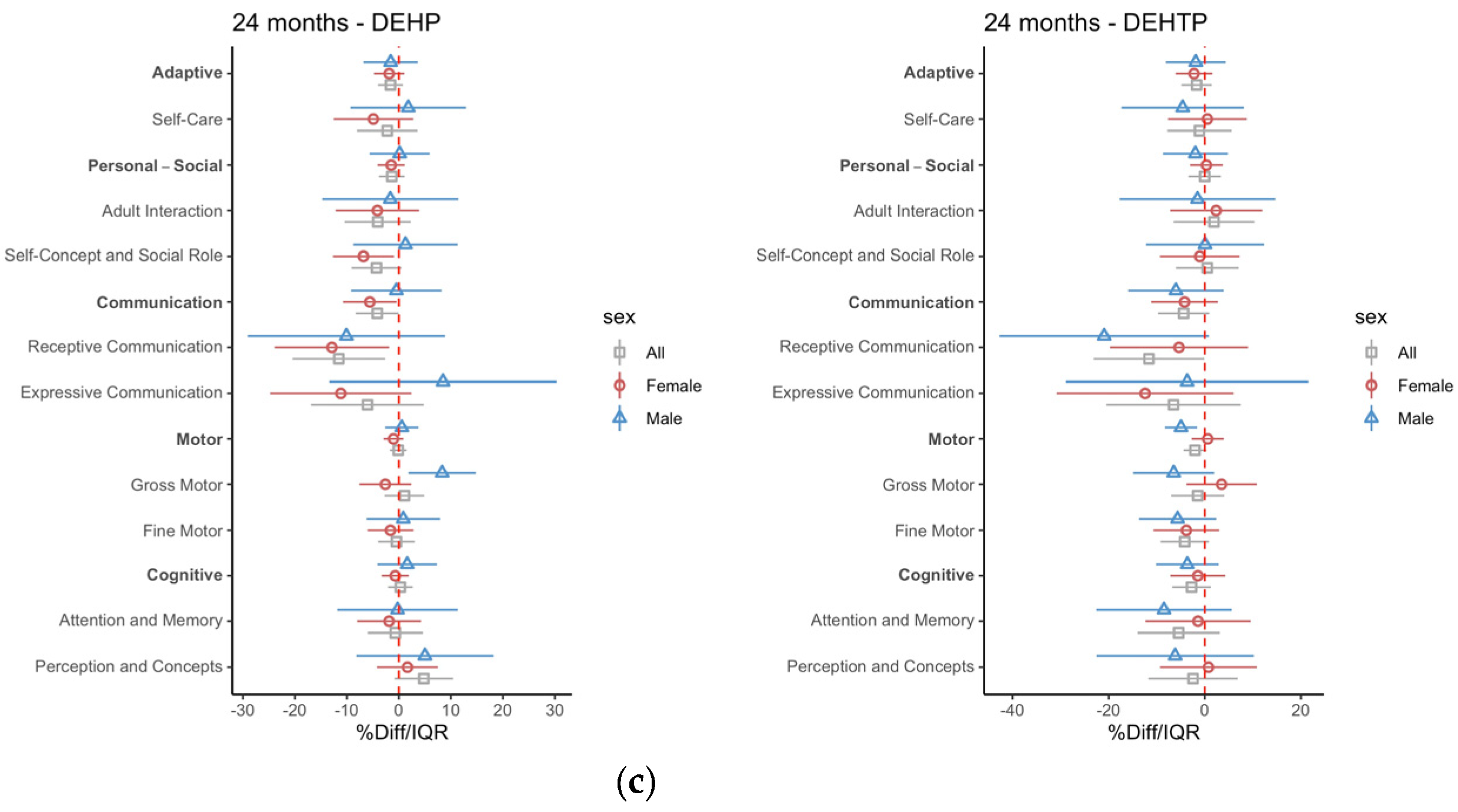
3.2. Intrauterine Phthalate Exposure and BDI-2 in Early Childhood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villagomez, A.N.; Muñoz, F.M.; Peterson, R.L.; Colbert, A.M.; Gladstone, M.; MacDonald, B.; Wilson, R.; Fairlie, L.; Gerner, G.J.; Patterson, J.; et al. Neurodevelopmental delay: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2019, 37, 7623–7641. [Google Scholar] [CrossRef] [PubMed]
- Zablotsky, B.; Bramlett, M.D.; Visser, S.N.; Danielson, M.L.; Blumberg, S.J. Latent Class Analysis of ADHD Neurodevelopmental and Mental Health Comorbidities. J. Dev. Behav. Pediatr. 2018, 39, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lamsal, R.; Zwicker, J.D. Economic Evaluation of Interventions for Children with Neurodevelopmental Disorders: Opportunities and Challenges. Appl. Health Econ. Health Policy 2017, 15, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Ghassabian, A.; Trasande, L. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front. Endocrinol. 2018, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Ejaredar, M.; Nyanza, E.C.; Eycke, K.T.; Dewey, D. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res. 2015, 142, 51–60, ISSN 0013-9351. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, H.; Pao, P.-C.; Lee, A.; Wang, J.; Chan, Y.S.; Iii, F.A.M.; Chan, S.W.; Cheng, S.H.; Chen, X. Exposure to phthalates impaired neurodevelopment through estrogenic effects and induced DNA damage in neurons. Aquat. Toxicol. 2020, 222, 105469, ISSN 0166-445X. [Google Scholar] [CrossRef]
- Lee, S.M.; Jeon, S.; Jeong, H.J.; Kim, B.-N.; Kim, Y. Dibutyl phthalate exposure during gestation and lactation in C57BL/6 mice: Maternal behavior and neurodevelopment in pups. Environ. Res. 2019, 182, 109025. [Google Scholar] [CrossRef]
- Harris, C.A.; Henttu, P.; Parker, M.G.; Sumpter, J.P. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997, 105, 802–811. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Tan, T.; Lee, S.T.; Cheng, S.H.; Lee, F.W.F.; Ho, K.C. Toxicity and estrogenic endocrine dis-rupting activity of phthalates and their mixtures. Int. J. Environ. Res. Public Health 2014, 11, 3156–3168. [Google Scholar] [CrossRef]
- Gray, L.E., Jr.; Ostby, J.; Furr, J.; Price, M.; Veeramachaneni, D.R.; Parks, L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000, 58, 350–365. [Google Scholar] [CrossRef]
- Christiansen, S.; Boberg, J.; Axelstad, M.; Dalgaard, M.; Vinggaard, A.M.; Metzdorff, S.B.; Hass, U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod. Toxicol. 2010, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, S.; Barrett, E.; Butts, S.; Wang, C.; Swan, S. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction 2014, 147, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, S.; Butts, S.; Wang, C.; Barrett, E.; Nguyen, R.; Schwartz, S.M.; Swan, S.H. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J. Clin. Endocrinol. Metab. 2017, 102, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Pacyga, D.C.; Gardiner, J.C.; Flaws, J.A.; Li, Z.; Calafat, A.M.; Korrick, S.A.; Schantz, S.L.; Strakovsky, R.S. Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ. Int. 2021, 155, 106676. [Google Scholar] [CrossRef] [PubMed]
