(Micro)Plastics Are Toxic Pollutants
Abstract
:1. Microplastics Occurrence
2. Chemicals in MPs including Microfibers (MFs)
3. MPs Accumulate Toxics from the Environment
3.1. Metal Contaminants
3.2. Organic Contaminants
3.3. Pesticides
3.4. PFAS
4. The Role of Biofilm in Affecting the Transfer of Contaminants onto and from MPs
5. Chemicals Transfer from MPs into Organisms
6. The Effects of Aging, Weathering, and Leachates
7. Toxicity Studies on MP Leachates
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Everaert, G.; De Rijcke, M.; Lonneville, B.; Janssen, C.R.; Backhaus, T.; Mees, J.; van Sebille, E.; Koelmans, A.A.; Catarino, A.I.; Vandegehuchte, M.B. Risks of floating microplastic in the global ocean. Environ. Pollut. 2020, 267, 115499. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Programme; Secretariat of the Basel, Rotterdam and Stockholm Conventions. Chemicals in Plastics: A Technical Report; United Nations Environment Programme: Geneva, Switzerland, 2023; 128p, Available online: https://www.unep.org/resources/report/chemicals-plastics-technical-report (accessed on 14 October 2023).
- Athey, S.N.; Carney Almroth, B.; Granek, E.F.; Hurst, P.; Tissot, A.G.; Weis, J.S. Unraveling physical and chemical effects of textile microfibers. Water 2022, 14, 3797. [Google Scholar] [CrossRef]
- Le Bihanic, F.; Clérandeau, C.; Cormier, B.; Crebassa, J.-C.; Keiter, S.H.; Beiras, R.; Morin, B.; Bégout, M.-L.; Cousin, X.; Cachot, J. Organic contaminants sorbed to microplastics affect marine medaka fish early life stages development. Mar. Pollut. Bull. 2020, 154, 111059. [Google Scholar] [CrossRef]
- Pannetier, P.; Cachot, J.; Clérandeau, C.; Faure, F.; Van Arkel, K.; de Alencastro, L.F.; Levasseur, C.; Sciacca, F.; Bourgeois, J.P.; Morin, B. Toxicity assessment of pollutants sorbed on environmental sample microplastics collected on beaches: Part I-adverse effects on fish cell line. Environ. Pollut. 2019, 248, 1088–1097. [Google Scholar] [CrossRef]
- Wiesinger, H.; Wang, Z.; Hellweg, S. Deep dive into plastic monomers, additives, and processing aids. Environ. Sci. Technol. 2021, 55, 9339–9351. [Google Scholar] [CrossRef]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef] [PubMed]
- Peretz, J.; Vrooman, L.; Ricke, W.; Hunt, P.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.; Swan, S.; VandeVoort, C.; et al. Bisphenol A and reproductive health: Update of experimental and human evidene, 2007–2013. Environ. Health Persp. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Gomez-De León, C.T.; Del Río-Araiza, V.H.; Morales-Montor, J. The detrimental effect of microplastics on critical periods of development in the neuroendocrine system. Birth Defects Res. 2020, 112, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-a induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef] [PubMed]
- Senyildiz, M.; Karaman, E.F.; Bas, S.S.; Pirincci, P.A.; Ozden, S. Effects of BPA on global DNA methylation and global histone 3 lysine modifications in SH-SY5Y cells: An epigenetic mechanism linking the regulation of chromatin modifiying genes. Toxicol. In Vitro 2017, 44, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Navarro, C.; Robles, V.; Herráez, M.P. Transgenerational inheritance of heart disorders caused by paternal bisphenol a exposure. Environ. Pollut. 2015, 206, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.M.; Leppert, B.; Jahreis, S.; Wissenbach, D.K.; Feltens, R.; Grützmann, K.; Thürmann, L.; Bauer, T.; Ishaque, H.; Schick, M.; et al. MEST Mediates the impact of prenatal bisphenol a exposure on long-term body weight development. Clin. Epigenet. 2018, 10, 58. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, H.; Peter, K.T.; González, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R.; et al. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic microfibers emissions to land rival those to waterbodies and are growing. PLoS ONE 2020, 15, e023783. [Google Scholar] [CrossRef]
- Lacasse, K.; Baumann, W. Environmental Considerations for Textile Processes and Chemicals. In Textile Chemicals: Environmental Data and Facts; Lacasse, K., Baumann, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 484–647. [Google Scholar]
