Health Risk Assessment of PAHs from Estuarine Sediments in the South of Italy
Abstract
:1. Introduction
2. Materials and Methods
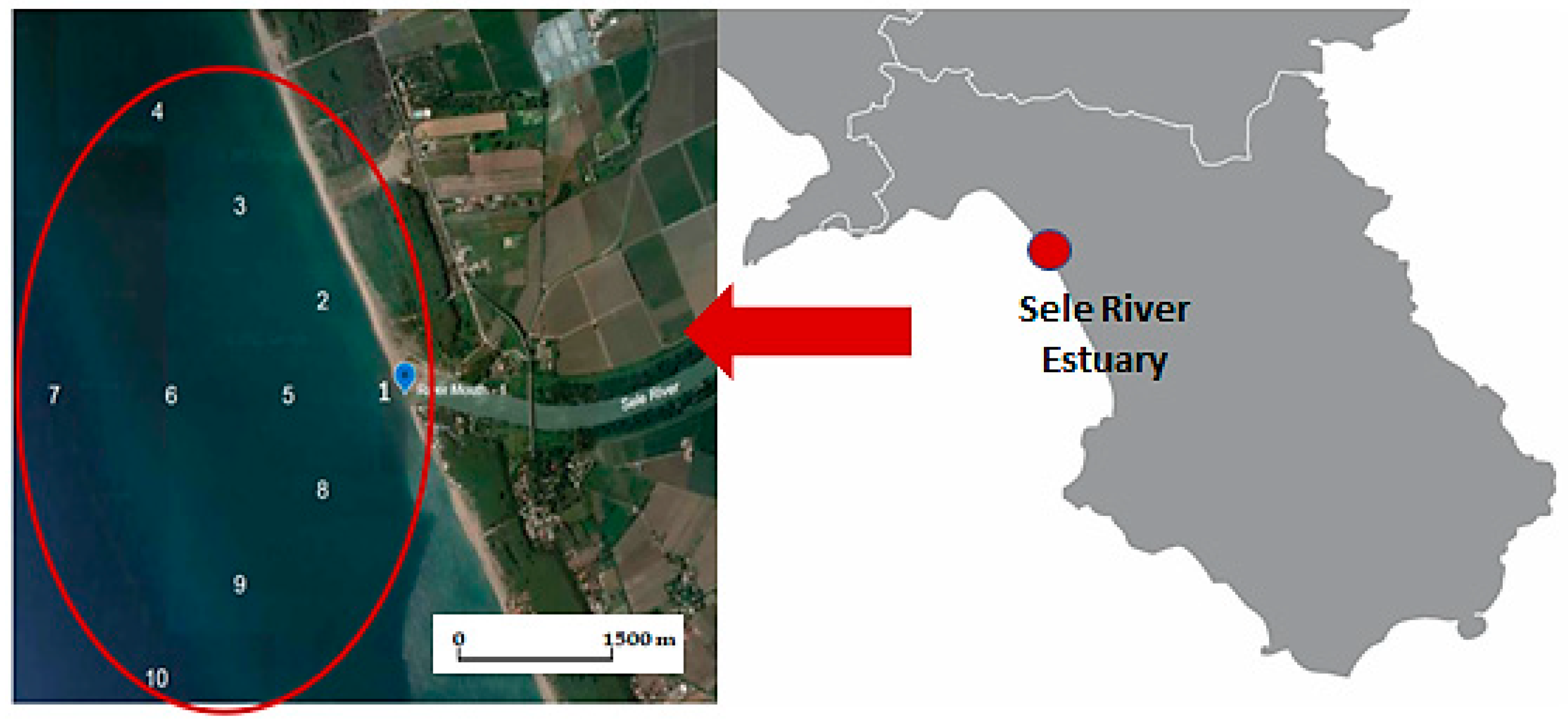
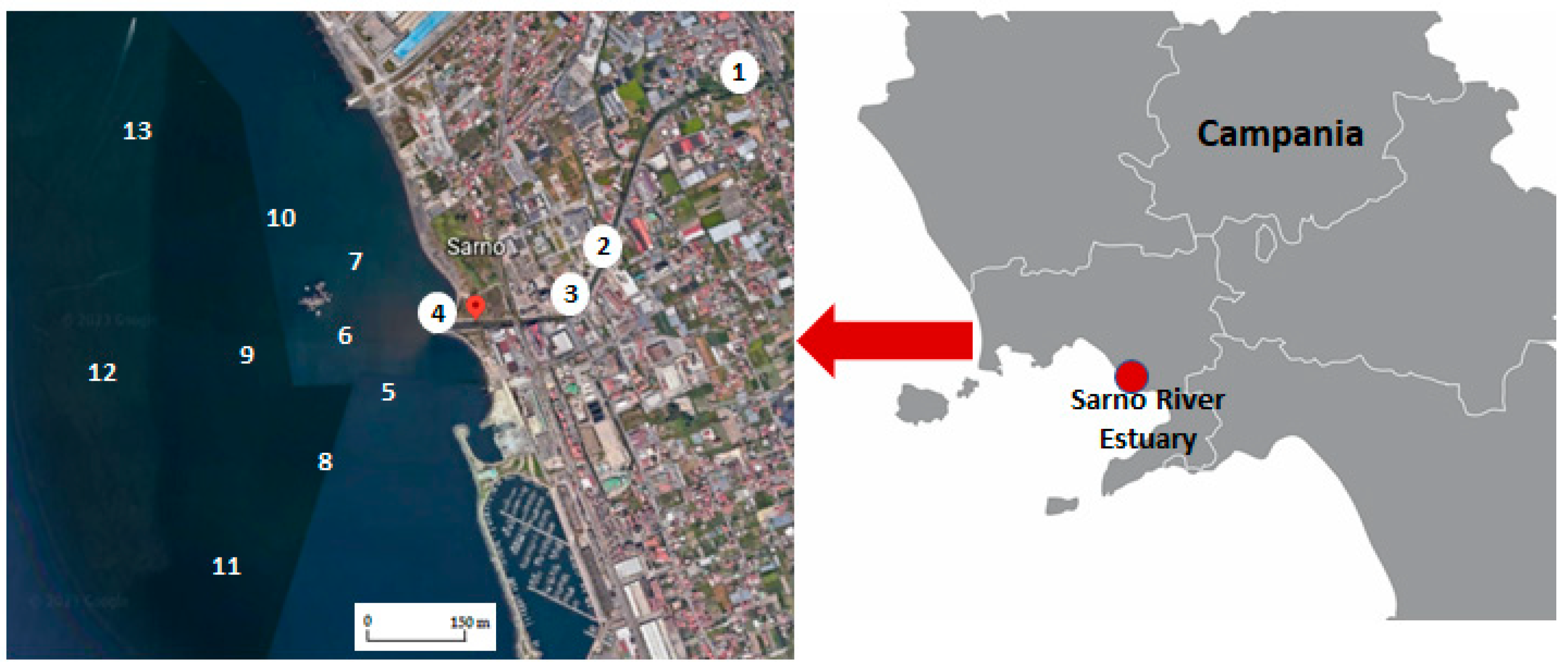
2.1. Study Area
2.2. Sampling
2.3. Extraction Procedure and Clean-Up
2.4. Instrumental Analysis, Quality Assurance, and Quality Control
2.5. Human Health Risk Assessment
- SFingestion (Kg-day/mg) indicates the oral slope factor.
