Fishing Cat Scats as a Biomonitoring Tool for Toxic Heavy Metal Contamination in Aquatic Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection of Scats of Fishing Cat
2.3. Preparation of Scat Samples for Metal Analysis
2.4. Digestion of Scats of Fishing Cat
2.5. Quality Control
2.6. Data Analysis
3. Results
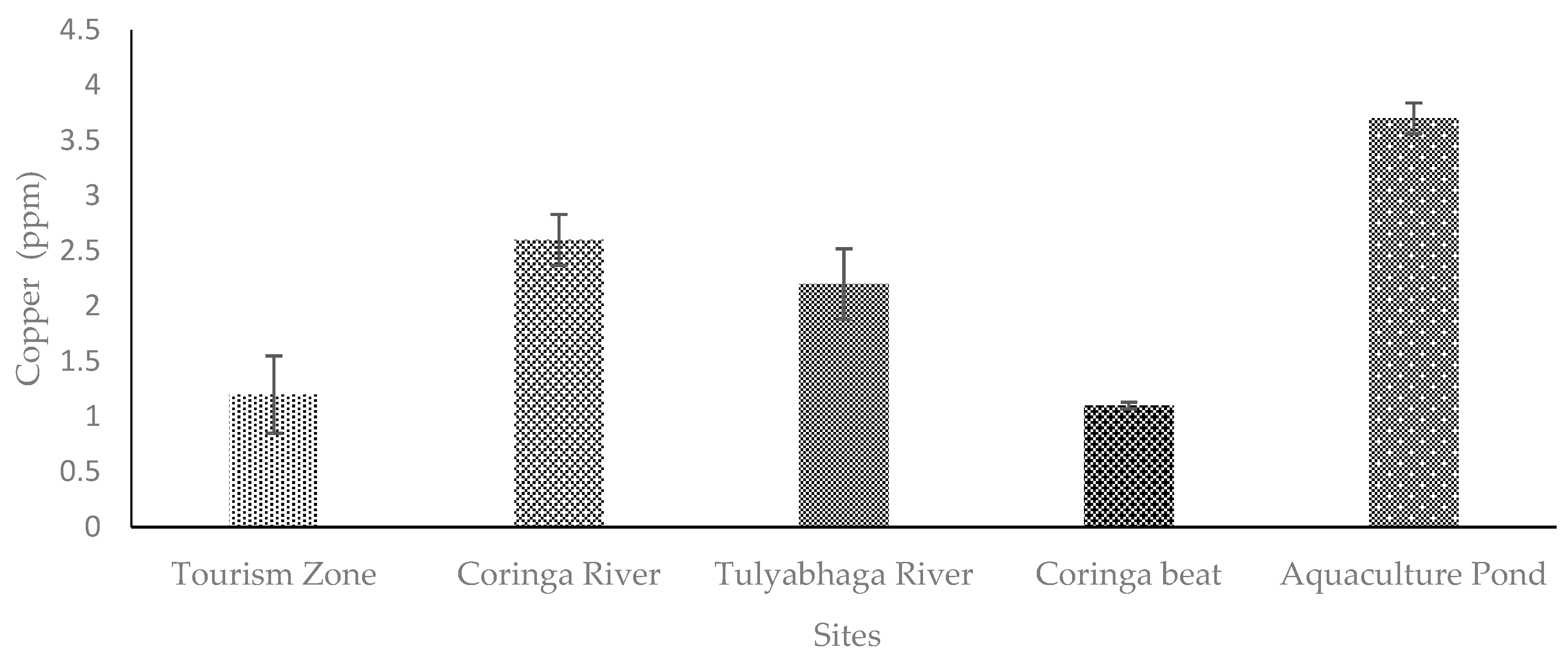
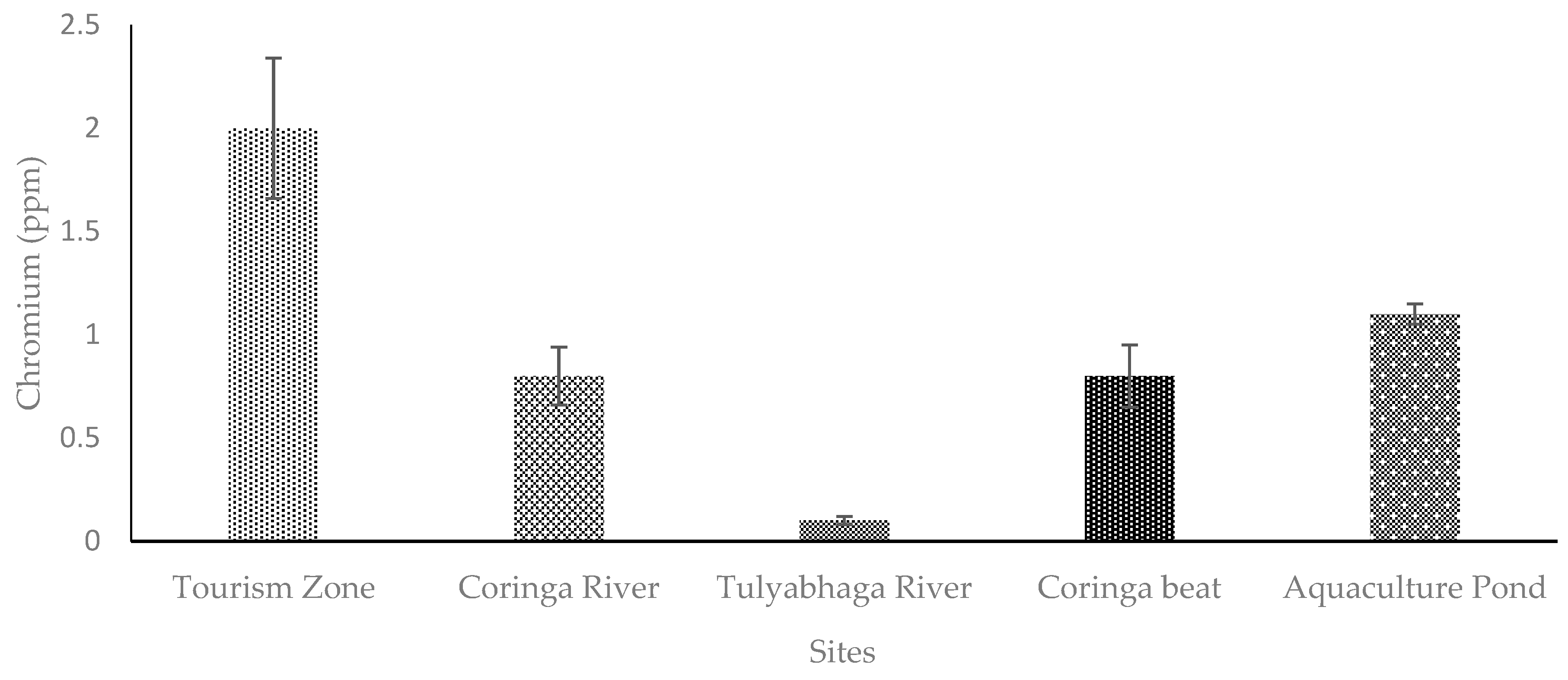
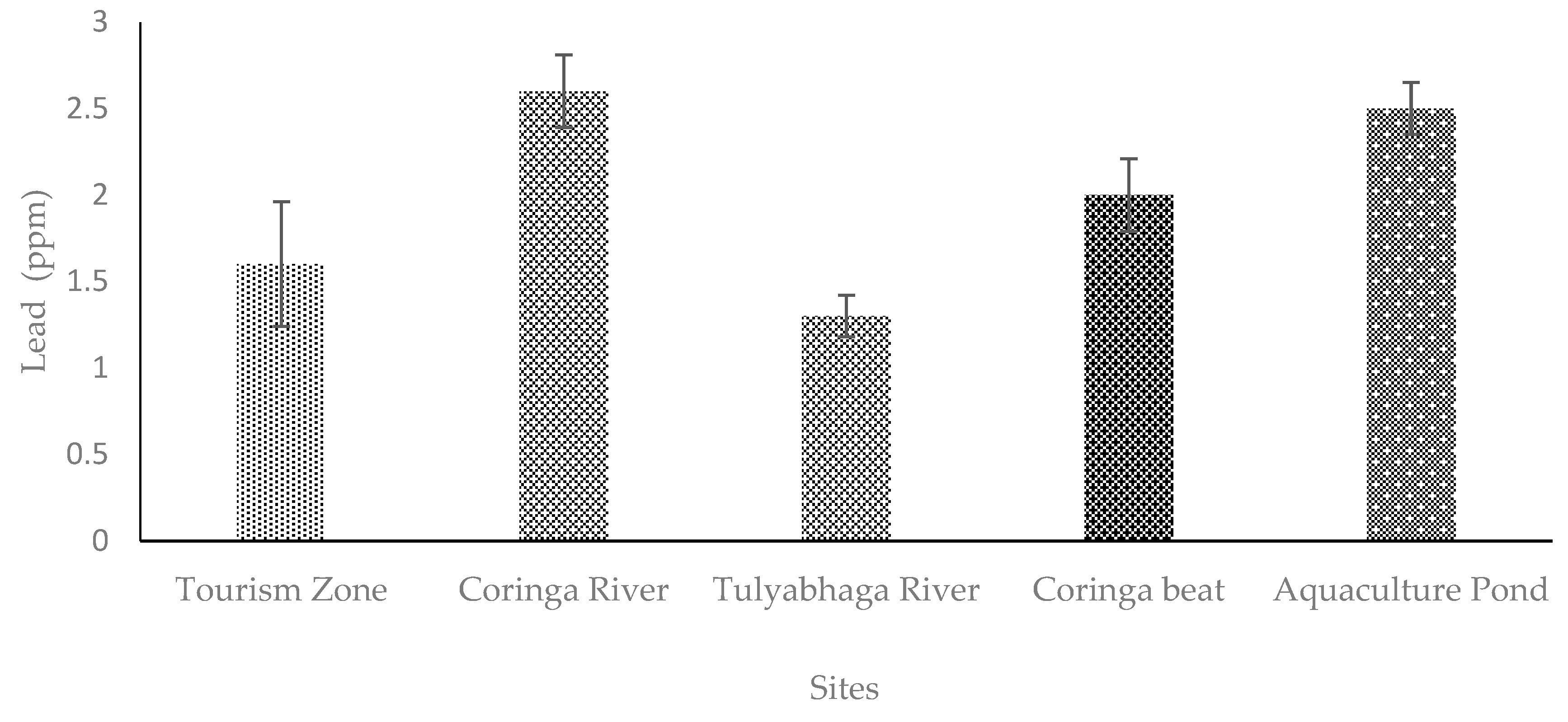
4. Discussion
4.1. Copper (Cu)
4.2. Chromium (Cr)
4.3. Lead (Pb)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burger, J.; Tsipoura, N.; Niles, J.; Gochfeld, M.; Dey, A.; Mizrahi, D. Mercury, Lead, Cadmium, Arsenic, Chromium