Effects of Metamifop on Defense Systems in Monopterus albus
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design
2.3. Sample Collection
2.4. White Blood Cell Count
2.5. ROS Production Assay
2.6. Related Enzyme Activity, MDA Content, and PCO Content Assay
2.7. Histological Analysis
2.8. Gene Expression
2.9. Statistical Analysis
3. Results
3.1. Solvent and Metamifop in Water
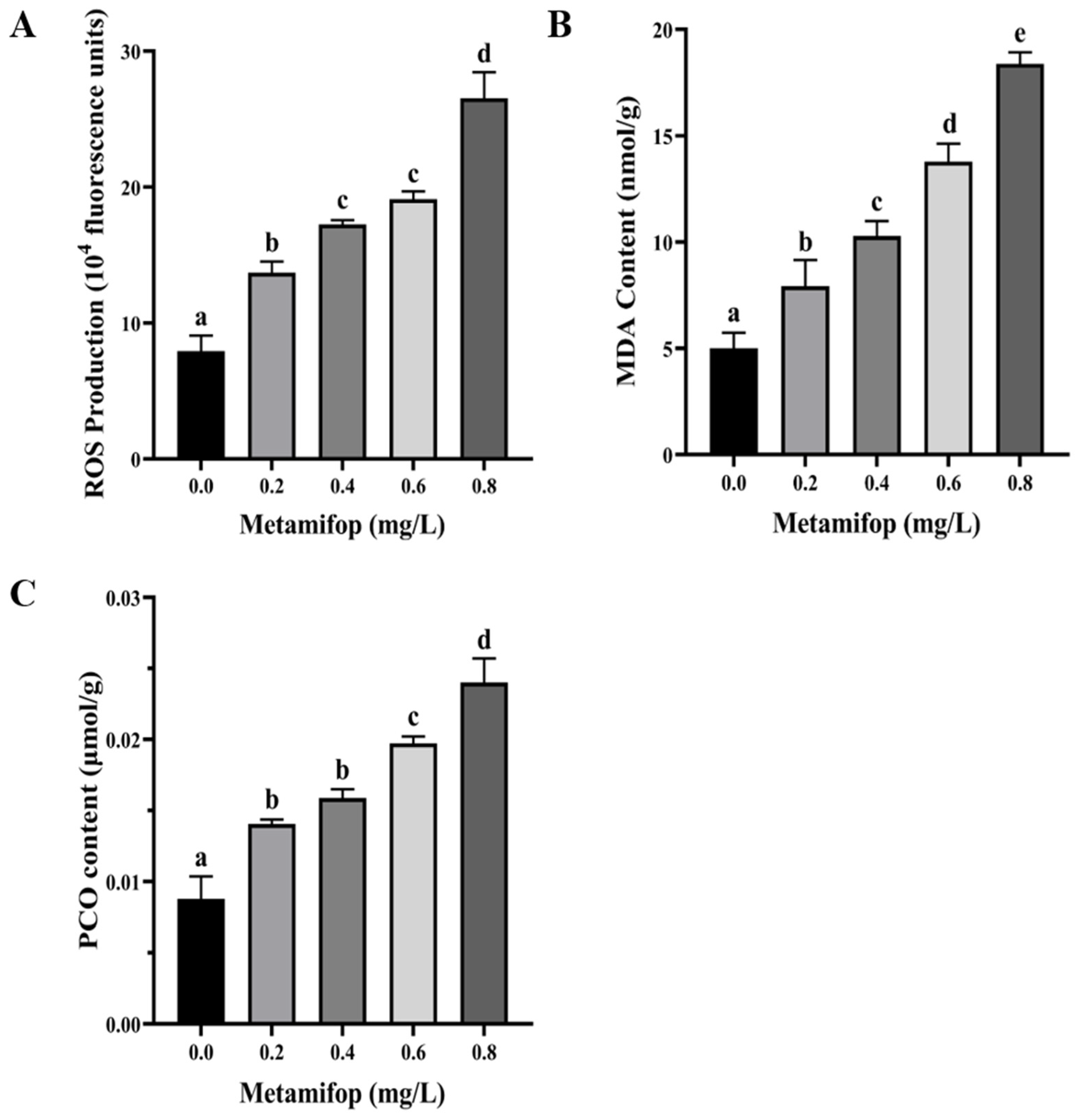
3.2. Effects of Metamifop on ROS Production, MDA Content, and PCO Content
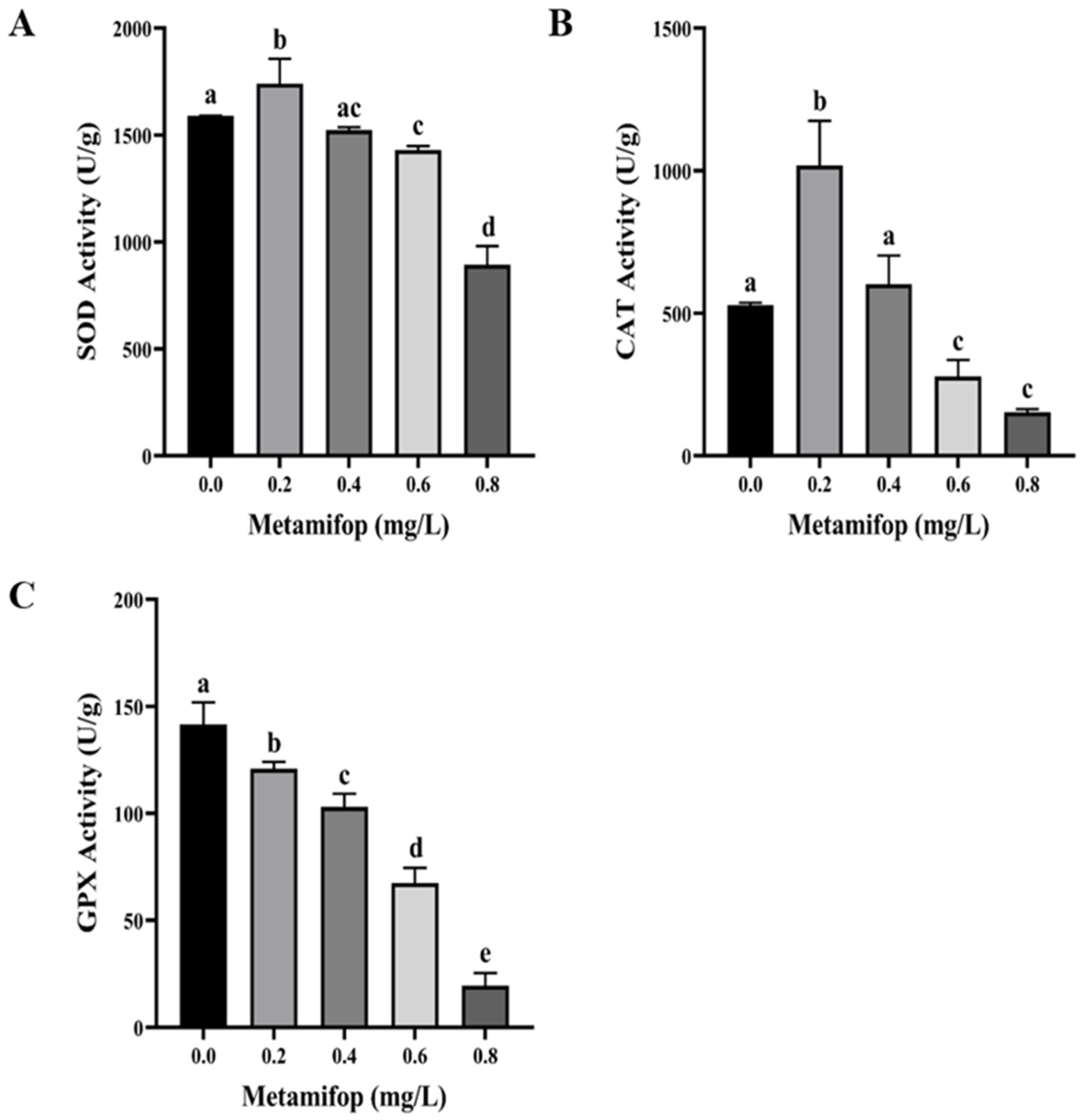
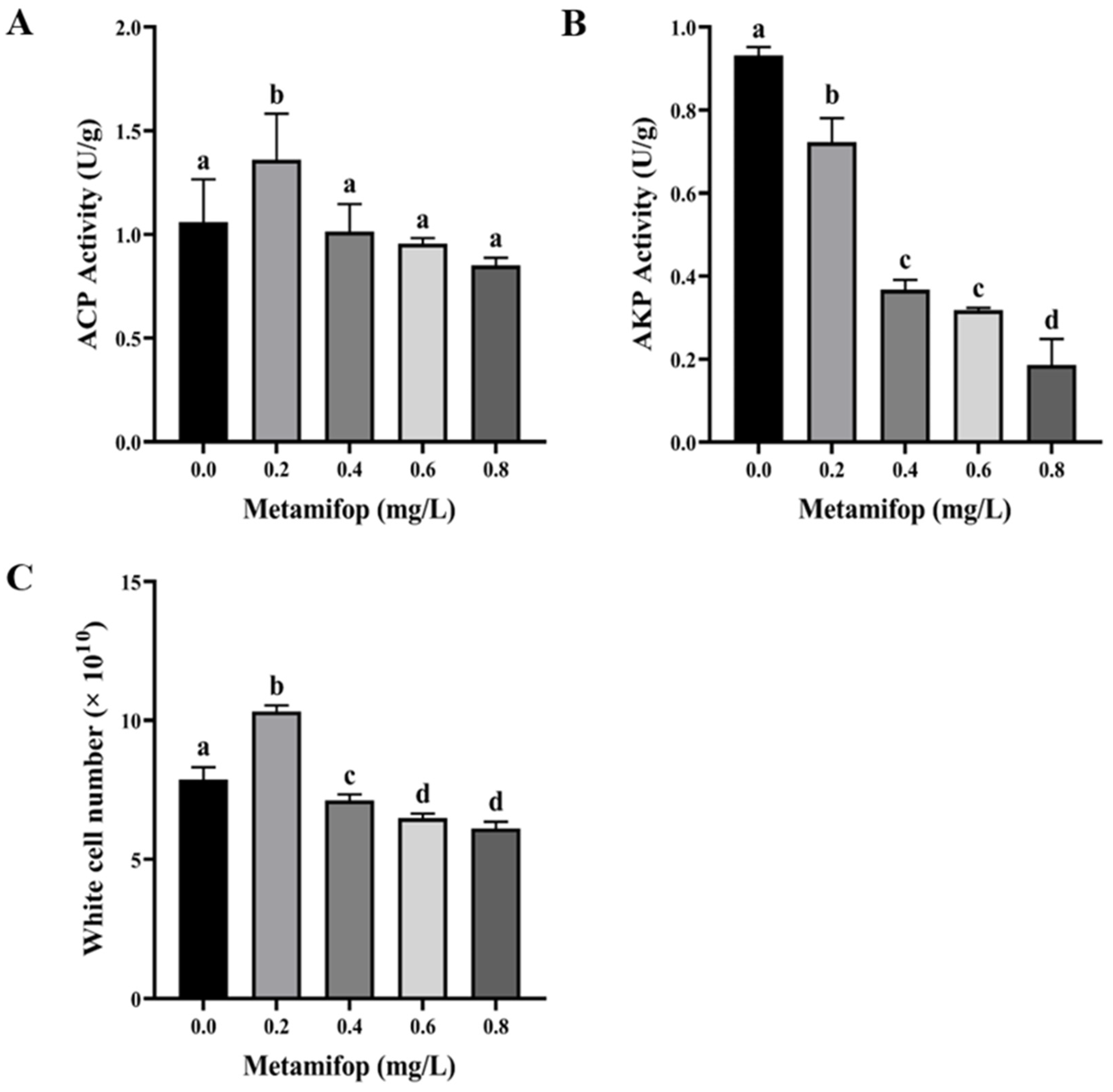
3.3. Effects of Metamifop on Enzyme Activity
3.4. Efftct of Metamifop on White Cell Number
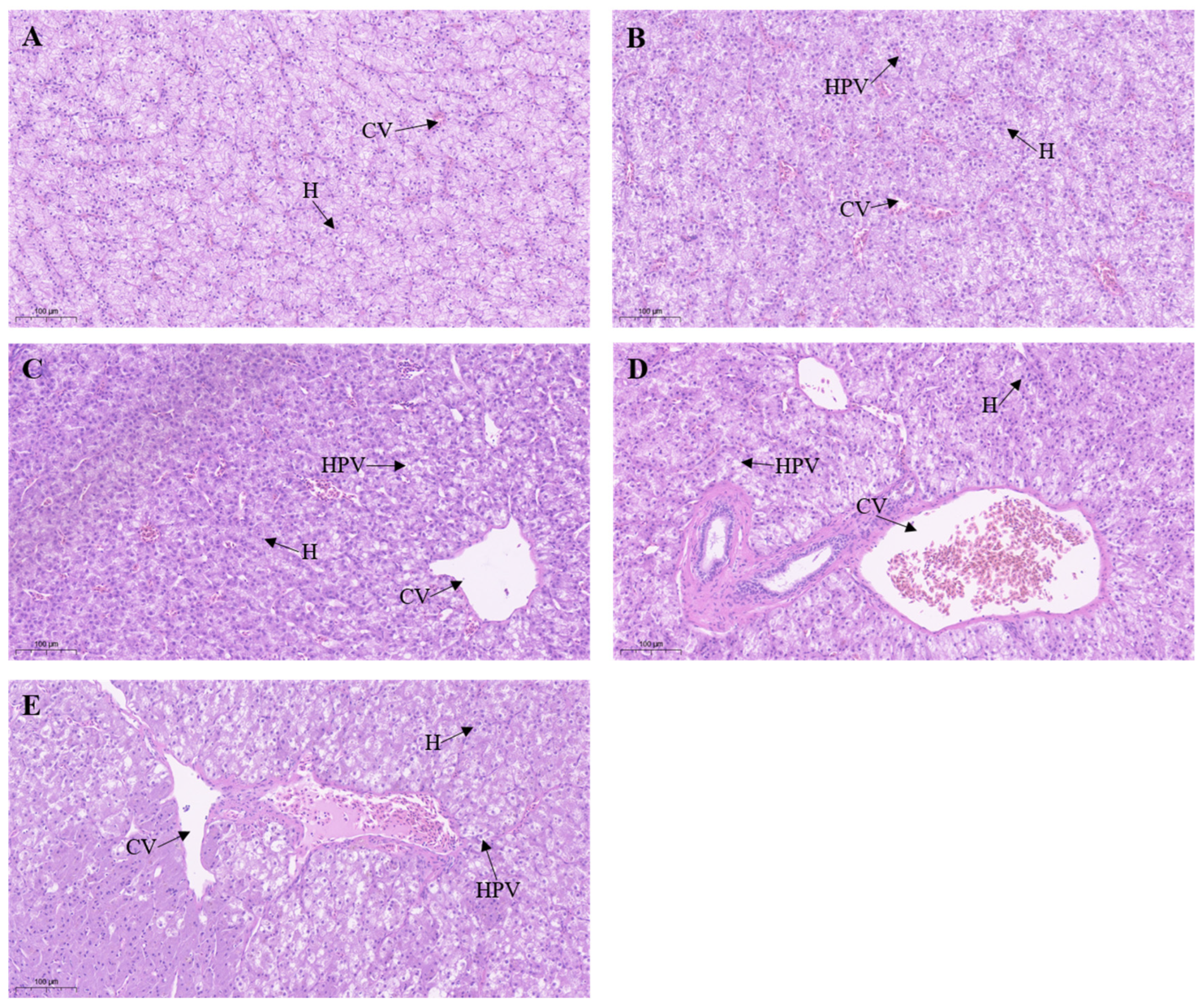
3.5. Effect of Metamifop on Histology
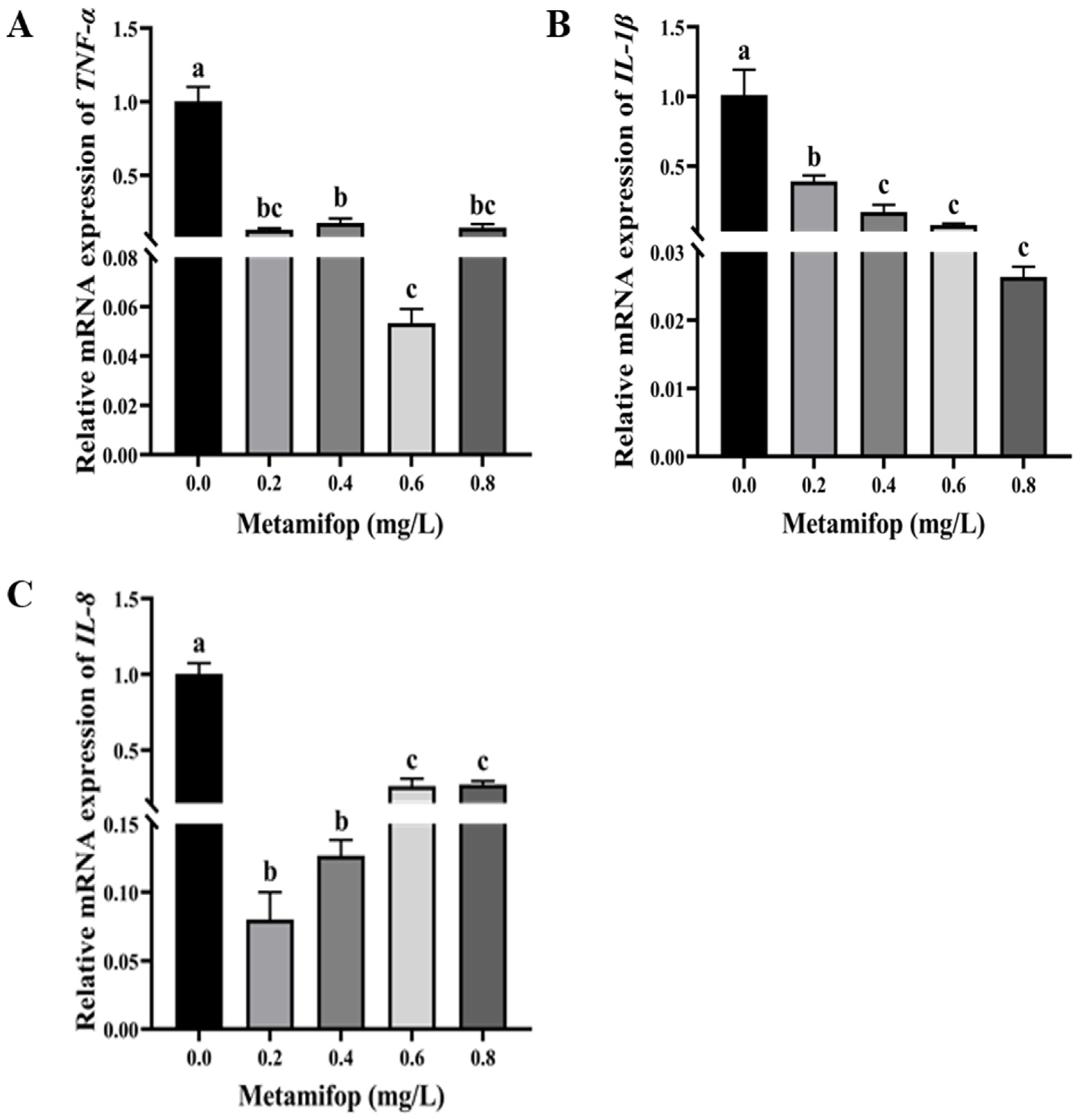
3.6. Effects of Metamifop on Inflammatory-Related Genes
3.7. Effects of Metamifop on Apoptosis-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. World Food and Agriculture–Statistical Yearbook 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Maum, M.A.; Barrett, S.C.H. The biology of canadian weeds. 77. Echinochloa crus-galli (L.). Beauv. Can. J. Plant Sci. 1986, 66, 739–759. [Google Scholar]
- Xia, X.D.; Tang, W.J.; He, S.; Kang, J.; Ma, H.J.; Li, J.H. Mechanism of metamifop inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase in Echinochloa crus-galli. Sci. Rep. 2016, 6, 34066. [Google Scholar] [CrossRef]
- Xu, Q.; Peng, X.; Guo, H.L.; Che, Y.; Dou, Z.; Xing, Z.P.; Hou, J.; Styles, D.; Gao, H.; Zhang, H.C. Rice-crayfish coculture delivers more nutrition at a lower environmental cost. Sustain. Prod. Consum. 2022, 29, 14–24. [Google Scholar] [CrossRef]
- Bao, J.; Jiang, H.B.; Li, X.D. Thirty years of rice-crab coculture in China-Research progress and prospects. Rev. Aquac. 2022, 14, 1597–1612. [Google Scholar] [CrossRef]