- SFdermal (Kg-day/mg) is the dermal slope factor.
- SA (cm2/kg) represents the area of dermal contact with the sediment.
- AF (mg/cm2) is the skin absorption coefficient for the sediment.
- ABS is the skin absorption coefficient for contaminants.
- AT (years) is the average lifespan.
3. Results
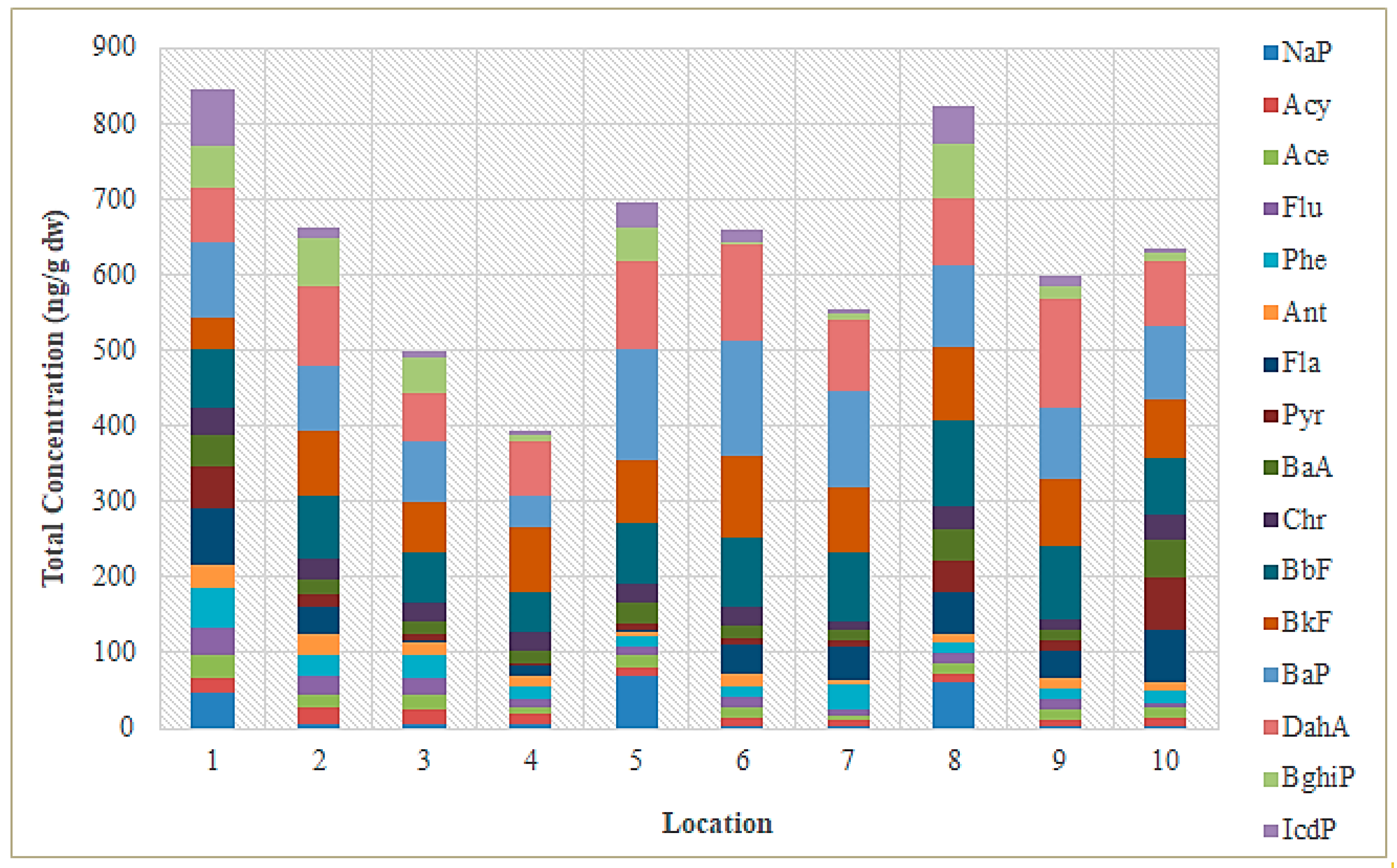
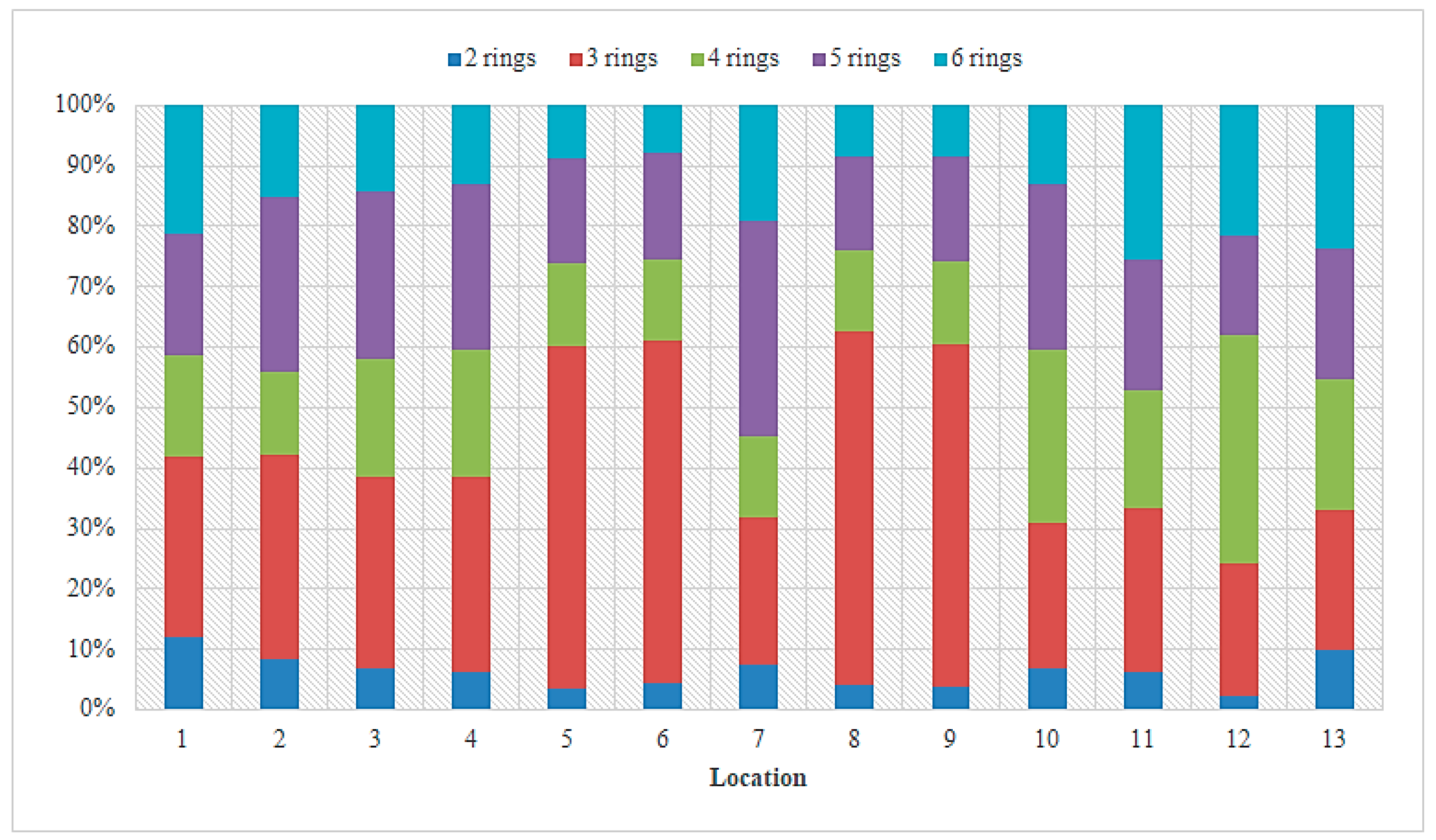
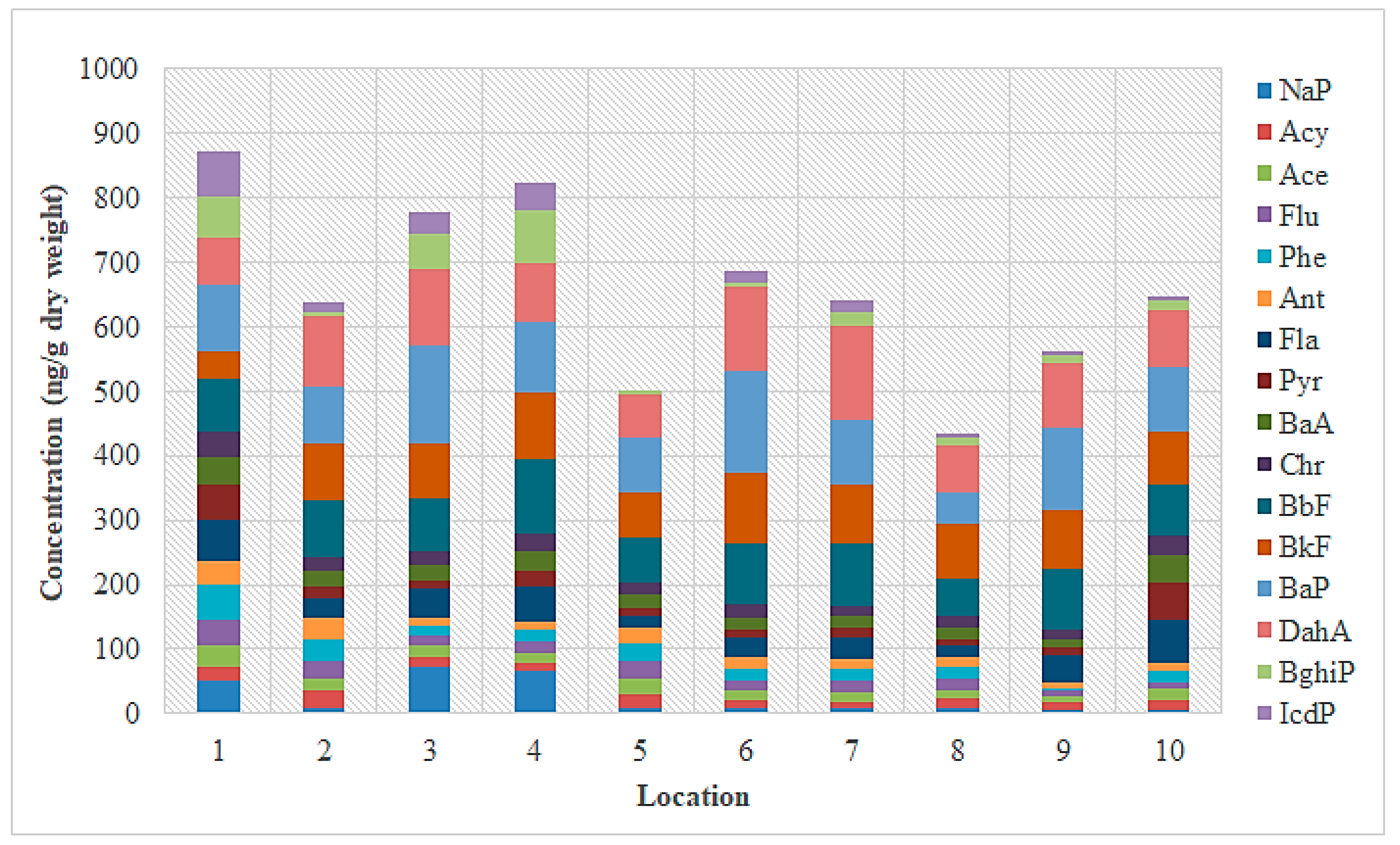
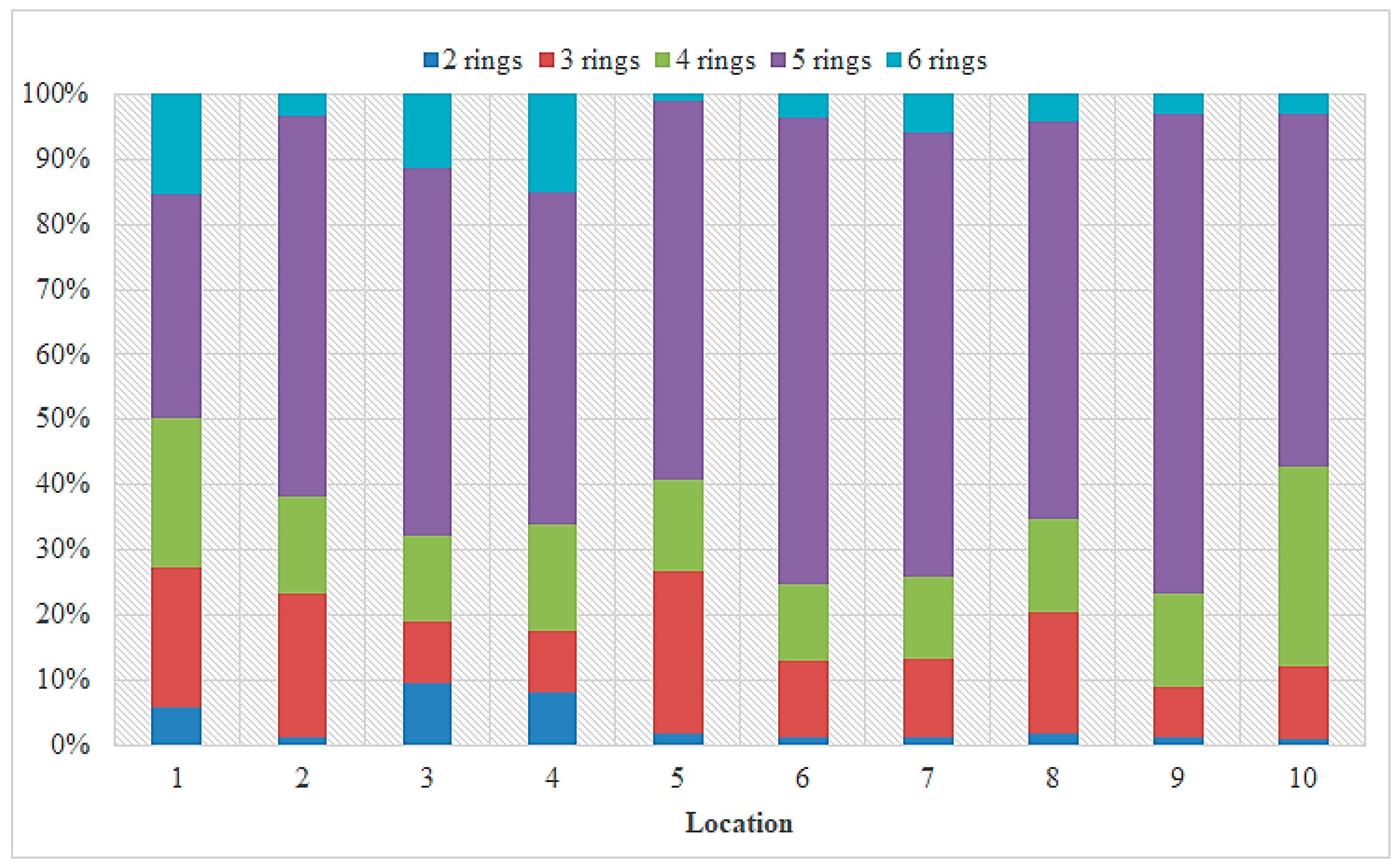
3.1. PAH Concentrations in Sediment from the Sele River
3.2. PAH Concentrations in Sediment from the Sarno River
3.3. PAH Concentrations in Sediment from the Volturno River
3.4. Evaluation of the Carcinogenic Risk for Human Health from Dermal and Accidental Ingestion Exposure to the PAHs Present in the Sediments of the Surface Waters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Zhang, W.; Pang, S.; Chen, J.; Bhatt, P.; Mishra, S.; Chen, S. Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ. Res. 2021, 194, 110660. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, S.; Wang, L.; Yang, X.; Zhao, Q.; Fan, J.; Yuan, X. Polycyclic aromatic hydrocarbons (PAHs) in seawater and sediments from the northern Liaodong Bay, China. Mar. Pollut. Bull. 2016, 113, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Brion, D.; Pelletier, É. Modelling PAHs adsorption and sequestration in freshwater and marine sediments. Chemosphere 2005, 61, 867–876. [Google Scholar] [CrossRef]
- Shahsavari, E.; Schwarz, A.; Aburto-Medina, A.; Ball, A.S. Biological degradation of polycyclic aromatic compounds (PAHs) in soil: A current perspective. Curr. Pollut. Rep. 2019, 5, 84–92. [Google Scholar] [CrossRef]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Wang, H. Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere 2007, 68, 93–104. [Google Scholar] [CrossRef]
- Awe, A.A.; Opeolu, B.O.; Olatunji, O.S.; Fatoki, O.S.; Jackson, V.A.; Snyman, R. Occurrence and probabilistic risk assessment of PAHs in water and sediment samples of the Diep River, South Africa. Heliyon 2020, 6, e04306. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, Y.; Qiu, X.; Li, R.; Fang, Y.; Wang, J.; Hu, D. Sources, transformation, and health implications of PAHs and their nitrated, hydroxylated, and oxygenated derivatives in PM2.5 in Beijing. J. Geophys. Res. Atmos. 2015, 120, 7219–7228. [Google Scholar] [CrossRef]
- CCME (Canadian Council of Ministers of the Environment). Canadian Soil Quality Guidelines for Carcinogenic and Other Polycyclic Aromatic Hydrocarbons (Environmental and Human Health Effects); Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2008. [Google Scholar]
- Albanese, S.; Fontaine, B.; Chen, W.; Lima, A.; Cannatelli, C.; Piccolo, A.; De Vivo, B. Polycyclic aromatic hydrocarbons in the soils of a densely populated region and associated human health risks: The Campania Plain (Southern Italy) case study. Environ. Geochem. Health 2015, 37, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Dai, Y.; Luo, Y.; Zhang, S. Health and ecotoxicological risk assessment for human and aquatic organism exposure to polycyclic aromatic hydrocarbons in the Baiyangdian Lake. Environ. Sci. Pollut. Res. 2021, 28, 574–586. [Google Scholar] [CrossRef]