- Hill, P.J.; Taylor, M.; Goswami, P.; Blackburn, R.S. Substitution of PFAS Chemistry in Outdoor Apparel and the Impact on Repellency Performance. Chemosphere 2017, 181, 500–507. [Google Scholar] [CrossRef]
- KEMI. Hazardous Chemical Substances in Textiles—Proposals for Risk Management Measures; Kemikalieinspektion: Sundbyberg, Sweden, 2016. [Google Scholar]
- Alipour, S.; Hashemi, S.H.; Alavian Petroody, S.S. Release of Microplastic Fibers from Carpet-Washing Workshops Wastewater. J. Water Wastewater 2021, 31, 27–33. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. COVID-19 Face Masks: A Potential Source of Microplastic Fibers in the Environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Briain, O.Ó.; Marques Mendes, A.R.; McCarron, S.; Healy, M.G.; Morrison, L. The Role of Wet Wipes and Sanitary Towels as a Source of White Microplastic Fibres in the Marine Environment. Water Res. 2020, 182, 116021. [Google Scholar] [CrossRef] [PubMed]
- Belzagui, F.; Buscio, V.; Gutiérrez-Bouzán, C.; Vilaseca, M. Cigarette Butts as a Microfiber Source with a Microplastic Level of Concern. Sci. Total Environ. 2021, 762, 144165. [Google Scholar] [CrossRef]
- Novotny, T.; Lum, K.; Smith, E.; Wang, V.; Barnes, R. Cigarettes Butts and the Case for an Environmental Policy on Hazardous Cigarette Waste. Int. J. Environ. Res. Public Health 2009, 6, 1691–1705. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and Biological Effects of Cigarette Litter in Marine Worms. Sci. Rep. 2015, 5, 14119. [Google Scholar] [CrossRef]
- Moerman, J.W.; Potts, G.E. Analysis of Metals Leached from Smoked Cigarette Litter. Tob. Control 2011, 20, i30–i35. [Google Scholar] [CrossRef]
- Torkashvand, J.; Farzadkia, M. A Systematic Review on Cigarette Butt Management as a Hazardous Waste and Prevalent Litter: Control and Recycling. Environ. Sci. Pollut. Res. 2019, 26, 11618–11630. [Google Scholar] [CrossRef]
- Montarsolo, A.; Mossotti, R.; Patrucco, A.; Caringella, R.; Zoccola, M.; Pozzo, P.; Tonin, C. Study on the microplastics release from fishing nets. Eur. Phys. J. Plus 2018, 133, 494. [Google Scholar] [CrossRef]
- Verla, A.W.; Enyoh, C.E.; Verla, E.N.; Nwarnorh, K.O. Microplastic-toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Appl. Sci. 2019, 1, 1400. [Google Scholar] [CrossRef]
- Llorca, M.; Gabriella, S.; Monica, M.; Damia, B.; Marinella, F. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ. Pollut. 2018, 235, 680–691. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Zhang, L.; Wang, K.; Yu, X.; Zheng, Z.; Zheng, R. Sorption Behaviors of Phenanthrene on the Microplastics Identified in a Mariculture Farm in Xiangshan Bay, Southeastern China. Sci. Total Environ. 2018, 628–629, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Costa, F.G.; Pereira, M.L. Heavy metals and human health. In Environmental Health—Emerging Issues and Practice; Oosthuizen, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 227–246. [Google Scholar] [CrossRef]
- Munier, B.; Bendell, L. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS ONE 2018, 13, e0191759. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Sait, S.T.L.; Sørensen, L.; Kubowicz, S.; Vike-Jonas, K.; Gonzalez, S.V.; Asimakopoulos, A.G.; Booth, A.M. Microplastic fibres from synthetic textiles: Environmental degradation and additive chemical content. Environ. Pollut. 2021, 268, 115745. [Google Scholar] [CrossRef]
- Lionetto, F.; Esposito Corcione, C. An overview of the sorption studies of contaminants on poly(Ethylene Terephthalate) microplastics in the marine environment. J. Mar. Sci. Eng. 2021, 9, 445. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.S.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, Desorption, and Transfer to Biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef]
- Kazmiruk, T. Sorption of trace elements (Cd, Cu, Hg, Pb, and Zn) by macro- and microplastics within intertidal sediments; A laboratory, field and modeling approach. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2023; 177p. Available online: https://sfu-primo.hosted.exlibrisgroup.com/permalink/f/usv8m3/01SFUL_ALMA51469874650003611 (accessed on 16 November 2023).