- Yuan, Q.; Lv, W.W.; Sun, X.L.; Huang, W.W.; Zhou, W.Z. Effects of chemical fertilizer application upon the water quality parameters of a rice-eel (Monopterus albus) coculture system. Aquac. Res. 2023, 2023, 9341799. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, M.N.; Guo, M.Y.; Duan, M.M.; Zheng, J.Y.; Chen, X.G.; Liu, Y.C.; Qiu, L.H. Effects of sublethal concentration of metamifop on hepatic lipid metabolism in adult zebrafish (Danio rerio). Aquat. Toxicol. 2021, 238, 105938. [Google Scholar] [CrossRef]
- Stara, A.; Machova, J.; Velisek, J. Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Environ. Toxicol. Pharmacol. 2012, 33, 334–343. [Google Scholar] [CrossRef]
- Gupta, P.; Verma, S.K. Evaluation of genotoxicity induced by herbicide pendimethalin in fresh water fish Clarias batrachus (linn.) and possible role of oxidative stress in induced DNA damage. Drug Chem. Toxicol. 2022, 45, 750–759. [Google Scholar] [CrossRef]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van, B.D.R.; Berneman, Z.N. Apoptosis: Mechanisms and relevance in cancer. Ann. Hematol. 2005, 84, 627–639. [Google Scholar] [CrossRef]
- Luo, S.Y.; Liu, C.; Ding, J.; Gao, X.M.; Wang, J.Q.; Zhang, Y.B.; Du, C.; Hou, C.C.; Zhu, J.Q.; Lou, B. Scavenging reactive oxygen species is a potential strategy to protect Larimichthys crocea against environmental hypoxia by mitigating oxidative stress. Zool. Res. 2021, 42, 592–605. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Kalhor, N.; Dawood, M.A.O.; Ahmadifar, M.; Moghadam, M.S.; Abarghouei, S.; Hedayati, A. Effects of polystyrene microparticles on inflammation, antioxidant enzyme activities, and related gene expression in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. Int. 2021, 28, 14909–14916. [Google Scholar] [CrossRef]
- He, K.W.; Luo, X.P.; Wen, M.; Wang, C.G.; Qin, C.J.; Shao, J.; Gan, L.; Dong, R.R.; Jiang, H.B. Effect of acute ammonia toxicity on inflammation, oxidative stress and apoptosis in head kidney macrophage of Pelteobagrus fulvidraco and the alleviation of curcumin. Comp. Biochem. Physiol. Part C 2021, 248, 109098. [Google Scholar] [CrossRef]