- Omar, W.A.M.; Mahmoud, H.M. Risk assessment of polycyclic aromatic hydrocarbons (PAHs) in River Nile up-and downstream of a densely populated area. J. Environ. Sci. Health A 2017, 52, 166–173. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, B.; Xia, W.; Gao, M.; Zheng, H.; Chen, J.; Tian, M. Assessing potential risks of aquatic polycyclic aromatic compounds via multiple approaches: A case study in Jialing and Yangtze Rivers in downtown Chongqing, China. Environ. Pollut. 2022, 294, 118620. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Levels of polycyclic aromatic hydrocarbons in the water and sediment of Buffalo River Estuary, South Africa and their health risk assessment. Arch. Environ. Contam. Toxicol. 2019, 76, 657–669. [Google Scholar] [CrossRef]
- Regional Office for Europe. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2000; Available online: https://apps.who.int/iris/handle/10665/107335 (accessed on 16 September 2022).
- Rocha, M.J.; Dores-Sousa, J.L.; Cruzeiro, C.; Rocha, E. PAHs in water and surface sediments from Douro River estuary and Porto Atlantic coast (Portugal)-impacts on human health. Environ. Monit. Assess. 2017, 189, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahsan, A.; Farooq, M.A.; Naveed, M.; Li, H. Role of polycyclic aromatic hydrocarbons as EDCs in metabolic disorders. In Endocrine Disrupting Chemicals-induced Metabolic Disorders and Treatment Strategies; Springer: Cham, Switzerland, 2021; pp. 323–341. [Google Scholar]
- Gaber, M.; Sequely, A.A.; Monem, N.A.; Balbaa, M. Effect of polyaromatic hydrocarbons on cellular cytochrome P450 1A induction. Ocean Coast Manag. 2021, 69, 21026. [Google Scholar] [CrossRef]
- Lee, T.Y.; Tseng, Y.H. The potential of phytochemicals in oral cancer prevention and therapy: A review of the evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Kobets, T.; Smith, B.P.; Williams, G.M. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods 2022, 11, 2828. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/Convention Task Force on the Health Aspects of Air Pollution; World Health Organization. Health Risks of Persistent Organic Pollutants from Long-Range Transboundary Air Pollution; WHO Regional Office for Europe: Copenhagen, Denmark, 2003; Available online: https://apps.who.int/iris/handle/10665/107471 (accessed on 16 September 2022).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Air pollution, Part 1, some non-heterocyclic polycyclic aromatic hydrocarbons and some related industrial exposure. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 1–853. Available online: https://pubmed.ncbi.nlm.nih.gov/21141735/ (accessed on 3 October 2022).
- Tay, C.K.; Doamekpor, L.K.; Mohammed, S.; Dartey, G.; Kuddy, R.; Fianyaglo, E.; Mawuena, M. Health risk assessment and source identification of Polycyclic Aromatic Hydrocarbons (PAHs) in commercially available singed cowhide within the Greater Accra Region, Ghana. West Afr. J. Appl. Ecol. 2022, 30, 13–34. [Google Scholar]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Campling, B.G.; El-Deiry, W.S. Clinical implications of p53 mutations in lung cancer. Lung Cancer 2003, 75, 53–78. [Google Scholar]