- Cathey, A.L.; Watkins, D.; Rosario, Z.Y.; Vélez, C.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations of Phthalates and Phthalate Replacements with CRH and Other Hormones Among Pregnant Women in Puerto Rico. J. Endocr. Soc. 2019, 3, 1127–1149. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Ferguson, K.K.; Soldin, O.P.; Cantonwine, D.E.; Rivera-González, L.O.; Del Toro, L.V.A.; Meeker, J.D. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: A longitudinal analysis. Reprod. Biol. Endocrinol. 2015, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Day, D.B.; Collett, B.R.; Barrett, E.S.; Bush, N.R.; Swan, S.H.; Wang, C.; TIDES Study Team. Prenatal sex hormones and behavioral outcomes in children. Psychoneuroendocrinology 2020, 113, 104547. [Google Scholar] [CrossRef]
- Baud, O.; Berkane, N. Hormonal Changes Associated with Intra-Uterine Growth Restriction: Impact on the Developing Brain and Future Neurodevelopment. Front. Endocrinol. 2019, 10, 179. [Google Scholar] [CrossRef]
- Firestein, M.R.; Romeo, R.D.; Winstead, H.; Goldman, D.A.; Grobman, W.A.; Haas, D.; Mercer, B.; Parker, C.; Parry, S.; Reddy, U.; et al. Elevated prenatal maternal sex hormones, but not placental aromatase, are associated with child neurodevelopment. Horm. Behav. 2022, 140, 105125. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Liu, X.; Rauh, V.A.; Calafat, A.M.; Just, A.C.; Hoepner, L.; Diaz, D.; Quinn, J.; Adibi, J.; Perera, F.P.; et al. Maternal Prenatal Urinary Phthalate Metabolite Concentrations and Child Mental, Psychomotor, and Behavioral Development at 3 Years of Age. Environ. Health Perspect. 2012, 120, 290–295. [Google Scholar] [CrossRef]
- Kobrosly, R.W.; Evans, S.; Miodovnik, A.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Swan, S.H. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ. Health Perspect. 2014, 122, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Eom, S.; Kim, H.J.; Lee, J.J.; Choi, G.; Choi, S.; Eun, S.H. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age-CHECK cohort study. Sci. Total Environ. 2018, 624, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.; Polańska, K.; Koch, H.M.; Pälmke, C.; Waszkowska, M.; Stańczak, A.; Wesołowska, E.; Hanke, W.; Bose-O’Reilly, S.; Calamandrei, G.; et al. Phthalate exposure and neurodevelopmental outcomes in early school age children from Poland. Environ. Res. 2019, 179, 108829. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.; McCray, N.L.; VanNoy, B.N.; Yau, A.; Geller, R.J.; Adamkiewicz, G.; Zota, A.R. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: A preliminary analysis. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 366–373. [Google Scholar] [CrossRef]
