Hawksbill Sea Turtle (Eretmochelys imbricata) Blood and Eggs Organochlorine Pesticides Concentrations and Embryonic Development in a Nesting Area (Yucatan Peninsula, Mexico)
Abstract
1. Introduction
2. Material and Methods
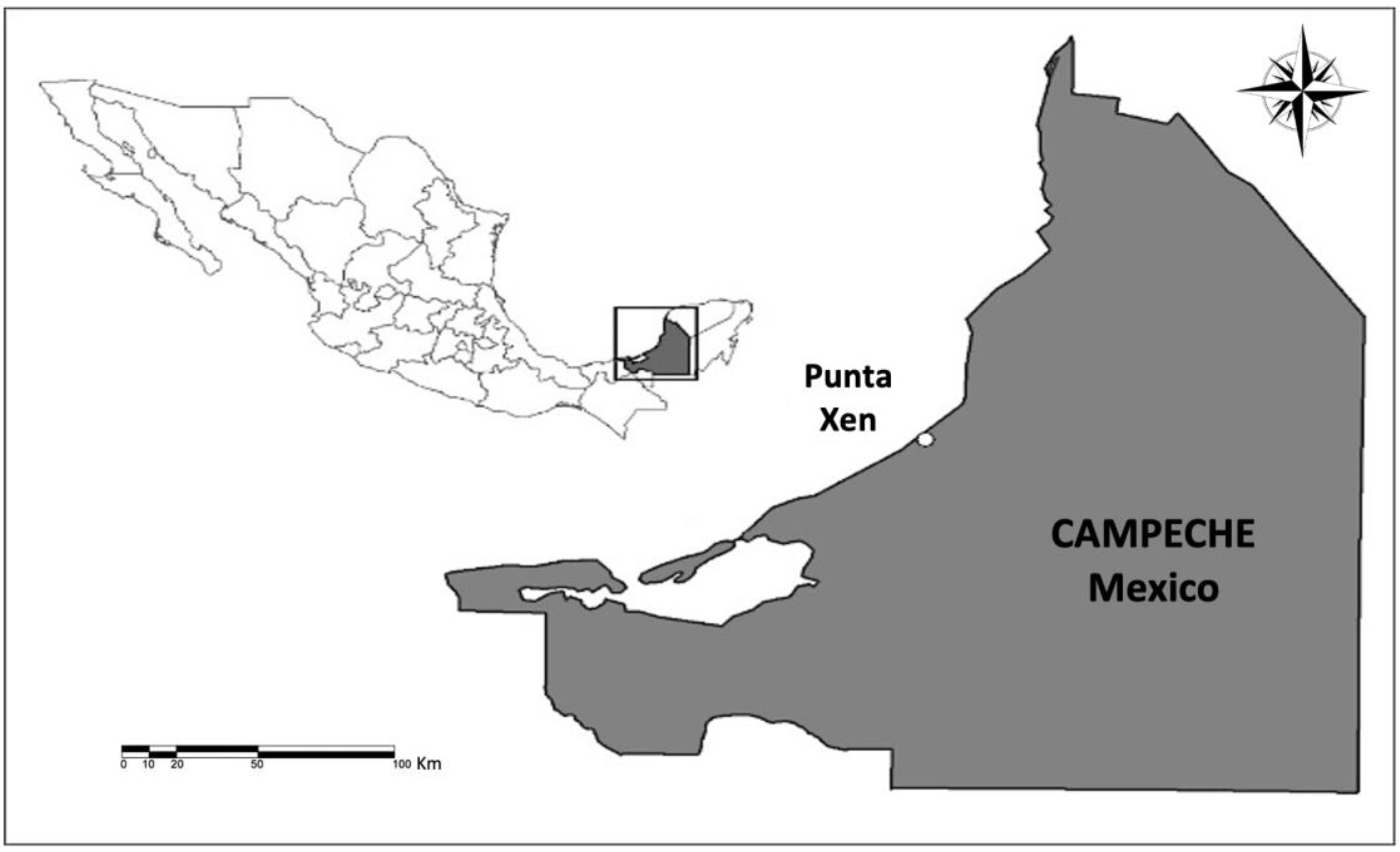
2.1. Study Area
2.2. Blood Sample Collection
2.3. Contaminant Analysis
2.4. Instrumental Analysis
2.5. Statistical Analysis
3. Results
3.1. Concentrations and Patterns
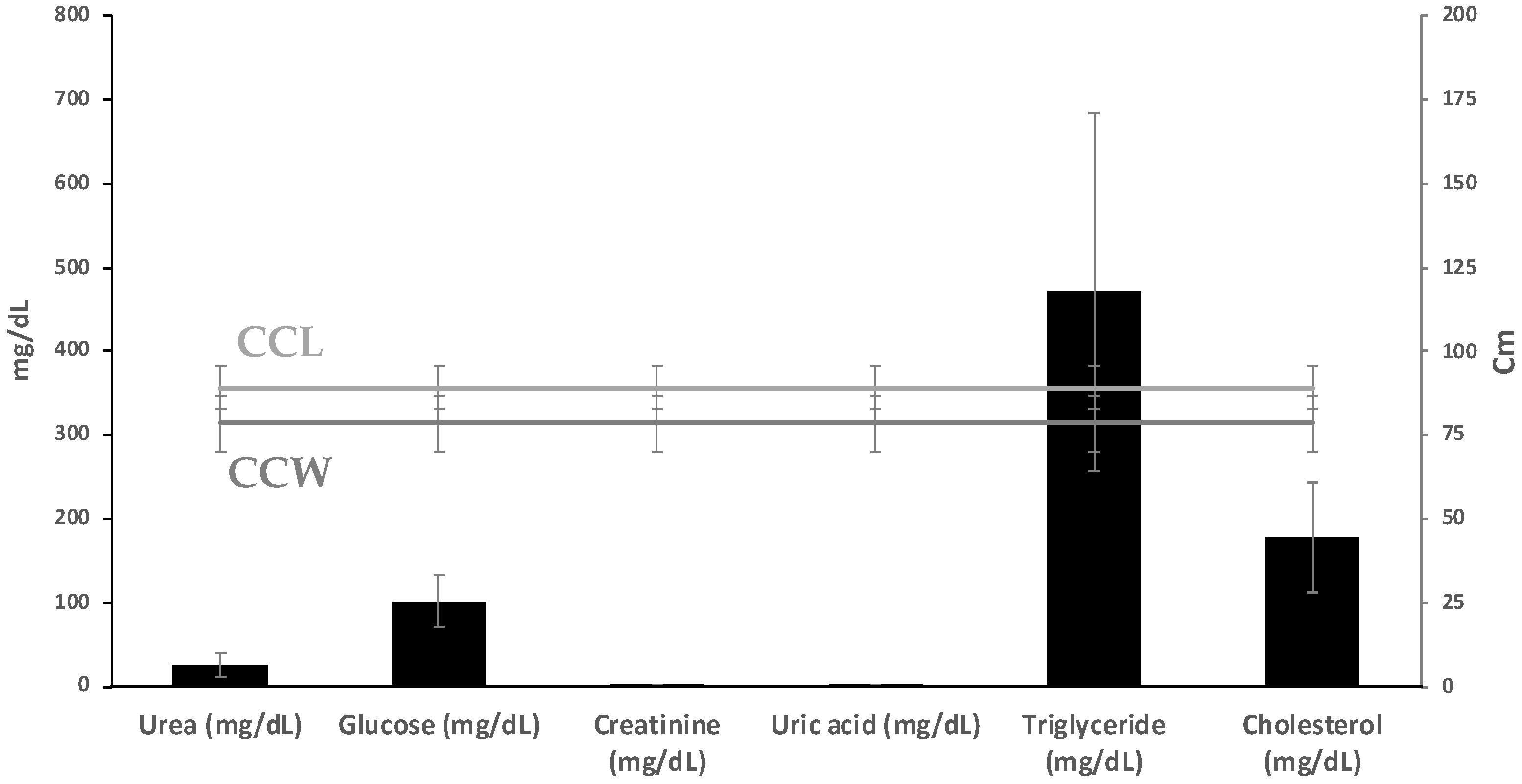
3.2. Hematology and Plasma Biochemistry
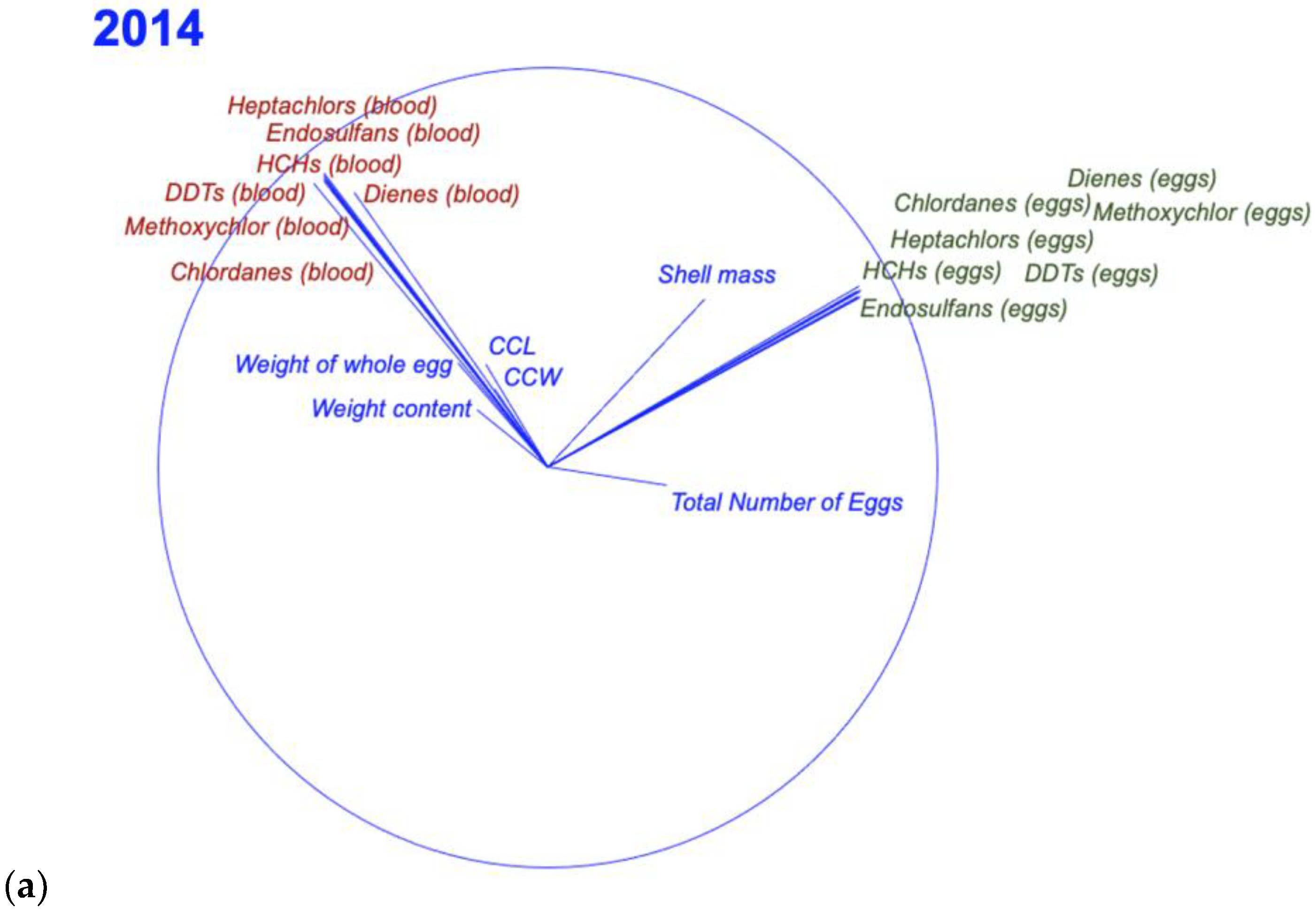
3.3. Relationship between Egg and Blood Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Houtan, K.S.; Hargrove, S.K.; Balazs, G.H. Modeling Sea Turtle Maturity Age from Partial Life History Records. Pac. Sci. 2014, 68, 465–477. [Google Scholar] [CrossRef]
- Secretariat, C.I.T.E.S. Status, scope and trends of the legal and illegal international trade in marine turtles, its conservation impacts, management options and mitigation priorities. In Proceedings of the 18th Meeting of the CITES Conference of the Parties, Geneva, Switzerland, 17–28 August 2019. [Google Scholar]
- Thomson, S.A. Turtles of the World: Annotated Checklist and Atlas of Taxonomy, Synonymy, Distribution, and Conservation Status. Phyllomedusa J. Herpetol. 2021, 20, 225–228. [Google Scholar] [CrossRef]