- Unwin, J.; Cocker, J.; Scobbie, E.; Chambers, H. An assessment of occupational exposure to polycyclic aromatic hydrocarbons in the UK. Ann. Occup. Hyg. 2006, 50, 395–403. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Divi, R.L.; Kulldorff, M.; Vermeulen, R.; Haverkos, K.J.; Kuo, M.M.; Sinha, R. Leukocyte polycyclic aromatic hydrocarbon–DNA adduct formation and colorectal adenoma. Carcinogenesis 2007, 28, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- John, K.; Ragavan, N.; Pratt, M.M.; Singh, P.B.; Al-Buheissi, S.; Matanhelia, S.S.; Martin, F.L. Quantification of phase I/II metabolizing enzyme gene expression and polycyclic aromatic hydrocarbon–DNA adduct levels in human prostate. The Prostate 2009, 69, 505–519. [Google Scholar] [CrossRef]
- Garcia-Suastegui, W.A.; Huerta-Chagoya, A.; Carrasco-Colín, K.L.; Pratt, M.M.; John, K.; Petrosyan, P.; Gonsebatt, M.E. Seasonal variations in the levels of PAH–DNA adducts in young adults living in Mexico City. Mutagenesis 2010, 26, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Diggs, D.L.; Harris, K.L.; Rekhadevi, P.V.; Ramesh, A. Tumor. microsomal metabolism of the food toxicant, benzo (a) pyrene, in Apc Min mouse model of colon cancer. Tumor. Biol. 2012, 33, 1255–1260. [Google Scholar] [CrossRef]
- Wells, P.G.; Lee, C.J.; McCallum, G.P.; Perstin, J.; Harper, P.A. Receptor-and reactive intermediate-mediated mechanisms of teratogenesis. In Adverse Drug Reactions; Springer: Berlin/Heidelberg, Germany, 2010; Volume 43, pp. 221–242. [Google Scholar] [CrossRef]
- Drwal, E.; Rak, A.; Gregoraszczuk, E.L. Polycyclic aromatic hydrocarbons (PAHs)—Action on placental function and health risks in future life of newborns. Toxicology 2019, 411, 133–142. [Google Scholar] [CrossRef]
- Anyahara, J.N. Effects of Polycyclic Aromatic Hydrocarbons (PAHs) on the environment: A systematic review. Int. J. Adv. Acad. Res. 2021, 7, e7303. [Google Scholar] [CrossRef]
- Ambade, B.; Sethi, S.S.; Giri, B.; Biswas, J.K.; Bauddh, K. Characterization, behavior, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the estuary sediments. Bull. Environ. Contam. Toxicol. 2022, 108, 243–252. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Hou, L.; Li, X.; Yin, G.; Sun, P.; Zheng, D. Geographical distribution of polycyclic aromatic hydrocarbons in estuarine sediments over China: Human impacts and source apportionment. Sci. Total Environ. 2021, 768, 145279. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Anthropogenic PAHs in lake sediments: A literature review (2002–2018). Environ. Sci. Process. Impacts 2018, 20, 1649–1666. [Google Scholar] [CrossRef] [PubMed]
- Montuori, P.; Triassi, M. Polycyclic aromatic hydrocarbons loads into the Mediterranean Sea: Estimate of Sarno River inputs. Mar. Pollut. Bull. 2012, 64, 512–520. [Google Scholar] [CrossRef]
- Montuori, P.; De Rosa, E.; Di Duca, F.; Provvisiero, D.P.; Sarnacchiaro, P.; Nardone, A.; Triassi, M. Estimation of Polycyclic Aromatic Hydrocarbons Pollution in Mediterranean Sea from Volturno River, Southern Italy: Distribution, Risk Assessment and Loads. Int. J. Environ. Res. Public Health. 2021, 18, 1383. [Google Scholar] [CrossRef]