- Cantonwine, D.E.; Cordero, J.F.; Rivera-González, L.O.; Del Toro, L.V.A.; Ferguson, K.K.; Mukherjee, B.; Calafat, A.M.; Crespo, N.; Jiménez-Vélez, B.; Padilla, I.Y.; et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: Distribution, temporal variability, and predictors. Environ. Int. 2014, 62, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Cantonwine, D.E.; Rivera-González, L.O.; Ferguson, K.; Mukherjee, B.; Calafat, A.M.; Ye, X.; Del Toro, L.V.A.; Crespo-Hernández, N.; Jiménez-Vélez, B.; et al. Distribution, Variability, and Predictors of Urinary Concentrations of Phenols and Parabens among Pregnant Women in Puerto Rico. Environ. Sci. Technol. 2013, 47, 3439–3447. [Google Scholar] [CrossRef]
- Manjourides, J.; Zimmerman, E.; Watkins, D.J.; Carpenito, T.; Vélez-Vega, C.M.; Huerta-Montañez, G.; Rosario, Z.; Ayala, I.; Vergara, C.; Feric, Z.; et al. Cohort profile: Center for Research on Early Childhood Exposure and Development in Puerto Rico. BMJ Open 2020, 10, e036389. [Google Scholar] [CrossRef]
- Meeker, J.D.; Hu, H.; Cantonwine, D.E.; Lamadrid-Figueroa, H.; Calafat, A.M.; Ettinger, A.S.; Hernandez-Avila, M.; Loch-Caruso, R.; Téllez-Rojo, M.M. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environ. Health Perspect. 2009, 117, 1587–1592. [Google Scholar] [CrossRef]
- Silva, M.J.; Samandar, E.; Calafat, A.M.; Ye, X. Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicol. Vitr. 2015, 29, 716–721. [Google Scholar] [CrossRef]
- Rodríguez-Carmona, Y.; Ashrap, P.; Calafat, A.M.; Ye, X.; Rosario, Z.; Bedrosian, L.D.; Huerta-Montanez, G.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; et al. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 56–69. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Zota, A.R.; Calafat, A.M.; Woodruff, T. Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 2014, 122, 235–241. [Google Scholar] [CrossRef]
- Varshavsky, J.R.; Morello-Frosch, R.; Woodruff, T.J.; Zota, A.R. Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ. Int. 2018, 115, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.J.; Obsekov, V.; Jacobson, M.H.; Kahn, L.G.; Trasande, L. Phthalates and Sex Steroid Hormones Among Men From NHANES, 2013–2016. J. Clin. Endocrinol. Metab. 2020, 105, e1225–e1234. [Google Scholar] [CrossRef]
- Bliss, S.L. Test Reviews: Newborg, J. (2005). Battelle Developmental Inventory—Second Edition. Itasca, IL: Riverside. J. Psychoeduc. Assess. 2007, 25, 409–415. [Google Scholar] [CrossRef]
- Newborg, J. Battelle Developmental Inventory, 2nd ed.; Riverside: Itasca, IL, USA, 2005. [Google Scholar] [CrossRef]
- Ferguson, K.K.; Rosen, E.M.; Rosario, Z.; Feric, Z.; Calafat, A.M.; McElrath, T.F.; Vega, C.V.; Cordero, J.F.; Alshawabkeh, A.; Meeker, J.D. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ. Int. 2019, 132, 105099. [Google Scholar] [CrossRef]