and Selenium in Feathers of Shorebirds during Migrating through Delaware Bay, New Jersey: Comparing the 1990s and 2011/2012. Toxics 2015, 3, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Sandilyan, S.; Kathiresan, K. Decline of mangroves–a threat of heavy metal poisoning in Asia. Ocean Coast. Manag. 2014, 102, 161–168. [Google Scholar] [CrossRef]
- Rao, K.N.; Saito, Y.; Nagakumar, K.C.V.; Demudu, G.; Rajawat, A.S.; Kubo, S.; Li, Z. Palaeogeography and evolution of the Godavari delta, east coast of India during the Holocene: An example of wave-dominated and fan-delta settings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 440, 213–233. [Google Scholar]
- Malla, G.; Sivakumar, K. The Coringa Mangroves-Realm of the fishing cat. Sanctuary Asia 2014, 34, 60–65. [Google Scholar]
- Malla, G. Ecology and conservation of Fishing Cat in Godavari mangroves of Andhra Pradesh. In Proceedings of the First International Fishing Cat Conservation Symposium, Kathmandu, Nepal, 25–29 November 2015; Volume 45, pp. 25–29. [Google Scholar]
- Mukherjee, S.; Appel, A.; Duckworth, J.W.; Sanderson, J.; Dahal, S.; Willcox, D.H.A.; Rahman, H. Prionailurus viverrinus. The IUCN Red List of Threatened Species. 2016. Available online: https://www.iucnredlist.org/species/18150/221434864 (accessed on 9 February 2023).