- Wu, X.Y.; Han, H.R.; Xie, K.M.; He, N.N.; Yang, Z.W.; Jin, X.H.; Ma, S.J. Difenoconazole disrupts carp intestinal physical barrier and causes inflammatory response via triggering oxidative stress and apoptosis. Pestic. Biochem. Physiol. 2023, 194, 105507. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, L.; Liu, Y.; Du, C.; Hou, C.C.; Xie, Q.P.; Tang, D.J.; Liu, F.; Lou, B.; Zhu, J.Q. Change to the transcriptomic profile, oxidative stress, apoptotic and immunity in the liver of small yellow croaker (Larimichthys polyactis) under hypoxic stress. Aquaculture 2023, 576, 739854. [Google Scholar] [CrossRef]
- Li, Y.M.; Liu, Z.Q.; Li, M.F.; Jiang, Q.C.; Wu, D.L.; Huang, Y.H.; Jiao, Y.; Zhang, M.; Zhao, Y.L. Effects of nanoplastics on antioxidant and immune enzyme activities and related gene expression in juvenile Macrobrachium nipponense. J. Hazard. Mater. 2020, 398, 122990. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, X.P.; Zhan, F.F.; Cheng, K. Effects of lead on acid phosphatase and alkaline phosphatase in Carassias auratus. Sichuan J. Zool. 2008, 27, 201–204. [Google Scholar]
- Allen, P. Soft tissue accumulation of lead in the blue tilapia, rordchrom is aureus (Steindachner), and the modifying effects of cadmium and mercury. Biol. Trace Elem. Res. 1995, 50, 193–208. [Google Scholar] [CrossRef]
- Atli, G.; Canli, M. Enzymatic responses to metal exposurses in a freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. Part C 2007, 145, 282–287. [Google Scholar]
- Sanchez, F.; Lozano-Munoz, I.; Munoz, S.; Diaz, N.; Neira, R.; Wacyk, J. Effect of dietary inclusion of microalgae (Nannochloropsis gaditana and Schizochytrium spp.) on non-specitic immunity and erythrocyte maturity in Atlantic salmon fingerlings. Fish Shellfish Immunol. 2023, 140, 108975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Nie, L.; Zhu, G.; Xiang, L.X.; Shao, J.Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.Q.; Wu, L.X.; Tang, C.; Wang, H.N.; Wei, Y.J. Autophagy-inflammation interplay during infection: Balancing pathogen clearance and host inflammation. Front. Pharmacol. 2022, 13, 832750. