Assessment and Management of Mercury Leaching from a Riverbank
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Passive Samplers and Instrumentations
2.2.1. DGT Samplers: THg and MeHg Analysis
2.2.2. Diffusion Samplers: Anions and velocity Measurement
Measurement of Inorganic Anions in Pore Water
Estimation of Porewater Velocity
2.2.3. Deployment and Retrieval of Diffusion Samplers
2.3. Dissolved Oxygen, Redox, PH and Sulfide
2.4. Laboratory Experiments
3. Results and Discussions
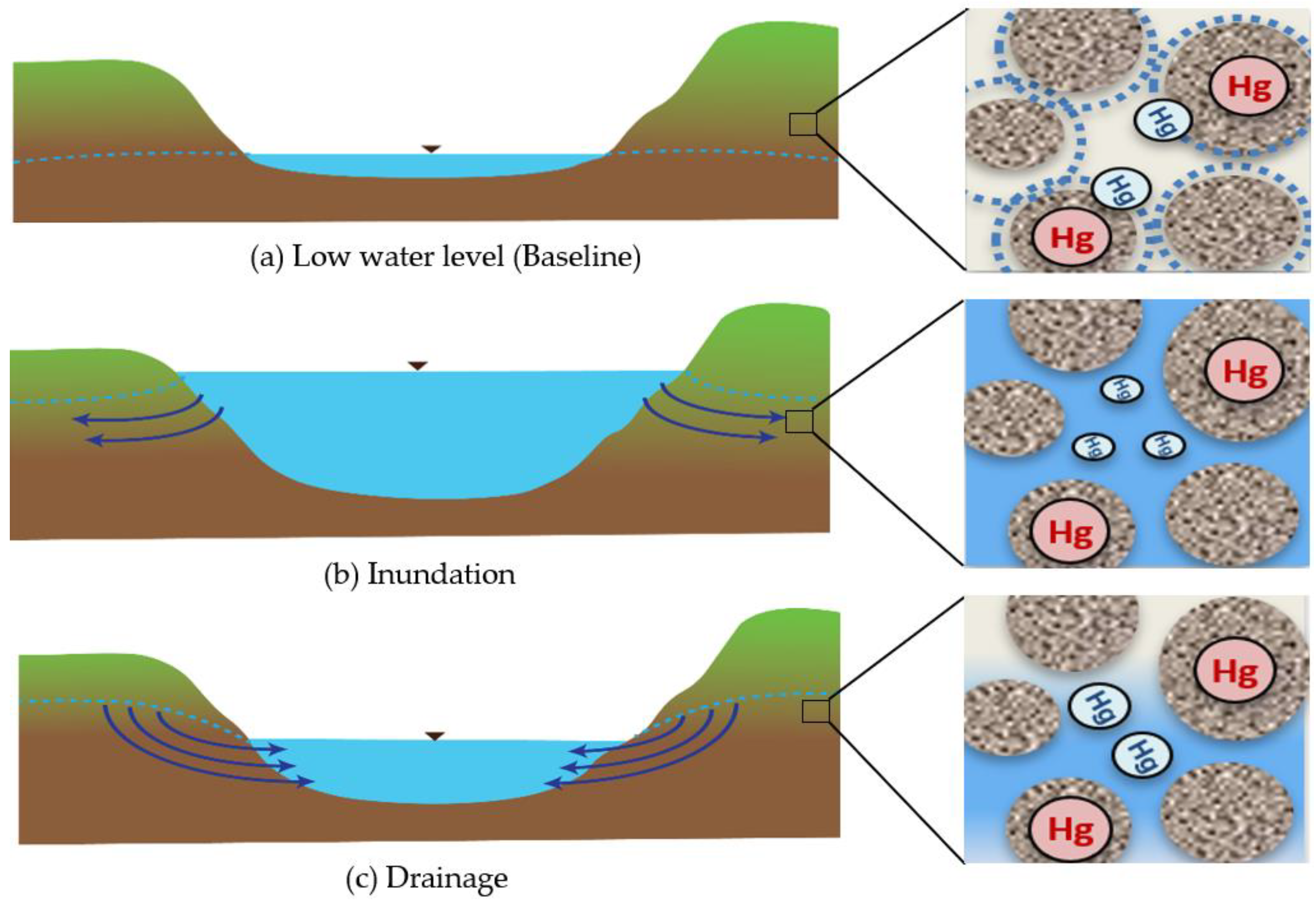
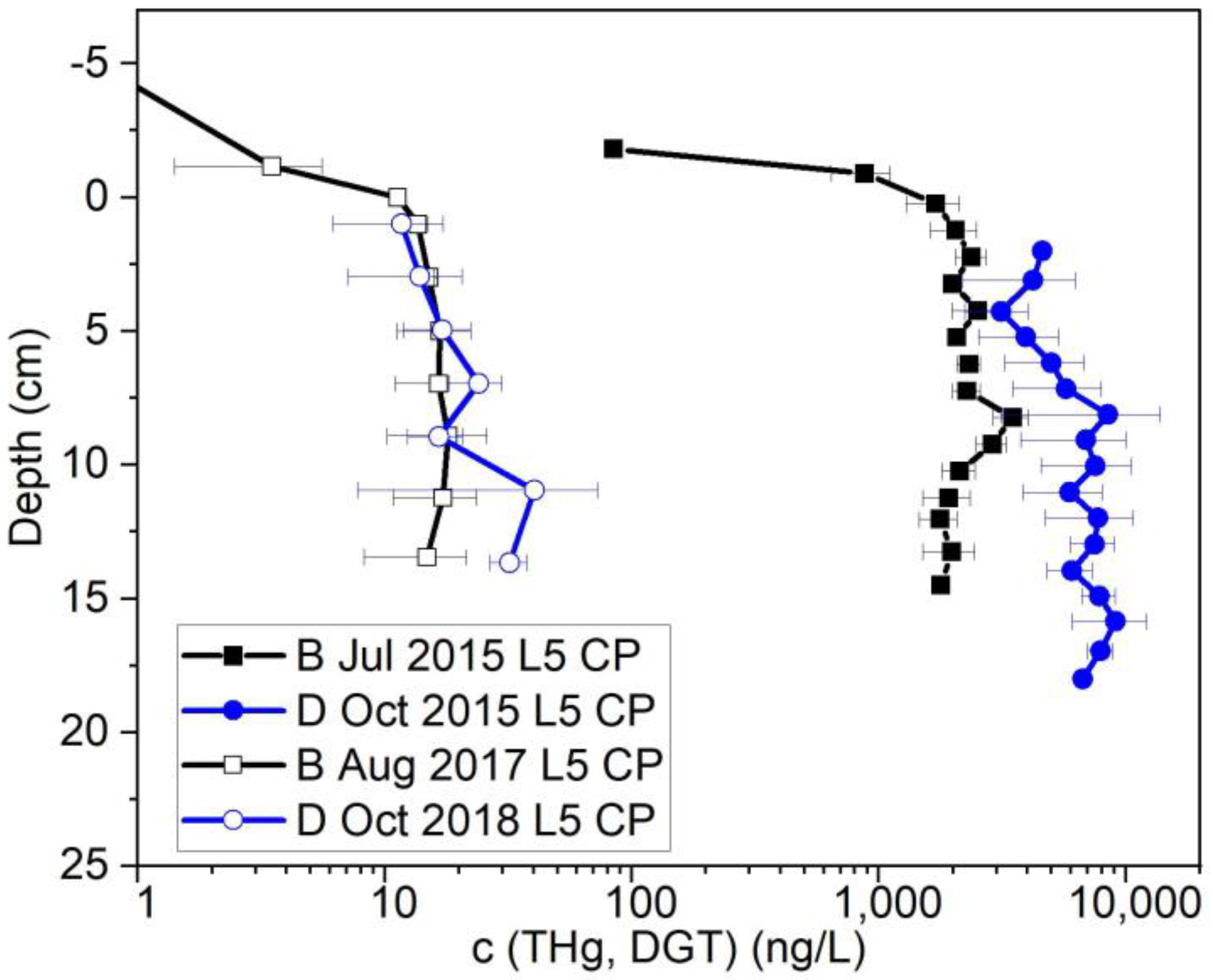
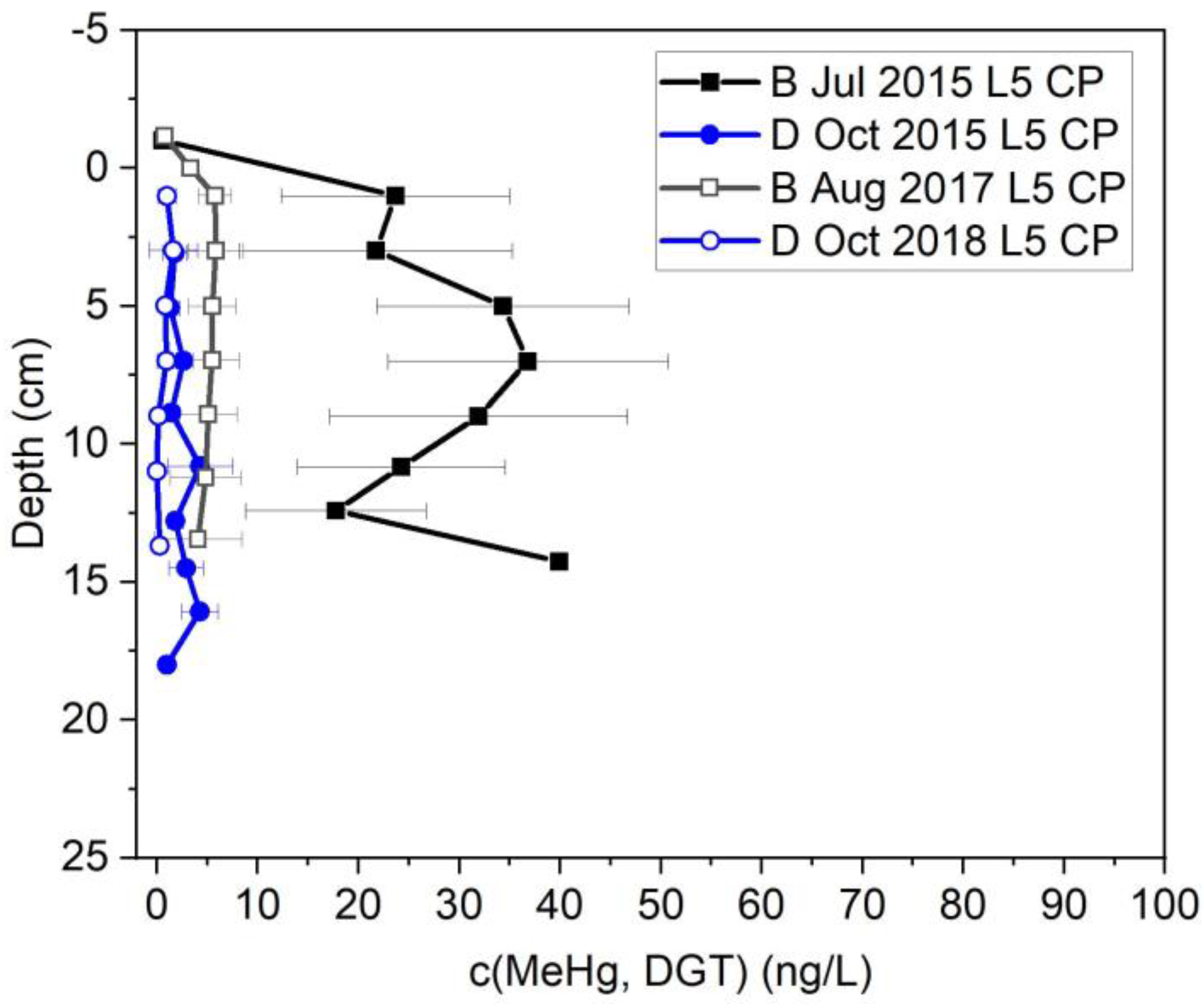
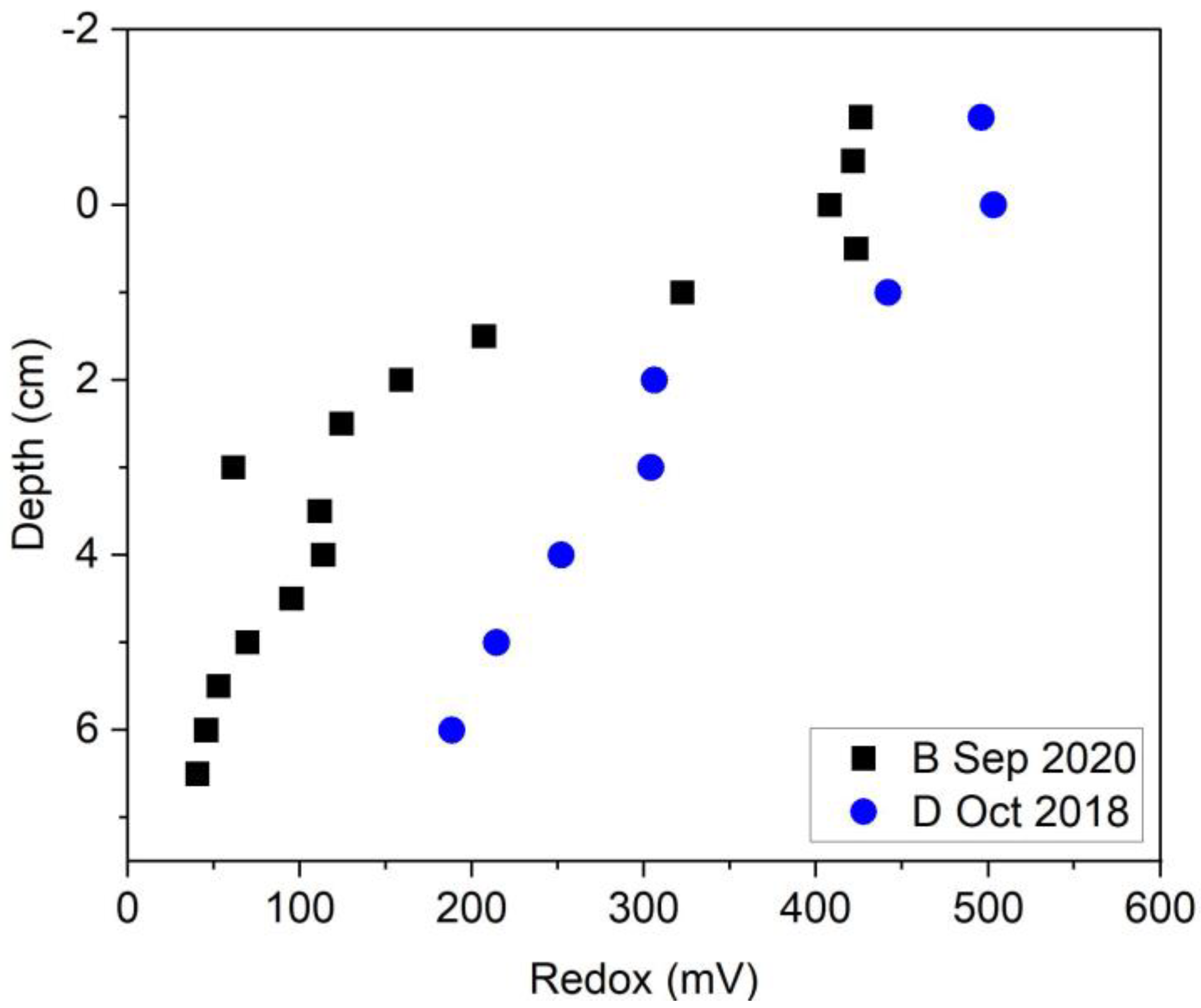
3.1. Drainage vs. Baseline Conditions
3.1.1. Field Results
3.1.2. Laboratory Results
3.2. Bank Stabilization Effect on THg
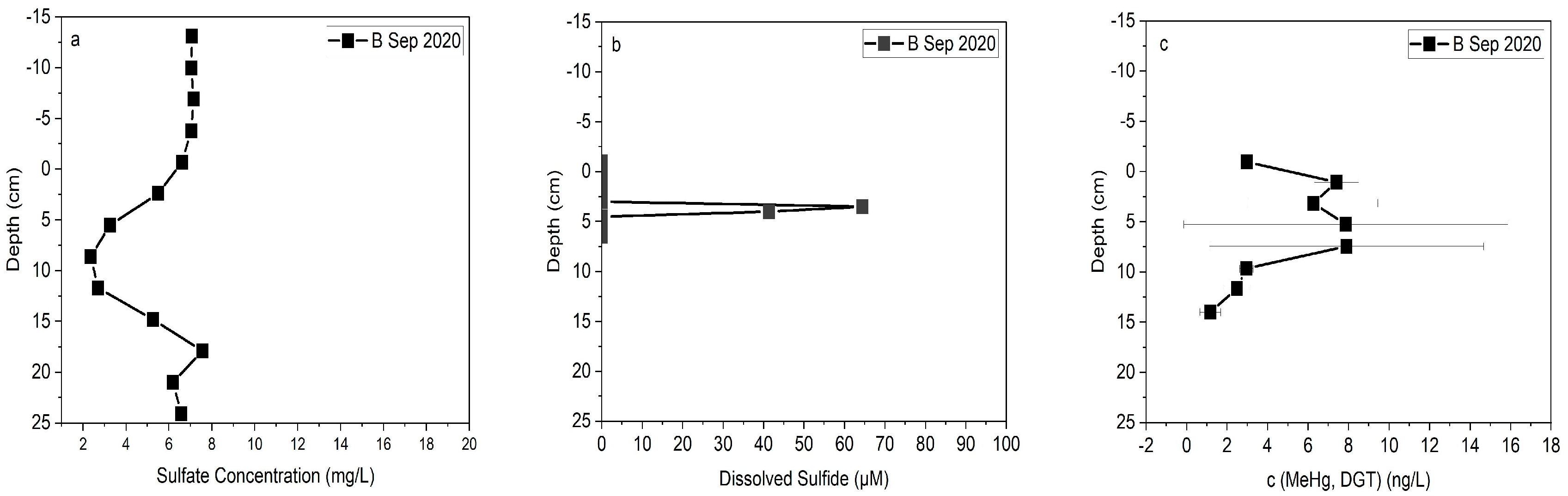
3.3. MeHg Behavior after Bank Stabilization