- Frias, J.P.G.L.; Sobral, P.; Ferreira, A.M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010, 60, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef]
- Heskett, M.; Takada, H.; Yamashita, R.; Yuyama, M.; Ito, M.; Geok, Y.B.; Ogata, Y.; Kwan, C.; Heckhausen, A.; Taylor, H.; et al. Measurement of persistent organic pollutants (POPs) in plastic resin pellets from remote islands: Toward establishment of background concentrations for International Pellet Watch. Mar. Pollut. Bull. 2012, 64, 445–448. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Siavalas, G.; Kalaitzidis, S.; Papatheodorou, G.; Christanis, K. Distribution of polycyclic aromatic hydrocarbons (PAHs) in the Gulf of Aliveri, Central Greece. In Proceedings of the 9th National Greek Symposium on Oceanography and Fishery, Patras, Greece, 13–16 May 2009; Volume 1, pp. 251–255. [Google Scholar]
- Yeo, B.G.; Takada, H.; Hosoda, J.; Kondo, A.; Yamashita, R.; Saha, M.; Maes, T. Polycyclic aromatic hydrocarbons (PAHs) and hopanes in plastic resin pellets as markers of oil pollution via international pellet watch monitoring. Arch. Environ. Contam. Toxicol. 2017, 73, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Booyatumanondo, R.; Zakaria, M.P.; Dung, L.Q.; et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Colabuono, F.; Taniguchi, S.; Montone, R. Polychlorinated biphenyls and organochlorine pesticides in plastics ingested by seabirds. Mar. Poll. Bull. 2010, 60, 630–634. [Google Scholar] [CrossRef]
- Parashar, N.; Mahanty, B.; Hait, S. Microplastics as carriers of per- and polyfluoroalkyl substances (PFAS) in aquatic environment: Interactions and ecotoxicological effects. Water Emerg. Contam. Nanoplastics 2023, 2, 15. [Google Scholar] [CrossRef]
- Llorca, M.; Farré, M.; Karapanagioti, H.K.; Barceló, D. Levels and fate of perfluoroalkyl substances in beached plastic pellets and sediments collected from Greece. Mar. Pollut. Bull. 2014, 87, 286–291. [Google Scholar] [CrossRef]
- Barlow, D.; Biffinger, J.; Estrella, L.; Lu, Q.; Hung, C.-S.; Nadeau, L.; Crouch, A.; Russell, J.; Crookes-Goodson, W. Edge-localized biodeterioration and secondary microplastic formation by Papiliotrema laurentii unsaturated biofilm cells on polyurethane films. Langmuir 2020, 36, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Pu, Z.; Chen, F.; Xu, H.; Cao, X.; Chen, C.C.; Wong, J.; Liao, Y.; Zhu, X.; Pan, K. Metal leaching from plastics in the marine environment: An ignored role of biofilm. Environ. Internat. 2023, 177, 107988. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Zhou, X.; Yan, H.; Han, X.; Wu, M.; Chen, F. Research progress on the role of biofilm in heavy metals adsorption-desorption characteristics of microplastics: A review. Environ. Pollut. 2023, 336, 122448. [Google Scholar] [CrossRef]
- Wong, X.; Bolan, N.; Tsang, D.; Sarkar, B.; Bradney, L.; Li, Y. A review of microplastics aggregation in aquatic environment: Influence factors, analytical methods, and environmental implications. J. Hazard. Mater. 2021, 402, 123496. [Google Scholar] [CrossRef]
- 4 Ockenden, A.; Northcott, G.L.; Tremblay, L.A.; Simon, K.S. Disentangling the influence of microplastics and their chemical additives on a model detritivore system. Environ. Pollut. 2022, 307, 119558. [Google Scholar] [CrossRef]
- Bakir, A.; O’Connor, I.A.; Rowland, S.J.; Hendriks, A.J.; Thompson, R.C. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environ. Pollut. 2016, 219, 56–65. [Google Scholar] [CrossRef]
- Diepens, N.J.; Koelmans, A.A. Accumulation of plastic debris and associated contaminants in aquatic food webs. Enviro. Sci. Technol. 2018, 52, 8510–8520. [Google Scholar] [CrossRef]