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; He, Y.; Zhou, R. Swamp eel (Monopterus albus). Trends Genet. 2021, 37, 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, T.Y.; Zhu, Q.Q.; Wang, L.; Pei, X.; Zhu, C.K.; Wang, H.; Li, J.L. Effects of metamifop on ammonia production and metabolism of Monopterus albus. Pestic. Biochem. Physiol. 2023, 193, 105446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, T.Y.; Wang, L.; Ma, X.T.; Zhu, C.K.; Wang, H.; Li, J.L. Metamifop as an estrogen-like chemical affects the pituitary-hypothalamic-gonadal (HPG) axis of female rice field eels (Monopterus albus). Front. Physiol. 2023, 14, 1088880. [Google Scholar] [CrossRef]
- Zhao, F.; Li, H.; Cao, F.J.; Chen, X.G.; Liang, Y.; Qiu, L.H. Short-term developmental toxicity and potential mechanisms of the herbicide metamifop to zebrafish (Danio rerio) embryos. Chemosphere 2019, 236, 124590. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals, Test No. 203: Fish, Acute Toxicity Test, Holding of Fish; OECD: Paris, France, 2019; pp. 5–6. [Google Scholar]
- Driver, A.S.; Kodavanti, P.R.S.; Mundy, W.R. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol. Teratol. 2000, 22, 175–181. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Dahms, H.U.; Dong, F.; Jing, W.X.; Wang, L. Immune-associated parameters and antioxidative responses to cadmium in the freshwater crab Sinopotamon henanense. Ecotoxicol. Environ. Saf. 2016, 129, 235–241. [Google Scholar] [CrossRef]
- Wang, C.X.; Harwood, J.D.; Zhang, Q.M. Oxidative stress and DNA damage in common carp (Cyprinus carpio) exposed to the herbicide mesotrione. Chemosphere 2018, 193, 1080–1086. [Google Scholar] [CrossRef]
- Xu, Z.H.; Regenstein, J.M.; Xie, D.D.; Lu, W.J.; Ren, X.C.; Yuan, J.J.; Mao, L.C. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Cui, Y.T.; Hou, Z.M.; Ren, Y.C.; Men, X.H.; Zheng, B.; Liu, P.; Xia, B. Effects of aerial exposure on oxidative stress, antioxidant and non-specific immune responses of juvenile sea cucumber Apostichopus japonicus under low temperature. Fish Shellfish Immunol. 