3.4. Flux of Mercury at Sediment-Water Interface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poulin, B.A.; Aiken, G.R.; Nagy, K.L.; Manceau, A.; Krabbenhoft, D.P.; Ryan, J.N. Mercury transformation and release differs with depth and time in a contaminated riparian soil during simulated flooding. Geochim. Cosmochim. Acta 2016, 176, 118–138. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Kucharzyk, K.H.; Zhang, T.; Deshusses, M.A. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: A critical review. Environ. Sci. Technol. 2013, 47, 2441–2456. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.F.; Lamborg, C.H.; Hammerschmidt, C.R. Marine biogeochemical cycling of mercury. Chem. Rev. 2007, 107, 641–662. [Google Scholar] [CrossRef]
- Bigham, G.N.; Murray, K.J.; Masue-Slowey, Y.; Henry, E.A. Biogeochemical controls on methylmercury in soils and sediments: Implications for site management. Integr. Environ. Assess. Manag. 2017, 13, 249–263. [Google Scholar] [CrossRef]
- Fornasaro, S.; Morelli, G.; Rimondi, V.; Fagotti, C.; Friani, R.; Lattanzi, P.; Costagliola, P. The extensive mercury contamination in soil and legacy sediments of the Paglia River basin (Tuscany, Italy): Interplay between Hg-mining waste discharge along rivers, 1960s economic boom, and ongoing climate change. J. Soils Sediments 2022, 22, 656–671. [Google Scholar] [CrossRef]
- Hošek, M.; Bednárek, J.; Popelka, J.; Elznicová, J.; Tůmová, Š.; Rohovec, J.; Navrátil, T.; Grygar, T.M. Persistent mercury hot spot in Central Europe and Skalka Dam reservoir as a long-term mercury trap. Environ. Geochem. Health 2020, 42, 1273–1290. [Google Scholar] [CrossRef]
- Lazareva, O.; Sparks, D.L.; Landis, R.; Ptacek, C.J.; Ma, J. Investigation of legacy industrial mercury in floodplain soils: South River, Virginia, USA. Environ. Earth Sci. 2019, 78, 246. [Google Scholar] [CrossRef]