- Moss, B. Marine Reptiles, Birds and Mammals and Nutrient Transfers among the Seas and the Land: An Appraisal of Current Knowledge. J. Exp. Mar. Biol. Ecol. 2017, 492, 63–80. [Google Scholar] [CrossRef]
- Lovich, J.E.; Ennen, J.R.; Agha, M.; Whitfield Gibbons, J. Where Have All the Turtles Gone, and Why Does It Matter? BioScience 2018, 68, 771–781. [Google Scholar] [CrossRef]
- De Pádua Almeida, A.; Santos, A.J.B.; Thomé, J.C.A.; Belini, C.; Baptistotte, C.; Marcovaldi, M.Â.; dos Santos, A.S.; Lopez, M. Avaliação Do Estado de Conservação Da Tartaruga Marinha Chelonia mydas (Linnaeus, 1758) No Brasil. Biodivers. Bras. 2011, 1, 12–19. [Google Scholar] [CrossRef]
- Casale, P.; Broderick, A.C.; Camiñas, J.A.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.J.; Godley, B.J.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea Turtles: Current Knowledge and Priorities for Conservation and Research. Endanger. Species Res. 2018. [Google Scholar] [CrossRef]
- Eckert, K.; Eckert, A. An Atlas of Sea Turtle Nesting Habitat for the Wider Caribbean Region Revised Edition; WIDECAST Technical Report; WIDECAST: Ballwin, MO, USA, 2019. [Google Scholar]
- Levasseur, K.E.; Stapleton, S.P.; Fuller, M.C.; Quattro, J.M. Exceptionally High Natal Homing Precision in Hawksbill Sea Turtles to Insular Rookeries of the Caribbean. Mar. Ecol. Prog. Ser. 2019, 620, 155–171. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Manuel, S.; Coates, K.A.; Kenworthy, W.J.; Smith, S.R. Effects of Excluding Sea Turtle Herbivores from a Seagrass Bed: Overgrazing May Have Led to Loss of Seagrass Meadows in Bermuda. Mar. Ecol. Prog. Ser. 2010, 419, 223–232. [Google Scholar] [CrossRef]
- Wabnitz, C.C.C.; Balazs, G.; Beavers, S.; Bjorndal, K.A.; Bolten, A.B.; Christensen, V.; Hargrove, S.; Pauly, D. Ecosystem Structure and Processes at Kaloko Honokōhau, Focusing on the Role of Herbivores, Including the Green Sea Turtle Chelonia mydas, in Reef Resilience. Mar. Ecol. Prog. Ser. 2010, 420, 27–44. [Google Scholar] [CrossRef]
- León, Y.M.; Bjorndal, K.A. Selective Feeding in the Hawksbill Turtle, an Important Predator in Coral Reef Ecosystems. Mar. Ecol. Prog. Ser. 2002, 245, 249–258. [Google Scholar] [CrossRef]
- Dodd, C.K.; Dreslik, M.J. Habitat Disturbances Differentially Affect Individual Growth Rates in a Long-Lived Turtle. J. Zool. 2008, 275, 18–25. [Google Scholar] [CrossRef]
- Chaloupka, M.Y.; Musick, J.A. Age, Growth, and Population Dynamics. In The Biology of Sea Turtles, Volume I; CRC Press: London, UK, 2017. [Google Scholar] [CrossRef]
- Kendall, W.L.; Stapleton, S.; White, G.C.; Richardson, J.I.; Pearson, K.N.; Mason, P. A Multistate Open Robust Design: Population Dynamics, Reproductive Effort, and Phenology of Sea Turtles from Tagging Data. Ecol. Monogr. 2019, 89, e01329. [Google Scholar] [CrossRef]