2020, 101, 58–65. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.B.; Wang, G.L.; Guan, T.Y.; Zhu, C.K.; Li, J.L.; Wang, H. Effects of cadmium on antioxidant and non-specific immunity of Macrobrachium nipponense. Ecotoxicol. Environ. Saf. 2021, 224, 112651. [Google Scholar] [CrossRef]
- Shen, W.Y.; Fu, L.L.; Li, W.F.; Zhu, Y.R. Effect of dietary supplementation with Bacillus subtilis on the growth, performance, immune response and antioxidant activities of the shrimp (Litopenaeus vannamei). Aqua. Res. 2010, 41, 1691–1698. [Google Scholar] [CrossRef]
- Dorts, J.; Silvestre, F.; Tu, H.T.; Tyberghein, A.E.; Phuong, N.T.; Kestemont, P. Oxidative stress, protein carbonylation and heat shock proteins in the black tiger shrimp, Penaeus monodon, following exposure to endosulfan and deltamethrin. Environ. Toxicol. Pharmacol. 2009, 28, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Bonetto, V. Redox proteomics: Identification of oxidatively modified proteins. J. Proteom. 2003, 3, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Braconi, D.; Bernardini, G.; Santucci, A. Linking protein oxidation to environmental pollutants: Redox proteomic approaches. J. Proteom. 2011, 74, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Moreno, I.; Pichardo, S.; Jos, A.; Moyano, R.; Monterde, J.G.; Camean, A. Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed subchronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon 2005, 46, 725–735. [Google Scholar] [CrossRef]
- Bojarski, B.; Ludwikowska, A.; Kurek, A.; Pawlak, K.; Tombarkiewicz, B.; Lutnicka, H. Hematological alterations in common carp (Cyprinus carpio L.) exposed to herbicides: Pendimethalin and ethofumesate tested separately and in mixture. Folia Biol. 2015, 63, 167–174. [Google Scholar] [CrossRef][Green Version]