- Montuori, P.; De Rosa, E.; Di Duca, F.; De Simone, B.; Scippa, S.; Russo, I.; Triassi, M. Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources. Toxics 2022, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dong, L.; Meng, X.; Li, Q.; Huang, Z.; Li, C.; Li, R.; Yang, W.; Crittenden, J. Distribution and sources of polycyclic aromatic hydrocarbons and phthalic acid esters in water and surface sediment from the Three Gorges Reservoir. J. Environ. Sci. 2018, 69, 271–280. [Google Scholar] [CrossRef] [PubMed]
- USEPA (United States Environmental Protection Agency). Method 3540C. Soxhlet Extraction; USEPA: Washington, DC, USA, 1996. [Google Scholar]
- US Environmental Protection Agency. Protocol for Equipment Leak Emission Estimates; EPA-453/R-95—17; US Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- Wcisło, E.; Ioven, D.; Kucharski, R.; Szdzuj, J. Human health risk assessment case study: An abandoned metal smelter site in Poland. Chemosphere 2002, 47, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, Y.G.; Liu, J.L.; Zeng, G.M.; Li, X. Effects of EDTA on lead uptake by Typha oreentalis Presl: A new lead-accumulating species in southern China. Bull. Environ. Contam. Toxicol. 2008, 81, 36–41. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Cao, Z.; Lin, C.; Yang, Z. Spatial distribution and health risk of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in the water of the Luanhe River basin, China. Environ. Monit. Assess. 2010, 163, 1–13. [Google Scholar] [CrossRef]
- Gerba, C.P.; Pepper, I.L.; Gerb, C.P.; Brusseau, M. Risk Assessment. Environmental and Pollution Science; Academic Press, Elsevier: San Diego, CA, California, 2006. [Google Scholar]
- Han, J.; Liang, Y.; Zhao, B.; Wang, Y.; Xing, F.; Qin, L. Polycyclic aromatic hydrocarbon (PAHs) geographical distribution in China and their source, risk assessment analysis. Environ. Pollut. 2019, 251, 312–327. [Google Scholar] [CrossRef]
- Akpan, A.D.; Okori, B.S.U.; Ekpechi, D.C. Human Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Water Samples around Eket Metropolis, Akwa Ibom State, Nigeria. Asian J. Environ. Sci. 2022, 19, 58–71. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Deelaman, W.; Choochuay, C.; Pongpiachan, S.; Han, Y. Ecological and health risks of polycyclic aromatic hydrocarbons in the sediment core of Phayao Lake, Thailand. J. Exp. Sci. Environ. Epidemiol. 2023, 2, 3. [Google Scholar] [CrossRef]
- Richardson, G.M. Compendium of Canadian Human Exposure Factors for Risk Assessment; O’Connor Associates Environmental Incorporated: Ottawa, ON, Canada, 1997. [Google Scholar]
- Khiari, N.; Charef, A.; Atoui, A.; Azouzi, R.; Khalil, N.; Khadhar, S. Southern Mediterranean coast pollution: Long-term assessment and evolution of PAH pollutants in Monastir Bay (Tunisia). Mar. Pollut. Bull. 2021, 167, 112268. [Google Scholar] [CrossRef] [PubMed]
- MassDEP. Technical Update: Calculation of Enhanced Soil Ingestion Rate; MassDEP: Boston, MA, USA, 2002. [Google Scholar]