- Zimmerman, E.; Watkins, D.J.; Huerta-Montanez, G.; Pabon, Z.R.; Feric, Z.; Manjourides, J.; Meeker, J.D. Associations of gestational phthalate exposure and non-nutritive suck among infants from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) birth cohort study. Environ. Int. 2021, 152, 106480. [Google Scholar] [CrossRef]
- Bellinger, D.C. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Saf. Health Work. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Nachman, R.M.; Cooper, G.S. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int. 2020, 137, 105408. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.-Z.; Huang, X.; Wang, M.; Wu, J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology 2019, 73, 199–212. [Google Scholar] [CrossRef]
- Minatoya, M.; Kishi, R. A review of recent studies on Bisphenol A and phthalate exposures and child neurodevelopment. Int. J. Environ. Res. Public Health 2021, 18, 3585. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ha, E.; Kim, E.; Park, H.; Ha, M.; Kim, J.; Hong, Y.; Chang, N.; Kim, B. Prenatal Exposure to Phthalates and Infant Development at 6 Months: Prospective Mothers and Children’s Environmental Health (MOCEH) Study. Environ. Health Perspect. 2011, 119, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Torres-Olascoaga, L.; Watkins, D.; Schnaas, L.; Meeker, J.; Solano-Gonzalez, M.; Osorio-Valencia, E.; Peterson, K.; Tellez-Rojo, M.; Tamayo-Ortiz, M. Early Gestational Exposure to High-Molecular-Weight Phthalates and Its Association with 48-Month-Old Children’s Motor and Cognitive Scores. Int. J. Environ. Res. Public Health 2020, 17, 8150. [Google Scholar] [CrossRef]
- Philippat, C.; Nakiwala, D.; Calafat, A.M.; Botton, J.; De Agostini, M.; Heude, B.; Slama, R.; The EDEN Mother–Child Study Group. Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ. Health Perspect. 2017, 125, 097014. [Google Scholar] [CrossRef]
- Téllez-Rojo, M.M.; Cantoral, A.; Cantonwine, D.E.; Schnaas, L.; Peterson, K.; Hu, H.; Meeker, J.D. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ. 2013, 461–462, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Doherty, B.T.; Engel, S.M.; Buckley, J.P.; Silva, M.J.; Calafat, A.M.; Wolff, M.S. Prenatal phthalate biomarker concentrations and performance on the Bayley scales of infant development-II in a population of young urban children. Environ. Res. 2017, 152, 51–58. [Google Scholar] [CrossRef]
- Loftus, C.T.; Bush, N.R.; Day, D.B.; Ni, Y.; Tylavsky, F.A.; Karr, C.J.; Kannan, K.; Barrett, E.S.; Szpiro, A.A.; Sathyanarayana, S.; et al. Exposure to prenatal phthalate mixtures and neurodevelopment in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Environ. Int. 2021, 150, 106409. [Google Scholar] [CrossRef]