- Strongin, K.; Polidoro, B.; Linardich, C.; Ralph, G.; Saul, S.; Carpenter, K. Translating Globally Threatened Marine Species Information into Regional Guidance for the Gulf of Mexico. Glob. Ecol. Conserv. 2020, 23, e01010. [Google Scholar] [CrossRef]
- Hamann, M.; Godfrey, M.H.; Seminoff, J.A.; Arthur, K.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Broderick, A.C.; Campbell, L.M.; Carreras, C.; et al. Global Research Priorities for Sea Turtles: Informing Management and Conservation in the 21st Century. Endanger. Species Res. 2010, 11, 245–269. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Abreu-Grobois, F.A.; Mortimer, J.A.; Seminoff, J.A.; Amorocho, D.; Bjorndal, K.A.; et al. Global Conservation Priorities for Marine Turtles. PLoS ONE 2011, 6, e24510. [Google Scholar] [CrossRef]
- Rees, A.F.; Alfaro-Shigueto, J.; Barata, P.C.R.; Bjorndal, K.A.; Bolten, A.B.; Bourjea, J.; Broderick, A.C.; Campbell, L.M.; Cardona, L.; Carreras, C.; et al. Are We Working towards Global Research Priorities for Management and Conservation of Sea Turtles? Endanger. Species Res. 2016, 31, 337–382. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2019-2. 2019. Available online: https://www.iucnredlist.org (accessed on 10 November 2022).