- Eggleston, J.R. Mercury Loads in the South River and Simulation of Mercury Total Maximum Daily Loads (TMDLs) for the South River, South Fork Shenandoah River, and Shenandoah River: Shenandoah Valley, Virginia; US Geological Survey: Reston, VA, USA, 2009. [Google Scholar]
- Chapman, P.M.; Wang, F.; Germano, J.D.; Batley, G. Pore water testing and analysis: The good, the bad, and the ugly. Mar. Pollut. Bull. 2002, 44, 359–366. [Google Scholar] [CrossRef]
- Ankley, G.T.; Thomas, N.A.; Di Toro, D.M.; Hansen, D.J.; Mahony, J.D.; Berry, W.J.; Swartz, R.C.; Hoke, R.A.; Garrison, A.W.; Allen, H.E. Assessing potential bioavailability of metals in sediments: A proposed approach. Environ. Manag. 1994, 18, 331–337. [Google Scholar] [CrossRef]
- Desrochers, K.A.; Paulson, K.M.; Ptacek, C.J.; Blowes, D.W.; Gould, W.D. Effect of electron donor to sulfate ratio on mercury methylation in floodplain sediments under saturated flow conditions. Geomicrobiol. J. 2015, 32, 924–933. [Google Scholar] [CrossRef]
- Gilmour, C.C.; Henry, E.A. Mercury methylation in aquatic systems affected by acid deposition. Environ. Pollut. 1991, 71, 131–169. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Heyes, A.; Mason, R.P.; Miller, C.L. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. Biogeochemistry of Environmentally Important Trace Elements. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 835. [Google Scholar] [CrossRef]
- URS Corporation. Revised Final RCRA Facility Investigation and Human Health Risk Assessment Reports for AOC-4. 2015. Available online: https://southriverwatershed.org/resources/technical-documents (accessed on 14 January 2023).