- Donat-Vargas, C.; Perez-Carrascosa, F.; Gomez-Peña, C.; Mustieles, V.; Salcedo-Bellido, I.; Frederiksen, H.; Åkesson, A.; Arrebola, J.P. Associations of serum phthalate metabolites with thyroid hormones in GraMo cohort, Southern Spain. Environ. Pollut. 2021, 287, 117606. [Google Scholar] [CrossRef]
- Huang, P.-C.; Kuo, P.-L.; Guo, Y.-L.; Liao, P.-C.; Lee, C.-C. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum. Reprod. 2007, 22, 2715–2722. [Google Scholar] [CrossRef]
- Yao, H.-Y.; Han, Y.; Gao, H.; Huang, K.; Ge, X.; Xu, Y.-Y.; Xu, Y.-Q.; Jin, Z.-X.; Sheng, J.; Yan, S.-Q.; et al. Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 2016, 157, 42–48. [Google Scholar] [CrossRef]
- Prezioso, G.; Giannini, C.; Chiarelli, F. Effect of Thyroid Hormones on Neurons and Neurodevelopment. Horm. Res. Paediatr. 2018, 90, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Jordan, C.L.; Breedlove, S.M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Bendahan, C.C.; van de Beek, C.; Berenbaum, S.A. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neurosci. Biobehav. Rev. 2005, 29, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Niculae, A.; Pavăl, D. From molecules to behavior: An integrative theory of autism spectrum disorder. Med. Hypotheses 2016, 97, 74–84. [Google Scholar] [CrossRef]
- Sellinger, E.P.; Riesgo, V.R.; Brinks, A.S.; Willing, J.; Juraska, J.M. Perinatal phthalate exposure increases devel-opmental apoptosis in the rat medial prefrontal cortex. NeuroToxicology 2021, 87, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Chen, Y.H.; Mukherjee, B.; Meeker, J.D. Urinary phthalate metabolites and bi-omarkers of oxidative stress in pregnant women: A repeated measures analysis. Environ. Health Perspect. 2015, 123, 210–216. [Google Scholar] [CrossRef]
- Feng, Z.; Zou, X.; Jia, H.; Li, X.; Zhu, Z.; Liu, X.; Bucheli, P.; Ballevre, O.; Hou, Y.; Zhang, W.; et al. Maternal Docosahexaenoic Acid Feeding Protects Against Impairment of Learning and Memory and Oxidative Stress in Prenatally Stressed Rats: Possible Role of Neuronal Mitochondria Metabolism. Antioxid. Redox Signal. 2012, 16, 275–289. [Google Scholar] [CrossRef]
- Akhtar, F.; Rouse, C.A.; Catano, G.; Montalvo, M.; Ullevig, S.L.; Asmis, R.; Kharbanda, K.; Maffi, S.K. Acute maternal oxidant exposure causes susceptibility of the fetal brain to inflammation and oxidative stress. J. Neuroinflamm. 2017, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Holland, N.; Huen, K.; Tran, V.; Street, K.; Nguyen, B.; Bradman, A.; Eskenazi, B. Urinary Phthalate Metabolites and Biomarkers of Oxidative Stress in a Mexican-American Cohort: Variability in Early and Late Pregnancy. Toxics 2016, 4, 7. [Google Scholar] [CrossRef]
- Hayashi, M.; Miyata, R.; Tanuma, N. Oxidative Stress in Developmental Brain Disorders. Neurodegener. Dis. 2012, 724, 278–290. [Google Scholar] [CrossRef]