- Health Canada. Federal Contaminated Site Risk Assessment in Canada, Part V: Guidance on Human Health Detailed Quantitative Risk Assessment for Chemicals (DQRAChem.); Health Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Haney Jr, J.T.; Forsberg, N.D.; Hoeger, G.C.; Magee, B.H.; Meyer, A.K. Risk assessment implications of site-specific oral relative bioavailability factors and dermal absorption fractions for polycyclic aromatic hydrocarbons in surface soils impacted by clay skeet target fragments. Regul. Toxicol. Pharmacol. 2020, 113, 104649. [Google Scholar] [CrossRef]
- Kissel, J.C.; Richter, K.Y.; Fenske, R.A. Field measurement of dermal soil loading attributable to various activities: Implications for exposure assessment. Risk Anal. 1996, 16, 115–125. [Google Scholar] [CrossRef]
- Albanese, S.; Taiani, M.V.E.; De Vivo, B.; Lima, A. An environmental epidemiological study based on the stream sediment geochemistry of the Salerno province (Campania region, Southern Italy). J. Geochem. Explor. 2013, 131, 59–66. [Google Scholar] [CrossRef]
- Alberico, I.; Amato, V.; Aucelli, P.P.C.; D’Argenio, B.; Di Paola, G.; Pappone, G. Historical shoreline change of the Sele Plain (Southern Italy): The 1870–2009 time window. J. Coast. Res. 2012, 28, 1638–1647. [Google Scholar] [CrossRef]
- Moody, R.P.; Joncasa, J.; Richardsonb, M.; Chua, I. Contaminated soils (I): In vitro dermal absorption of benzo[a]pyrene in human skin. J. Toxicol. Environ. Health Part A 2007, 70, 1858–1865. [Google Scholar] [CrossRef]
- Knafla, A.; Phillipps, K.A.; Brecher, R.W.; Petrovic, S.; Richardson, M. Development of a dermal cancer slope factor for benzo[a]pyrene. Regul. Toxicol. Pharmacol. 2006, 45, 159–168. [Google Scholar] [CrossRef]
- Grmasha, R.A.; Al-sareji, O.J.; Salman, J.M.; Hashim, K.S. Polycyclic aromatic hydrocarbons (PAHs) in urban street dust within three land-uses of Babylon governorate, Iraq: Distribution, sources, and health risk assessment. J. King Saud Univ. Eng. Sci. 2020, 34, 231–239. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Bi, X.; Chaemfa, C.; Ren, Z.; Wang, X.; Fu, J. Phase distribution, sources and risk assessment of PAHs, NPAHs and OPAHs in a rural site of Pearl River Delta region, China. Atmos. Pollut. Res. 2014, 5, 210–218. [Google Scholar] [CrossRef]
- Srivastava, P.; Sreekrishnan, T.R.; Nema, A.K. Human health risk assessment and PAHs in a stretch of river Ganges near Kanpur. Environ. Monit. Assess. 2017, 189, 445. [Google Scholar] [CrossRef] [PubMed]
- USEPA (US Environmental Protection Agency). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final; EPA/540/R/99/005 OSWER 9285.7-02EP PB99-963312 July 2004; Office of Superfund Remediation and Technology Innovation: Washington, DC, USA, 2004. [Google Scholar]
- Cheng, J.; Wang, X.; Zheng, B.; Zhang, X.; Han, J. Evaluation of distribution characteristics and health risk of polycyclic aromatic hydrocarbons in sediments: From the perspective of land-ocean coordination. J. Hydrol 2022, 607, 127514. [Google Scholar] [CrossRef]




| ID | Location | Coordinates | ID | Location | Coordinates |
|---|---|---|---|---|---|
| 1 | Sele River mouth | 40°28′55″ N 14°56′33″ E | 6 | 1000 m west | 40°28′55″ N 14°55′50″ E |
| 2 | 500 m north | 40°29′04″ N 14°56′14″ E | 7 | 1000 m south | 40°28′39″ N 14°55′56″ E |
| 3 | 500 m west | 40°28′55″ N 14°56′12″ E | 8 | 1500 m north | 40°29′20″ N 14°55′38″ E |