- Wilcox, D.; Whitehurst, M.; Atwood, R.; Bsumek, P.; Wiggins, B. Mercury Pollution and Cleanup in the South River, VirginiaUnderstanding the Role of Fate and Transport in the Decision-Making Process for Environmental Remediation. Case Stud. Environ. 2020, 4, 962226. [Google Scholar] [CrossRef]
- Anchor QEA. Final Remedial Proposal for the South River and a Segment of the South Fork Shenandoah River, Virginia. 2013. Available online: https://southriverwatershed.org/resources/technical-documents (accessed on 14 January 2023).
- Bland, G.D.; Rao, B.; Reible, D. Evaluating the transport of Hg (II) in the presence of natural organic matter through a diffusive gradient in a thin-film passive sampler. Sci. Total Environ. 2020, 749, 141217. [Google Scholar] [CrossRef]
- Bireta, P.J.H. Application of Diffusive Gradient in Thin-Film Passive Samplers to Assess Mercury Availability and Mobility in a Fresh Water River System. Doctoral Dissertation, The University of Texas at Austin, Austin, TX, USA, 2015. Available online: https://repositories.lib.utexas.edu (accessed on 14 January 2023).
- Eckley, C.S.; Luxton, T.P.; Goetz, J.; McKernan, J. Water-level fluctuations influence sediment porewater chemistry and methylmercury production in a flood-control reservoir. Environ. Pollut. 2017, 222, 32–41. [Google Scholar] [CrossRef]
- Bradley, P.M.; Journey, C.A.; Chapelle, F.H.; Lowery, M.A.; Conrads, P.A. Flood hydrology and methylmercury availability in Coastal Plain rivers. Environ. Sci. Technol. 2010, 44, 9285–9290. [Google Scholar] [CrossRef]
- Kelly, T.J.; Hamilton, E.; Watts, M.J.; Ponting, J.; Sizmur, T. The effect of flooding and drainage duration on the release of trace elements from floodplain soils. Environ. Toxicol. Chem. 2020, 39, 2124–2135. [Google Scholar] [CrossRef]
- Vrtlar, T. Optimization of DGT Methyl Mercury Recovery, Bank Leaching Assessment and Evaluation of Stabilization Efforts on Mercury Fate and Transport in Freshwater Systems. Doctoral Dissertation, Texas Tech University, Lubbock, TX, USA, 2018. Available online: https://ttu-ir.tdl.org/ (accessed on 14 January 2023).
- AECOM. Commonwealth of Virginia, Construction Completion Report, Constitution Park Bank Management Area, Area of Concern 4; Former DuPont Waynesboro Plant: Waynesboro, Virginia, 2018; Available online: https://southriverwatershed.org/resources/technical-documents (accessed on 14 January 2023).