- Wardrop, P.; Shimeta, J.; Nugegode, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Acosta-Dacal, A.; Pérez Luzardo, O.; Martínez, I.; Rapp, J.; Reinold, S.; Montesdeoca-Esponda, S.; Montero, D.; Gómez, M. Bioaccumulation of additives and chemical contaminants from environmental microplastics in European seabass (Dicentrarchus labrax). Sci. Total Environ. 2022, 822, 153396. [Google Scholar] [CrossRef] [PubMed]
- Beckingham, B.; Ghosh, U. Differential bioavailability of polychlorinated biphenyls associated with environmental particles: Microplastic in comparison to wood, coal and biochar. Environ. Pollut. 2017, 220, 150–158. [Google Scholar] [CrossRef]
- Thaysen, C.; Sorais, M.; Verreault, J.; Diamond, M.L.; Rochman, C.M. Bidirectional transfer of halogenated flame retardants between the gastrointestinal tract and ingested Plastics in urban-adapted ring-billed gulls. Sci. Total Environ. 2020, 730, 138887. [Google Scholar] [CrossRef]
- Artham, T.; Sudhakar, M.; Venkatesan, R.; Madhavan Nair, C.; Murty, K.V.G.K.; Doble, M. Biofouling and stability of synthetic polymers in sea water. Int. Biodeterior. Biodegrad. 2009, 63, 884–890. [Google Scholar] [CrossRef]
- Borrirukwisitsak, S.; Keenan, H.E.; Gauchotte-Lindsay, C. Effects of salinity, pH and temperature on the octanol-water partition coefficient of bisphenol A. Int. J. Environ. Sci. Dev. 2012, 3, 460–464. [Google Scholar] [CrossRef]
- Luo, H.; Tu, C.; He, D.; Zhang, A.; Sun, J.; Li, J.; Xu, J.; Pan, X. Interactions between microplastics and contaminants: A review focusing on the effect of aging process. Sci. Total Environ. 2023, 899, 165615. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, P.; Morin, B.; Clérandeau, C.; Laurent, J.; Chapelle, C.; Cachot, J. Toxicity assessment of pollutants sorbed on environmental microplastics collected on beaches: Part II-adverse effects on Japanese medaka early life stages. Environ. Pollut. 2019, 248, 1098–1107 . [Google Scholar] [CrossRef]
- Seuront, L. Microplastic Leachates Impair Behavioural Vigilance and Predator Avoidance in a Temperate Intertidal Gastropod. Biol. Lett. 2018, 14, 20180453. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuster, B.; Alomar, C.; Viñas, L.; Campillo, J.A.; Pérez-Fernández, B.; Álvarez, E.; Compa, M.; Deudero, S. Organochlorine Pesticides (OCPs) and Polychlorinated Biphenyls (PCBs) Occurrence in Sparus aurata Exposed to Microplastic Enriched Diets in Aquaculture Facilities. Mar. Pollut. Bull. 2021, 173, 113030. [Google Scholar] [CrossRef] [PubMed]
- Schur, C.; Weil, C.; Baum, M.; Wallraff, J.; Schrier, M.; Oehlmann, J.; Wagner, M. Incubation in wastewater reduces the multigenerational effects of microplastics in Daphnia magna. Env. Sci. Technol. 2021, 55, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A. Microplastics in the marine environment. Mar. Poll. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Fries, E.; Suhring, R. he unusual suspects: Screening for persistent, mobile and toxic plastic additives in plastic leachates. Environ. Pollut. 2023, 335, 122263. [Google Scholar] [CrossRef]
- Li, H.-X.; Getzinger, G.; Ferguson, P.L.; Orihuela, B.; Zhy, M.; Rittschoff, D. Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle Amphibalanus Amphitrite. Environ. Sci. Tech. 2016, 50, 924–931. [Google Scholar] [CrossRef]
- Capolupo, M.; Sorensen, L.; Jayasena, K.; Booth, A.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Bejgarn, S.; MacLeod, M.; Bogdal, C.; Breitholtz, M. Toxicity of leachate from weathering plastics: An exploratory screening study with Nitocra spinipes. Chemosphere 2015, 132, 114–119. [Google Scholar] [CrossRef]