- Jnawali, S.R.; Baral, H.S.; Lee, S.; Acharya, K.P.; Upadhyaya, G.P.; Pandey, M.; Shrestha, R. The Status of Nepal’s Mammals: The National Red List Series. Department of National Parks and Wildlife Conservation, Kathmandu, Nepal; IUCN: Gland, Switzerland, 2011.
- Hossain, M.B.; Miazie, M.R.; Nur, A.A.U.; Paul, S.K.; Bakar, M.A.; Paray, B.A.; Arai, T. Assessment of Metal Contamination in Water of Freshwater Aquaculture Farms from a South Asian Tropical Coastal Area. Toxics 2022, 10, 536. [Google Scholar] [CrossRef]
- Hussain, J.; Husain, I.; Arif, M.; Gupta, N. Studies on heavy metal contamination in Godavari river basin. Appl. Water Sci. 2017, 7, 4539–4548. [Google Scholar] [CrossRef]
- Ray, A.K.; Tripathy, S.C.; Patra, S.; Sarma, V.V. Assessment of Godavari estuarine mangrove ecosystem through trace metal studies. Environ. Int. 2006, 32, 219–223. [Google Scholar] [CrossRef]
- Laskowski, R. Are the top carnivores endangered by heavy metal biomagnification? Oikos 1991, 60, 387–390. [Google Scholar] [CrossRef]
- Pandiyan, J.; Jagadheesan, R.; Karthikeyan, G.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Krishnappa, K.; Elumalai, K.; Govindarajan, M. Probing of heavy metals in the feathers of shorebirds of Central Asian Flyway wintering grounds. Sci. Rep. 2020, 10, 22118. [Google Scholar] [CrossRef]
- Shankar, A.; Salaria, N.; Sanil, R.; Chackaravarthy, S.D.; Shameer, T.T. Spatio-temporal association of fishing cats with the mammalian assemblages in the East Godavari mangrove delta, India. Mammal Study 2020, 45, 303–313. [Google Scholar] [CrossRef]
- De Barba, M.; Waits, L.P.; Genovesi, P.; Randi, E.; Chirichella, R.; Cetto, E. Comparing opportunistic and systematic sampling methods for non-invasive genetic monitoring of a small translocated brown bear population. J. Appl. Ecol. 2010, 47, 172–181. [Google Scholar] [CrossRef]
- Navaneethan, B.; Sankar, K.; Manjrekar, M.; Qureshi, Q. Food habits of tiger (Panthera tigris tigris) as shown by scat analysis in Bandhavgarh Tiger Reserve, Central India. Asian J. Conserv. Biol. 2019, 8, 224–227. [Google Scholar]
- Shin, D.M.; Oh, J.M.; Kim, J. Metal concentrations in Eurasian eagle owl pellets as a function of reproductive variables in Korea. Arch. Environ. Contam. Toxicol. 2018, 74, 298–304. [Google Scholar] [CrossRef]
- Jayakumar, R.; Muralidharan, S. Metal contamination in select species of birds in Nilgiris District, Tamil Nadu, India. Bull. Environ. Contam. Toxicol. 2011, 87, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, J.; Mahboob, S.; Jagadheesan, R.; Elumalai, K.; Krishnappa, K.; Al-Misned, F.; Govindarajan, M. A novel approach to assess the heavy metal content in the feathers of shorebirds: A perspective of environmental research. J. King Saud. Univ. Sci. 2020, 32, 3065–3071. [Google Scholar] [CrossRef]