- Shabnam, A.; Badre, A.A. Effect of dimethoate on the activities of acid and alkline phosphatases in the gilland liver of zebrafish, Danio rerio. Trend Biosci. 2013, 6, 612–616. [Google Scholar]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Sudova, E. Effects of acute exposure to metribuzin on some hematological, biochemical and histopathological parameters of common carp (Cyprinus carpio L.). Bull. Environ. Contam. Toxicol. 2009, 82, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, L.C.; Barcellos, L.J.G.; Valle, S.D.; Silva, T.D.; Anziliero, D.; dos Santos, E.D.; Pivato, M.; Zanatta, R. Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immunol. 2011, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Wang, T.; Hong, S.; Peddie, S.; Crampe, M.; Laing, K.J.; Cunningham, C.; Zou, J. Cytokines and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Peddie, S.; Scapigliati, G.; Zhang, Y.; Bols, N.C.; Ellis, A.E.; Secombes, C.J. Functional characterisation of the recombinant tumor necrosis factors in rainbow trout, Oncorhynchus mykiss. Dev. Comp. Immunol. 2003, 27, 813–822. [Google Scholar] [CrossRef]
- Lam, F.W.S.; Wu, S.Y.; Lin, S.J.; Lin, C.C.; Chen, Y.M.; Wang, H.C.; Chen, T.Y.; Lin, H.T.; Lin, J.H.Y. The expression of two novel orange-spotted grouper (Epinephelus coioides) TNF genes in peripheral blood leukocytes, various organs, and fish larvae. Fish Shellfish Immunol. 2011, 30, 618–629. [Google Scholar] [CrossRef]
- Li, Y.T.; Kon, N.; Jiang, L.; Tan, M.J.; Ludwig, T.; Zhao, Y.M.; Baer, R.; Gu, W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef]
- Bou, M.; Todorcevic, M.; Rodriguez, J.; Capilla, E.; Gutierrez, J.; Navarro, I. Interplay of adiponectin, TNFalpha and insulin on gene expression, glucose uptake and PPARgamma, AKT and TOR pathways in rainbow trout cultured adipocytes. Gen. Comp. Endocrinol. 2014, 205, 218–225. [Google Scholar] [CrossRef]
- Tanekhy, M.; Matsuda, S.; Itano, T.; Kawakami, H.; Kono, T.; Sakai, M. Expression of cytokine genes in head kidney and spleen cells of Japanese flounder (Paralichthys olivaceus) infected with Nocardia seriolae. Vet. Immunol. Immunopathol. 2010, 134, 178–183. [Google Scholar] [CrossRef]