- Schaffert, A.; Arnold, J.; Karkossa, I.; Blüher, M.; von Bergen, M.; Schubert, K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells 2021, 10, 2367. [Google Scholar] [CrossRef] [PubMed]




| PROTECT (N = 1576) | Analysis Group (N = 274) | ||
|---|---|---|---|
| Maternal Characteristics | n (%) | n (%) | |
| Alcohol Use | Never | 836 (53) | 125 (46) |
| Pre-pregnancy | 608 (39) | 123 (45) | |
| Currently Employed | No | 573 (36) | 75 (27) |
| Yes | 953 (60) | 185 (68) | |
| Maternal Education | GED or less | 337 (21) | 29 (11) |
| Some college | 515 (33) | 98 (36) | |
| Bachelors or higher | 674 (43) | 130 (47) | |
| Annual Income | <10 k | 431 (27) | 52 (19) |
| 10 k– < 30 k | 413 (26) | 84 (31) | |
| 30 k– < 50 k | 300 (19) | 63 (23) | |
| ≥50 k | 195 (12) | 34 (12) | |
| Maternal Age (years) | 18–24 | 583 (37) | 68 (25) |
| 25–29 | 490 (31) | 91 (33) | |
| 30–34 | 324 (21) | 58 (21) | |
| 35–41 | 173 (11) | 43 (16) | |
| Marital Status | Single | 302 (19) | 27 (10) |
| Married | 805 (51) | 151 (55) | |
| Cohabitating | 422 (27) | 78 (28) | |
| BMI | ≤25 | 755 (48) | 128 (47) |
| >25 to <30 | 427 (27) | 76 (28) | |
| ≥30 | 289 (18) | 43 (16) | |
| Number of Children | 0 | 651 (41) | 109 (40) |
| 1 | 534 (34) | 79 (29) | |
| 2–5 | 347 (22) | 72 (26) | |
| Child Characteristics | Mean ± SD or n (%) | ||
| Gestational Age (weeks) | 38.9 ± 1.9 | 39.0 ± 1.7 | |
| Birth Weight (grams) | 3174 ± 547 | 3162.47 ± 564 | |
| Sex | Female | 521 (33) | 137 (50) |
| Male | 566 (36) | 137 (50) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Zimmerman, E.; Huerta-Montañez, G.; Rosario-Pabón, Z.; Vélez-Vega, C.M.; Cordero, J.F.; Alshwabekah, A.; Meeker, J.D.; Watkins, D.J. Gestational Exposure to Phthalates and Phthalate Replacements in Relation to Neurodevelopmental Delays in Early Childhood. Toxics 2023, 11, 65. https://doi.org/10.3390/toxics11010065
Park S, Zimmerman E, Huerta-Montañez G, Rosario-Pabón Z, Vélez-Vega CM, Cordero JF, Alshwabekah A, Meeker JD, Watkins DJ. Gestational Exposure to Phthalates and Phthalate Replacements in Relation to Neurodevelopmental Delays in Early Childhood. Toxics. 2023; 11(1):65. https://doi.org/10.3390/toxics11010065
Chicago/Turabian StylePark, Seonyoung, Emily Zimmerman, Gredia Huerta-Montañez, Zaira Rosario-Pabón, Carmen M. Vélez-Vega, José F. Cordero, Akram Alshwabekah, John D. Meeker, and Deborah J. Watkins. 2023. "Gestational Exposure to Phthalates and Phthalate Replacements in Relation to Neurodevelopmental Delays in Early Childhood" Toxics 11, no. 1: 65. https://doi.org/10.3390/toxics11010065
APA StylePark, S., Zimmerman, E., Huerta-Montañez, G., Rosario-Pabón, Z., Vélez-Vega, C. M., Cordero, J. F., Alshwabekah, A., Meeker, J. D., & Watkins, D. J. (2023). Gestational Exposure to Phthalates and Phthalate Replacements in Relation to Neurodevelopmental Delays in Early Childhood. Toxics, 11(1), 65. https://doi.org/10.3390/toxics11010065