- Pandiyan, J.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Krishnappa, K. An assessment of level of heavy metals pollution in the water, sediment and aquatic organisms: A perspective of tackling environmental threats for food security. Saudi J. Biol. Sci. 2021, 28, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.I. Biometry: The Principles and Practice of Statistics in Biological Research; W.H. Freeman: San Francisco, CA, USA, 1969; 776p. [Google Scholar]
- Ullah, K.; Hashmi, M.Z.; Malik, R.N. Heavy-metal levels in feathers of cattle egret and their surrounding environment: A case of the Punjab province, Pakistan. Arch. Environ. Contam. Toxicol. 2014, 66, 139–153. [Google Scholar] [CrossRef]
- Gupta, V.; Bakre, P.P. Metal contamination in mammalian fauna of Sariska tiger reserve, Alwar, India. J. Ecophysiol. Occup. Health 2012, 12, 43–48. [Google Scholar]
- Gaumat, V.; Bakre, P.P. Mammalian dung as a bioindicator of heavy metal contamination. Proc. Acad. Environ. Biol. 1998, 7, 99–102. [Google Scholar]
- Gupta, V. Mammalian Scat as a Bio-indicator of Heavy Metals Contamination in Western Rajasthan, India. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Rodríguez-Estival, J.; Ortiz-Santaliestra, M.E.; Mateo, R. Assessment of Eco-toxicological risks to river otters from ingestion of invasive red swamp crayfish in metal contaminated areas: Use of feces to estimate dietary exposure. Environ. Res. 2020, 181, 108907. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci. Total Environ. 2000, 257, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Pandiyan, J.; Poiyamozhi, A.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Manzoor, I.; Govindarajan, M. Assessment of the Toxic Effects of Heavy Metals on Waterbirds and Their Prey Species in Freshwater Habitats. Toxics 2022, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S.; Thompson, D.J. Ecotoxicology of copper and cadmium in a contaminated grassland ecosystem.III. Small mammals. J. Apple. Ecol. 1987, 24, 601–614. [Google Scholar]
- Lee, C.S.L.; Li, X.; Shi, W.; Cheung, S.C.; Thornton, I. Metal contamination in urban, suburban and country park soils of Hong Kong: A study based on GIS and multivariate statistics. Sci. Total Environ. 2006, 356, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.N.; Zeb, N. Assessment of environmental contamination using feathers of Bubulcus ibis as a biomonitor of heavy metal pollution, Pakistan. Ecotoxicology 2009, 18, 522–536. [Google Scholar] [CrossRef]
- Janssens, E.; Dauwe, T.; Bervoets, L.; Eens, M. Inter and intra-clutch variability in heavy metals in feathers of Great tit nestlings (Parus major) along a pollution gradient. Arch. Environ. Contam. Toxicol. 2002, 43, 323–329. [Google Scholar] [CrossRef]
- Worrall, D.H. Diet of the Dunlin Calidris alpina in the Severn estuary. Bird Study 1984, 31, 203–212. [Google Scholar] [CrossRef]
- Al Sayegh Petkovšek, S.; Kopušar, N.; Kryštufek, B. Small mammals as biomonitors of metal pollution: A case study in Slovenia. Environ. Monit. Assess. 2014, 186, 4261–4274. [Google Scholar] [CrossRef]
- Ghani, A. Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egyptian Acad. J. BiolSci. 2011, 2, 9–15. [Google Scholar]