| 4 | 500 m south | 40°28′47″ N 14°56′16″ E | 9 | 1500 m west | 40°28′55″ N 14°55′28″ E |
| 5 | 1000 m north | 40°29′12″ N 14°55′56″ E | 10 | 1500 m south | 40°28′30″ N 14°55′38″ E |
| ID | Location | Coordinates | ID | Location | Coordinates |
|---|---|---|---|---|---|
| 1 | Source of Sarno River | 40°48′54.03″ N 14°36′45.36″ E | 8 | 150 m south | 40°43′35.68″ N 14°28′02.94″ E |
| 2 | Before junction with Alveo Comune | 40°46′42.73″ N 14°34′00.48″ E | 9 | 150 m west | 40°43′42.25″ N 14°27′59.97″ E |
| 3 | After junction with Alveo Comune | 40°46′00.34″ N 14°33′10.68″ E | 10 | 150 m north | 40°43′49.26″ N 14°27′30.31″ E |
| 4 | Sarno River mouth | 40°46′10.68″ N 14°28′07.89″ E | 11 | 500 m south | 40°43′30.31″ N 14°27′58.94″ E |
| 5 | 50 m south | 40°43′40.11″ N 14°28′06.45″ E | 12 | 500 m west | 40°43′42.29″ N 14°27′46.41″ E |
| 6 | 50 m west | 40°43′42.46″ N 14°28′05.03″ E | 13 | 500 m north | 40°43′57.85″ N 14°27′48.68″ E |
| 7 | 50 m north | 40°43′45.09″ N 14°28′05.17″ E |
| ID | Location | Coordinates | ID | Location | Coordinates |
|---|---|---|---|---|---|
| 1 | Volturno River mouth | 40°48′54.03″ N 14°36′45.36″ E | 6 | 1000 m west | 40°43′42.46″ N 14°28′05.03″ E |
| 2 | 500 m north | 40°46′42.73″ N 14°34′00.48″ E | 7 | 1000 m south | 40°43′45.09″ N 14°28′05.17″ E |
| 3 | 500 m west | 40°46′00.34″ N 14°33′10.68″ E | 8 | 1500 m north | 40°43′35.68″ N 14°28′02.94″ E |
| 4 | 500 m south | 40°43′42.62″ N 14°28′07.89″ E | 9 | 1500 m west | 40°43′42.25″ N 14°27′59.97″ E |
| 5 | 1000 m north | 40°43′40.11″ N 14°28′06.45″ E | 10 | 1500 m south | 40°43′49.26″ N 14°27′59.82″ E |
| Name | Abbreviation | Molecular Formula | Number of Rings | Chemical Structure | Molecular Weight |
|---|---|---|---|---|---|
| Naphthalene | Nap | C10H8 | 2 | 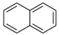 | 128.2 g mol−1 |
| Acenaphthylene | Acy | C12H8 | 3 |  | 152.2 g mol−1 |
| Acenaphthalene | Ace | C12H10 | 3 | 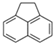 | 154.2 g mol−1 |
| Fluorene | Flu | C13H10 | 3 | 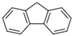 | 166.2 g mol−1 |
| Phenanthrene | Phe | C14H10 | 3 |  | 178.2 g mol−1 |
| Anthracene | Ant | C14H10 | 3 |  | 178.2 g mol−1 |
| Fluoranthene | Fla | C16H10 | 4 | 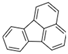 | 202.3 g mol−1 |
| Pyrene | Pyr | C16H10 | 4 | 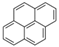 | 202.3 g mol−1 |
| Benzo[a]anthracene | BaA | C18H12 | 4 | 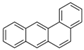 | 228.3 g mol−1 |
| Crysene | Chr | C18H12 | 4 | 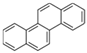 | 228.3 g mol−1 |
| Benzo[b]fluoranthene | BbF | C20H12 | 5 | 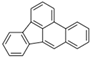 | 252.3 g mol−1 |
| Benzo[k]fluoranthene | BkF | C20H12 | 5 | 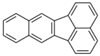 | 252.3 g mol−1 |
| Benzo[a]pyrene | BaP | C20H12 | 5 | 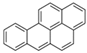 | 252.3 g mol−1 |
| Indeno [123-cd]pyrene | IcdP | C22H12 | 6 | 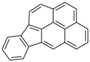 | 276.3 g mol−1 |
| Benzo[ghi]perylene | BghiP | C22H12 | 6 | 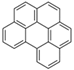 | 276.3 g mol−1 |
| Dibenzo[a,h]anthracene | DahA | C22H14 | 5 | 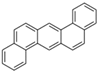 | 278.3 g mol−1 |
| Compound | TEF |
|---|---|