- Gilmour, C.C.; Henry, E.A.; Mitchell, R. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 1992, 26, 2281–2287. [Google Scholar] [CrossRef]
- Benoit, J.M.; Gilmour, C.C.; Mason, R.P.; Heyes, A. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ. Sci. Technol. 1999, 33, 951–957. [Google Scholar] [CrossRef]
- Yu, R.-Q.; Flanders, J.; Mack, E.E.; Turner, R.; Mirza, M.B.; Barkay, T. Contribution of coexisting sulfate and iron reducing bacteria to methylmercury production in freshwater river sediments. Environ. Sci. Technol. 2012, 46, 2684–2691. [Google Scholar] [CrossRef]
- Xu, J.; Bland, G.D.; Gu, Y.; Ziaei, H.; Xiao, X.; Deonarine, A.; Reible, D.; Bireta, P.; Hoelen, T.P.; Lowry, G.V. Impacts of Sediment Particle Grain Size and Mercury Speciation on Mercury Bioavailability Potential. Environ. Sci. Technol. 2021, 55, 12393–12402. [Google Scholar] [CrossRef] [PubMed]
- Davison, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Davison, W. Diffusive Gradients in Thin-Films for Environmental Measurements; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Amirbahman, A.; Massey, D.I.; Lotufo, G.; Steenhaut, N.; Brown, L.E.; Biedenbach, J.M.; Magar, V.S. Assessment of mercury bioavailability to benthic macroinvertebrates using diffusive gradients in thin films (DGT). Environ. Sci. Process. Impacts 2013, 15, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry, Revision E (August 2002). 2002; pp. 1–38. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_1631e_2002.pdf (accessed on 14 January 2023).
- US EPA. Method 1630, Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and CVAFS. EPA-821-R-01-020. 2001. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_1630_1998.pdf (accessed on 14 January 2023).
- Simon, N.; Kennedy, M.; Massoni, C. Evaluation and use of a diffusion-controlled sampler for determining chemical and dissolved oxygen gradients at the sediment-water interface. Hydrobiologia 1985, 126, 135–141. [Google Scholar] [CrossRef]
- Webster, I.T.; Teasdale, P.R.; Grigg, N.J. Theoretical and experimental analysis of peeper equilibration dynamics. Environ. Sci. Technol. 1998, 32, 1727–1733. [Google Scholar] [CrossRef]
- Thomas, B.; Arthur, M.A. Correcting porewater concentration measurements from peepers: Application of a reverse tracer. Limnol. Oceanogr. Methods 2010, 8, 403–413. [Google Scholar] [CrossRef]
- Garza-Rubalcava, U.; Hatzinger, P.B.; Schanzle, D.; Lavorgna, G.; Hedman, P.; Jackson, W.A. Improved assessment and performance monitoring of a biowall at a chlorinated solvent site using high-resolution passive sampling. J. Contam. Hydrol. 2022, 246, 103962. [Google Scholar] [CrossRef]
- Schneider, H.A.; Jackson, W.A.; Rainwater, K.; Reible, D.; Morse, S.; Hatzinger, P.B.; Garza-Rubalcava, U. Estimation of Interstitial Velocity Using a Direct Drive High-Resolution Passive Profiler. Groundwater 2019, 57, 915–924. [Google Scholar] [CrossRef]
- Bloom, N.S.; Preus, E.; Katon, J.; Hiltner, M. Selective extractions to assess the biogeochemically relevant fractionation of inorganic mercury in sediments and soils. Anal. Chim. Acta 2003, 479, 233–248. [Google Scholar] [CrossRef]
- Calmano, W.; Hong, J.; Förstner, U. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar] [CrossRef]
- Lynn, D.; Bonatti, E. Mobility of manganese in diagenesis of deep-sea sediments. Mar. Geol. 1965, 3, 457–474. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaei, H.; Rao, B.; Wood, T.V.; Garza-Rubalcava, U.; Alborzi, A.; Zhou, H.; Bireta, P.; Grosso, N.; Reible, D. Assessment and Management of Mercury Leaching from a Riverbank. Toxics 2023, 11, 179. https://doi.org/10.3390/toxics11020179
Ziaei H, Rao B, Wood TV, Garza-Rubalcava U, Alborzi A, Zhou H, Bireta P, Grosso N, Reible D. Assessment and Management of Mercury Leaching from a Riverbank. Toxics. 2023; 11(2):179. https://doi.org/10.3390/toxics11020179
Chicago/Turabian StyleZiaei, Hasti, Balaji Rao, Tea V. Wood, Uriel Garza-Rubalcava, Ashkan Alborzi, Huayun Zhou, Paul Bireta, Nancy Grosso, and Danny Reible. 2023. "Assessment and Management of Mercury Leaching from a Riverbank" Toxics 11, no. 2: 179. https://doi.org/10.3390/toxics11020179
APA StyleZiaei, H., Rao, B., Wood, T. V., Garza-Rubalcava, U., Alborzi, A., Zhou, H., Bireta, P., Grosso, N., & Reible, D. (2023). Assessment and Management of Mercury Leaching from a Riverbank. Toxics, 11(2), 179. https://doi.org/10.3390/toxics11020179