- Koski, M.; Søndergaard, J.; Christensen, A.M.; Nielsen, T.G. Effect of environmentally relevant concentrations of potentially toxic microplastic on coastal copepods. Aquat. Toxicol. 2021, 230, 105713. [Google Scholar] [CrossRef]
- Manzo, S.; Schiavo, S. Physical and chemical threats posed by micro (nano) plastic to sea urchins. Sci. Total Environ. 2022, 808, 152105. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cormier, B.; Gambardella, C.; Tato, T.; Perdriat, Q.; Costa, E.; Veclin, C.; Le Bihanic, F.L.; Grassl, B.; Dubocq, F.; Kärrman, A.; et al. Chemicals sorbed to environmental microplastics are toxic to early life stages of aquatic organisms. Ecotoxicol. Environ. Saf. 2021, 208, 111665. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, M.; Provenza, F.; Grazioli, E.; Cavallo, A.; Terlizzi, A.; Renzi, M. PET microplastics toxicity on marine key species is influenced by pH, particle size and food variations. Sci. Total Environ. 2020, 715, 136947. [Google Scholar] [CrossRef] [PubMed]
- Rendell-Bhatti, F.; Paganos, P.; Pouch, A.; Mitchell, C.; D’Aniello, S.; Godley, B.J.; Paztro, K.; Arnone, M.I.; Jimenez-Guri, E. Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ. Pollut. 2021, 269, 115744. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Zhang, Y.; Zhang, L.; Wang, Q.; Zhao, Y. The toxicity of microplastics and their leachates to embryonic development of the sea cucumber Apostichopus japonicus. Mar. Environ. Res. 2023, 190, 106114. [Google Scholar] [CrossRef]
- Seuront, L.; Nicastro, K.R.; McQuaid, C.D.; Zardi, G.I. Microplastic leachates induce species-specific trait strengthening in intertidal mussels. Ecol. Appl. 2021, 31, e02222. [Google Scholar] [CrossRef]
- Ricorte, M.; Prots, E.; Montemurra, N.; Bedrossiantz, J.; Bellot, M.; Gomez-Canela, C.; Roldua, D. Environmental concentrations of tire rubber-derived 6PPD-quinone alter CNS function in Zebrafish larvae. Sci. Total Environ. 2023, 896, 165240. [Google Scholar] [CrossRef]
- Haram, L.E.; Carlton, J.T.; Ruiz, G.M.; Maximenko, N.A. A plasticene lexicon. Mar. Pollut. Bull. 2020, 150, 110714. [Google Scholar] [CrossRef]
- Porcino, N.; Bottari, T.; Mancuso, M. Is wild marine biota affected by microplastics? Animals 2023, 13, 147. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Arp, H.P.H.; Kühnel, D.; Rummel, C.; MacLeod, M.; Potthoff, A.; Reichelt, S.; Rojo-Nieto, E.; Schmitt-Jansen, M.; Sonnenberg, J.; Toorman, E.; et al. Weathering plastics as a planetary boundary threat: Exposure, fate, and hazards. Environ. Sci. Technol. 2021, 55, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Nielsen, T. Microplastic exposure studies should be environmentally realistic. Proc. Nat. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.S.; Palmquist, K.H. Reality check: Experimental studies on microplastics lack realism. Appl. Sci. 2021, 11, 8529. [Google Scholar] [CrossRef]
- Alava, J.J.; Jahnke, A.; Bergmann, M.; Aguirre-Martínez, G.V.; Bendell, L.; Calle, P.; Domínguez, G.A.; Faustman, E.M.; Falman, J.; Kazmiruk, T.N.; et al. A Call to Include Plastics in the Global Environment in the Class of Persistent, Bioaccumulative, and Toxic (PBT) Pollutants. Environ. Sci. Technol. 2023, 57, 8185–8188. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Assembly of the United Nations Environment Programme. End Plastic Pollution: Towards an International Legally Binding Instrument; UNEA Resolution 5/14, Dakar, Senegal. 2022. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/39812/OEWG_PP_1_INF_1_UNEA%20resolution.pdf (accessed on 17 October 2023).
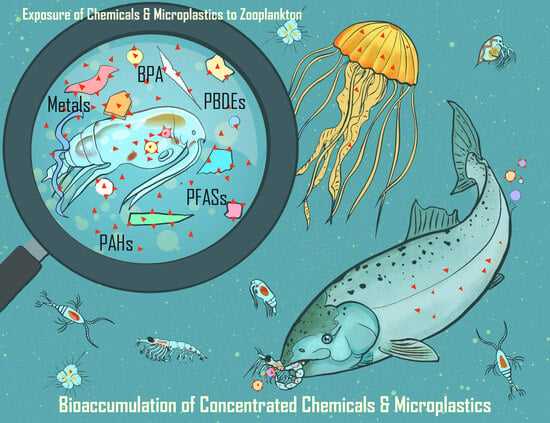
 = microplastics (MPs);
= microplastics (MPs);  = chemical contaminant or pollutant; and
= chemical contaminant or pollutant; and  = microplastic with sorbed pollutant.