- Carriero, M.M.; Henrique-Silva, F.; Meira, C.M.; Gato, I.M.Q.; Caetano, A.R.; Lobo, F.P.; Alves, A.L.; Varela, E.S.; Maia, A.A.M. Molecular characterization and gene expression analysis of the pro-inflammatory cytokines IL-1β and IL-8 in the South American fish Piaractus mesopotamicus challenged with Aeromonas dhakensis. Genet. Mol. Biol. 2020, 43, e20200006. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1. Cytokine Growth Factor Rev. 1984, 8, 253–265. [Google Scholar] [CrossRef]
- Wang, G.L.; Wang, M.C.; Zhang, X.W.; Chang, M.X.; Xie, H.X.; Nie, P. Molecular cloning, biological effect, and tissue distribution of interleukin-8 protein in mandarin fish (Siniperca chuasti) upon Flavobacterium columnare infection. Fish Shellfish Immunol. 2017, 66, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Prevot-D’Alvise, N.; Bunet, R.; Simide, R.; Couvray, S.; Coupe, S.; Grillasca, J.P. Effect of a glyphosate-based herbicide on gene expressions of the cytokines interleukin-1 beta and interleukin-10 and of heme oxygenase-1 in european sea bass, Dicentrarchus labrax L. Bull. Environ. Contam. Toxicol. 2014, 92, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cao, F.J.; Li, H.; Teng, M.M.; Liang, Y.; Qiu, L.H. The effects of a short-term exposure to propiconazole in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. Int. 2020, 27, 38212–38220. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.Y.; Han, Q.; Xu, Y.M.; Jiang, H.J.; Xing, H.J.; Teng, X.H. Impaired immune function and structural integrity in the gills of common carp (Cyprinus carpio L.) caused by chlorpyrifos exposure: Through oxidative stress and apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, S.J.; Choi, Y.J.; Kim, N.N.; Choi, C.Y.; Kim, J.W. Effects of different light wavelengths from LEDs on oxidative stress and apoptosis in olive flounder (Paralichthys olivaceus) at high water temperatures. Fish Shellfish Immunol. 2016, 55, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Finkel, E. The motochondrion: Is it central to apoptosis? Science 2001, 292, 624–626. [Google Scholar] [CrossRef]