- Lam, M.H.W.; Tjia, A.Y.W.; Chan, C.C.; Chan, W.P.; Lee, W.S. Speciation study of chromium, copper and nickel in coastal estuarine sediments polluted by domestic and industrial effluents. Mar. Pollut. Bull. 1997, 34, 949–959. [Google Scholar] [CrossRef]
- Catsiki, V.A.; Katsilieri, C.; Gialamas, V. Chromium distribution in benthic species from a gulf receiving tannery wastes (Gulf of Geras—Lesbos island, Greece). Sci. Total Environ. 1994, 145, 173–185. [Google Scholar] [CrossRef]
- Jerez, S.; Motas, M.; Palacios, M.J.; Valera, F.; Cuervo, J.J.; Barbosa, A. Concentration of trace elements in feathers of three Antarctic penguins: Geographical and interspecific differences. Environ. Pollut. 2011, 159, 2412–2419. [Google Scholar] [CrossRef]
- Markowski, M.; Kaliński, A.; Skwarska, J.; Wawrzyniak, J.; Bańbura, M.; Markowski, J.; Bańbura, J. Avian feathers as bioindicators of the exposure to heavy metal contamination of food. Bull. Environ. Contam. Toxicol. 2013, 91, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.C.; Posa, M.R.C. Distribution and prey of migratory shorebirds on the northern coastline of Singapore. Raffles Bull. Zool. 2014, 62, 701–717. [Google Scholar]
- Battley, P.F.; Rogers, D.I.; Piersma, T.; Koolhaas, A. Behavioural evidence for heat-load problems in great knots in tropical Australia fuelling for long-distance flight. Emu 2003, 103, 97–103. [Google Scholar] [CrossRef]
- Finkelstein, M.E.; George, D.; Scherbinski, S.; Gwiazda, R.; Johnson, M.; Burnett, J.; Smith, D.R. Feather lead concentrations and 207Pb/206Pb ratios reveal lead exposure history of California condors (Gymnogyps californianus). Environ. Sci. Technol. 2010, 44, 2639–2647. [Google Scholar] [CrossRef]
- Mado-Filho, G.M.; Salgado, L.T.; Rebelo, M.F.; Rezende, C.E.; Karez, C.S.; Pfeiffer, W.C. Heavy metals in benthic organisms from Todosos Santos Bay, Brazil. Braz. J. Biol. 2008, 68, 95–100. [Google Scholar] [CrossRef]
- Way, C.A.; Schroder, G.D. Accumulation of lead and cadmium in wild populations of the commensal rat, Rattus norvegicus. Arch. Environ. Contam. Toxicol. 1982, 11, 407–417. [Google Scholar] [CrossRef]
- Zook, B.C.; Sauer, R.M.; Garner, F.M. Lead poisoning in captive wild animals. J. Wildl. Dis. 1972, 8, 264–272. [Google Scholar] [CrossRef]
- Ogwuegbu, M.O.C.; Muhanga, W. Investigation of lead concentration in the blood of people in the copper belt province of Zambia. J. Environ. 2005, 1, 66–75. [Google Scholar]
- Udedi, S.S. From guinea worm scourge to metal toxicity in Ebonyi State. Nigeria Magazine 2003, 2, 13–14. [Google Scholar]
- Damek-Poprawa, M.; Sawicka-Kapusta, K. Damage to the liver and testis with reference to burden of heavy metals in yellownecked mice from areas around steelworks and zinc smelter in Poland. Toxicology 2003, 186, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, L.A.; Mikshevich, N.V. Accumulation of heavy metals by small mammals the background and polluted territories of the Urals. Vestn. Zool. 2017, 51, 325. [Google Scholar] [CrossRef]