= microplastic with sorbed pollutant.
 = microplastics (MPs);
= microplastics (MPs);  = chemical contaminant or pollutant; and
= chemical contaminant or pollutant; and  = microplastic with sorbed pollutant.
= microplastic with sorbed pollutant.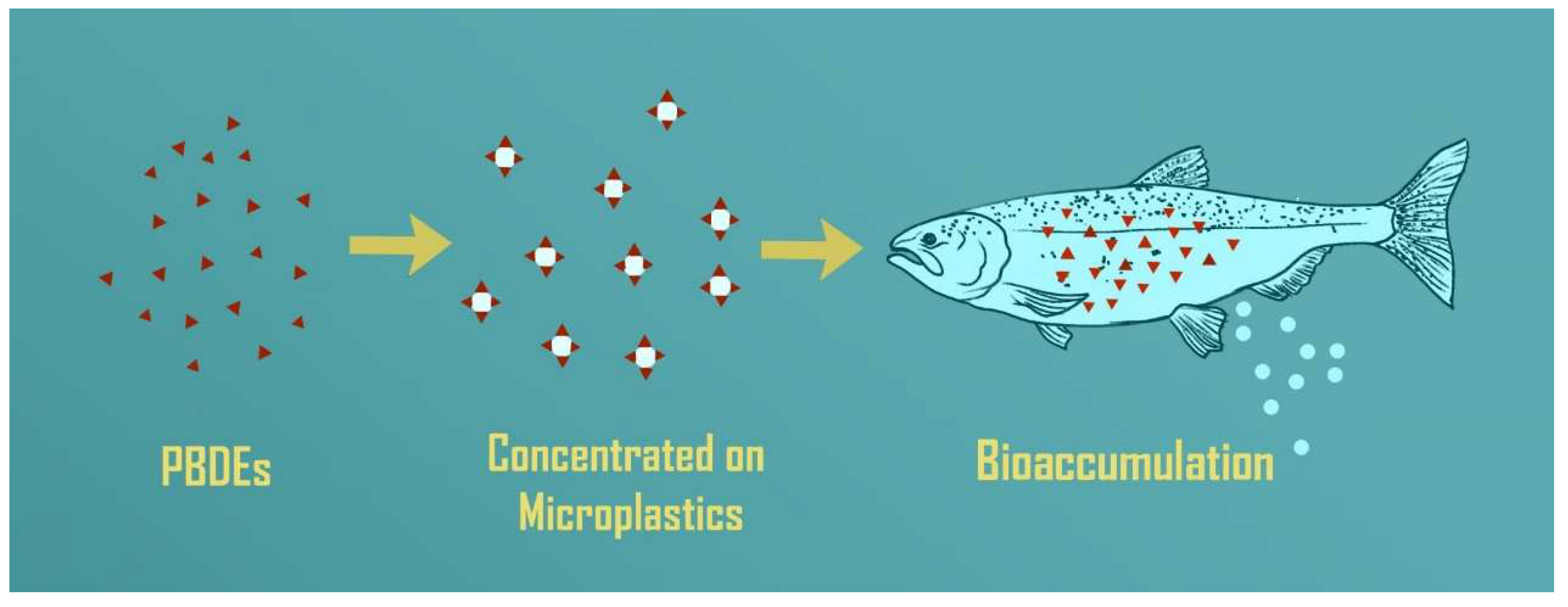
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, J.S.; Alava, J.J. (Micro)Plastics Are Toxic Pollutants. Toxics 2023, 11, 935. https://doi.org/10.3390/toxics11110935
Weis JS, Alava JJ. (Micro)Plastics Are Toxic Pollutants. Toxics. 2023; 11(11):935. https://doi.org/10.3390/toxics11110935
Chicago/Turabian StyleWeis, Judith S., and Juan José Alava. 2023. "(Micro)Plastics Are Toxic Pollutants" Toxics 11, no. 11: 935. https://doi.org/10.3390/toxics11110935
APA StyleWeis, J. S., & Alava, J. J. (2023). (Micro)Plastics Are Toxic Pollutants. Toxics, 11(11), 935. https://doi.org/10.3390/toxics11110935