| Acenaphthalene (Ace) | 0.001 |
| Acenaphthylene (Acy) | 0.001 |
| Anthracene (Ant) | 0.01 |
| Benzo[a]anthracene (BaA) | 0.1 |
| Benzo[a]pyrene (BaP) | 1 |
| Benzo[b]fluoranthene (BbF) | 0.1 |
| Benzo[g,h,i]perylene (BghiP) | 0.01 |
| Benzo[k]fluoranthene (BkF) | 0.1 |
| Crysene (Chr) | 0.01 |
| Dibenzo[a,h]anthracene (DahA) | 1 |
| Fluoranthene (Fla) | 0.001 |
| Fluorene (Flu) | 0.001 |
| Indeno [1,2,3-cd] pyrene (IcdP) | 0.1 |
| Naphtalene (Nap) | 0.001 |
| Fenanthrene (Phe) | 0.001 |
| Pyrene (Pyr) | 0.001 |
| Parameter | Unit of Measure | Value | References |
|---|---|---|---|
| BW | Kg | 70.7 | [53] |
| IRingestion | Kg/days | 2.00 × 10−5 | [54,55] |
| AT | Years | 80 | [56,57] |
| SAh | cm2 | 890 | [53] |
| SLh | Kg/cm2-event | 1.00 × 10−7 | [58] |
| Dhours | Hours | 0–16/16 h | [59] |
| Ddays | Days | 0–7/7 days | [59] |
| Dweeks | Weeks | 0–52/52 weeks | [59] |
| RAForal | - | 1 | [60] |
| EDyears | Years | 60 | [60] |
| RAFderm a | - | 0.148 | [61] |
| SFingestion a | Kg-day/mg | 2.3 | [60] |
| SFdermal a | Kg-day/mg | 25 | [62] |
| EF | (event/day) | 1 | [63] |
| SA | cm2/kg | 5000 | [64] |
| AF | mg/cm2 | 0.04 | [64] |
| ABS | / | 0.1 | [64] |
| River | Cs (mg Kg−1 dw) | Doseingestion (mg Kg−1/day) | Dosedermal (mg Kg−1/day) | BaPeq (mg Kg−1 dw) |
|---|---|---|---|---|
| Sele | 0.6360 | 7.86 × 10−4 | 3.23 × 10−5 | 2.23 × 10−1 |
| Sarno | 0.2677 | 3.31 × 10−4 | 1.36 × 10−5 | 3.38 × 10−2 |
| Volturno | 0.6577 | 8.13 × 10−4 | 3.35 × 10−5 | 2.30 × 10−1 |
| River | ILCRingestion (mg Kg−1 dw) | ILCRdermal (mg Kg−1 dw) | ILCRingestion/dermal [66] Cancerogenic Risk |
|---|---|---|---|
| Sele | 3.11 × 10−13 | 3.38 × 10−6 | If ILCR < 1 × 10−6 Low or Zero Risk |
| Sarno | 1.31 × 10−13 | 1.42 × 10−6 | If 1 × 10−6 < ILCR < 1 × 10−4 Medium Risk |
| Volturno | 3.22 × 10−13 | 3.50 × 10−6 | If ILCR >1 × 10−4 High Risk |
| River | ILCRingestion (mg Kg−1 dw) | ILCRdermal (mg Kg−1 dw) | ILCRingestion/dermal [66] Cancerogenic Risk |
|---|---|---|---|
| Sele | 4.14 × 10−13 | 4.50 × 10−6 | If ILCR < 1 × 10−6 Low or Zero Risk |
| Sarno | 3.33 × 10−13 | 3.61 × 10−6 | If 1 × 10−6 < ILCR < 1 × 10−4 Medium Risk |
| Volturno | 4.27 × 10−13 | 4.64 × 10−6 | If ILCR >1 × 10−4 High Risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Duca, F.; Montuori, P.; Trama, U.; Masucci, A.; Borrelli, G.M.; Triassi, M. Health Risk Assessment of PAHs from Estuarine Sediments in the South of Italy. Toxics 2023, 11, 172. https://doi.org/10.3390/toxics11020172
Di Duca F, Montuori P, Trama U, Masucci A, Borrelli GM, Triassi M. Health Risk Assessment of PAHs from Estuarine Sediments in the South of Italy. Toxics. 2023; 11(2):172. https://doi.org/10.3390/toxics11020172
Chicago/Turabian StyleDi Duca, Fabiana, Paolo Montuori, Ugo Trama, Armando Masucci, Gennaro Maria Borrelli, and Maria Triassi. 2023. "Health Risk Assessment of PAHs from Estuarine Sediments in the South of Italy" Toxics 11, no. 2: 172. https://doi.org/10.3390/toxics11020172
APA StyleDi Duca, F., Montuori, P., Trama, U., Masucci, A., Borrelli, G. M., & Triassi, M. (2023). Health Risk Assessment of PAHs from Estuarine Sediments in the South of Italy. Toxics, 11(2), 172. https://doi.org/10.3390/toxics11020172