| Prey Species (mg) | Prey Composition | Different Sites | ||||
|---|---|---|---|---|---|---|
| Tourism Zone (n = 6) | Coringa River (n = 6) | Tulyabhaga River (n = 6) | Coringa Beat (n = 6) | Aquafarms (n = 6) | ||
| Crustaceans | Shells | 3.3 ± 1.11 | 3.3 ± 0.99 | 0.3 ± 0.28 | 1.5 ± 0.61 | 3.5 ± 0.98 |
| Fish | Scales | 4.1 ± 0.73 | 3.1 ± 0.86 | 3.3 ± 0.95 | 5.4 ± 0.87 | 5.4 ± 1.07 |
| Fins | 1.4 ± 0.68 | 2.1 ± 0.85 | 0.5 ± 0.36 | 3.1 ± 0.67 | 6.5 ± 1.54 | |
| Bones | 2.8 ± 0.71 | 1.8 ± 0.61 | 1.7 ± 0.68 | 2.1 ± 0.80 | 1.4 ± 0.07 | |
| Bird | Feathers | 2.9 ± 0.80 | 2.4 ± 0.63 | 2.0 ± 0.71 | 1.7 ± 0.60 | 1.1 ± 0.90 |
| Bones | 1.8 ± 0.71 | 1.7 ± 0.71 | 2.0 ± 0.86 | 0.5 ± 0.29 | 0 | |
| Claws | 1.1 ± 0.57 | 1.0 ± 0.43 | 1.2 ± 0.54 | 1.2 ± 0.62 | 4.5 ± 1.23 | |
| Rodent | Hair | 5.2 ± 1.02 | 4.5 ± 1.10 | 4.0 ± 0.98 | 5.8 ± 0.63 | 1.4 ± 0.58 |
| Bone | 1.9 ± 0.75 | 2.0 ± 0.80 | 1.3 ± 0.73 | 2.2 ± 0.62 | 0.1 ± 0.15 | |
| Teeth | 1.8 ± 0.80 | 3.4 ± 1.11 | 2.4 ± 1.16 | 1.8 ± 0.85 | 0 | |
| Nails | 1.2 ± 0.64 | 1.3 ± 0.66 | 0.7 ± 0.47 | 0.3 ± 0.27 | 3.5 ± 0.86 | |
| Plant | Leaves | 2.3 ± 0.64 | 2.0 ± 0.84 | 1.4 ± 0.59 | 1.8 ± 0.62 | 1.7 ± 0.95 |
| Grass | 4.1 ± 0.98 | 3.0 ± 0.97 | 3.9 ± 0.94 | 4.4 ± 0.81 | 0.3 ± 0.27 | |
| Heartwood | 0.5 ± 0.37 | 1.1 ± 0.61 | 1.2 ± 1.02 | 0.9 ± 0.59 | 0 | |
| Thorny spikes | 1.8 ± 0.63 | 1.4 ± 0.71 | 1.2 ± 0.69 | 1.0 ± 0.51 | 0 | |
| Unidentified | Fauna/floral crumbles | 1.4 ± 0.82 | 1.9 ± 0.90 | 0.6 ± 0.40 | 1.0 ± 0.44 | 1.7 ± 0.60 |
| Others | Plastics/polythene/nylon | 61.0 ± 3.87 | 61.5 ± 2.49 | 61.3 ± 7.56 | 64.0 ± 2.13 | 66.9 ± 1.85 |
| Digested weight | Filtered/purely soluble | 0 | 0 | 9.9 ± 9.91 | 0 | 0 |
| Total weight | Dry weight | 4.1 ± 0.73 | 3.1 ± 0.86 | 3.3 ± 0.95 | 5.4 ± 0.87 | 3.5 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harika, T.L.; Al-Ghanim, K.A.; Riaz, M.N.; Krishnappa, K.; Pandiyan, J.; Govindarajan, M. Fishing Cat Scats as a Biomonitoring Tool for Toxic Heavy Metal Contamination in Aquatic Ecosystems. Toxics 2023, 11, 173. https://doi.org/10.3390/toxics11020173
Harika TL, Al-Ghanim KA, Riaz MN, Krishnappa K, Pandiyan J, Govindarajan M. Fishing Cat Scats as a Biomonitoring Tool for Toxic Heavy Metal Contamination in Aquatic Ecosystems. Toxics. 2023; 11(2):173. https://doi.org/10.3390/toxics11020173
Chicago/Turabian StyleHarika, Thirupati Lakshmi, Khalid A. Al-Ghanim, Mian Nadeem Riaz, Kaliyamoorthy Krishnappa, Jeganathan Pandiyan, and Marimuthu Govindarajan. 2023. "Fishing Cat Scats as a Biomonitoring Tool for Toxic Heavy Metal Contamination in Aquatic Ecosystems" Toxics 11, no. 2: 173. https://doi.org/10.3390/toxics11020173
APA StyleHarika, T. L., Al-Ghanim, K. A., Riaz, M. N., Krishnappa, K., Pandiyan, J., & Govindarajan, M. (2023). Fishing Cat Scats as a Biomonitoring Tool for Toxic Heavy Metal Contamination in Aquatic Ecosystems. Toxics, 11(2), 173. https://doi.org/10.3390/toxics11020173