- Desagher, S. Mitochondir as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Tie, H.M.; Yu, D.W.; Jiang, Q.X.; Yang, F.; Xu, Y.S.; Xia, W.S. Research on apoptotic mechanism and related pathways involved in postmortem grass carp (Ctenopharyngodon idellus) muscle. J. Sci. Food Agric. 2023, 103, 298–307. [Google Scholar] [CrossRef]

| Genes | Primer Sequence (5′–3′) |
|---|---|
| Bax | F: GGAGCAAGGTGGCTGGGTAA |
| R: GTGGACTCCCAATCCTTAGACA | |
| Bcl-2b | F: AGCCCACAAAACCACCACA |
| R: GACCACACAACCACCATCTCA | |
| caspase-9 | F: ATGTTGATGATGGTTGGTGCC |
| R: CTTTGCGTGGGTGATGCTT | |
| caspase-3 | F: GGTTCTGACCCTTACCGCTAC |
| R: TGTCCCATCTGCTAACGTGGA | |
| TNF-α | F:CCTTAGCCACACAGTGATGCG |
| R:CCCAGGCTCATCTTCCAGGT | |
| IL-1β | F:ACCTCATTATCGCCACGGAG |
| R:ATTTTACGGTTGTCGCTGCC | |
| IL-8 | F:TACTGGTTCTGCTTACTGTCGC |
| R:CAAATCTTTTGCCCATCCCT | |
| β-actin | F:TCAACACGCCTGCCATGTAT |
| R:CGCTCAGCTGTGGTAGTGAA |
| Actual Concentration (mg/L) | Nominal Metamifop Concentration (mg/L) | |||
|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | |
| 0 h | 0.24 ± 0.02 | 0.43 ± 0.04 | 0.66 ± 0.02 | 0.85 ± 0.01 |
| 24 h | 0.19 ± 0.01 | 0.38 ± 0.04 | 0.56 ± 0.05 | 0.76 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, T.; Zhang, Y.; Zhu, Q.; Wang, L.; Feng, J.; Wang, H.; Li, J. Effects of Metamifop on Defense Systems in Monopterus albus. Toxics 2023, 11, 811. https://doi.org/10.3390/toxics11100811
Guan T, Zhang Y, Zhu Q, Wang L, Feng J, Wang H, Li J. Effects of Metamifop on Defense Systems in Monopterus albus. Toxics. 2023; 11(10):811. https://doi.org/10.3390/toxics11100811
Chicago/Turabian StyleGuan, Tianyu, Yi Zhang, Qianqian Zhu, Long Wang, Jianbin Feng, Hui Wang, and Jiale Li. 2023. "Effects of Metamifop on Defense Systems in Monopterus albus" Toxics 11, no. 10: 811. https://doi.org/10.3390/toxics11100811
APA StyleGuan, T., Zhang, Y., Zhu, Q., Wang, L., Feng, J., Wang, H., & Li, J. (2023). Effects of Metamifop on Defense Systems in Monopterus albus. Toxics, 11(10), 811. https://doi.org/10.3390/toxics11100811