- Kouerey Oliwina, C.K.; Honarvar, S.; Girard, A.; Casale, P. (Eds.) Sea Turtles in the West Africa/East Atlantic Region; MTSG Annual Regional Report 2020; IUCN-SSC Marine Turtle Specialist Group: Ross, CA, USA, 2020. [Google Scholar]
- Schofield, G.; Hobson, V.J.; Fossette, S.; Lilley, M.K.S.; Katselidis, K.A.; Hays, G.C. Biodiversity Research: Fidelity to Foraging Sites, Consistency of Migration Routes and Habitat Modulation of Home Range by Sea Turtles. Divers. Distrib. 2010, 16, 840–853. [Google Scholar] [CrossRef]
- Hamann, M.; Schäuble, C.S.; Simon, T.; Evans, S. Demographic and Health Parameters of Green Sea Turtles Chelonia mydas Foraging in the Gulf of Carpentaria, Australia. Endanger. Species Res. 2006, 2, 81–88. [Google Scholar] [CrossRef]
- Wallace, B.P.; DiMatteo, A.D.; Hurley, B.J.; Finkbeiner, E.M.; Bolten, A.B.; Chaloupka, M.Y.; Hutchinson, B.J.; Alberto Abreu-Grobois, F.; Amorocho, D.; Bjorndal, K.A.; et al. Regional Management Units for Marine Turtles: A Novel Framework for Prioritizing Conservation and Research across Multiple Scales. PLoS ONE 2010, 5, e15465. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Schofield, G.; Gkazinou, C.; Almpanidou, V.; Hays, G.C. Global Sea Turtle Conservation Successes. Sci. Adv. 2017, 3, e1600730. [Google Scholar] [CrossRef]
- Esteban, N.; Mortimer, J.A.; Hays, G.C. How Numbers of Nesting Sea Turtles Can Be Overestimated by Nearly a Factor of Two. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.M.; Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Bucklin, D.; Shaver, D.J. Marine Threats Overlap Key Foraging Habitat for Two Imperiled Sea Turtle Species in the Gulf of Mexico. Front. Mar. Sci. 2018, 5, 336. [Google Scholar] [CrossRef]
- Kocmoud, A.R.; Wang, H.H.; Grant, W.E.; Gallaway, B.J. Population Dynamics of the Endangered Kemp’s Ridley Sea Turtle Following the 2010 Oil Spill in the Gulf of Mexico: Simulation of Potential Cause-Effect Relationships. Ecol. Model. 2019, 392, 159–178. [Google Scholar] [CrossRef]
- Leung, M.-R.; Marchand, M.; Stykel, S.; Huynh, M.; Flores, J.D. Effect of Localized Oil Spills on Atlantic Loggerhead Population Dynamics. Open J. Ecol. 2012, 2. [Google Scholar] [CrossRef]
- Iverson, A.R.; Benscoter, A.M.; Fujisaki, I.; Lamont, M.M.; Hart, K.M. Migration Corridors and Threats in the Gulf of Mexico and Florida Straits for Loggerhead Sea Turtles. Front. Mar. Sci. 2020, 7, 208. [Google Scholar] [CrossRef]
- Caillouet, C.W.; Raborn, S.W.; Shaver, D.J.; Putman, N.F.; Gallaway, B.J.; Mansfield, K.L. Did Declining Carrying Capacity for the Kemp’s Ridley Sea Turtle Population within the Gulf of Mexico Contribute to the Nesting Setback in 2010–2017? Chelonian Conserv. Biol. 2018, 17, 123–133. [Google Scholar] [CrossRef]
- Ceriani, S.A.; Casale, P.; Brost, M.; Leone, E.H.; Witherington, B.E. Conservation Implications of Sea Turtle Nesting Trends: Elusive Recovery of a Globally Important Loggerhead Population. Ecosphere 2019, 10, e02936. [Google Scholar] [CrossRef]
- Uribe-Martínez, A.; Liceaga-Correa, M.d.l.A.; Cuevas, E. Critical In-Water Habitats for Post-Nesting Sea Turtles from the Southern Gulf of Mexico. J. Mar. Sci. Eng. 2021, 9, 793. [Google Scholar] [CrossRef]
- Burgess, M.G.; McDermott, G.R.; Owashi, B.; Peavey Reeves, L.E.; Clavelle, T.; Ovando, D.; Wallace, B.P.; Lewison, R.L.; Gaines, S.D.; Costello, C. Protecting Marine Mammals, Turtles, and Birds by Rebuilding Global Fisheries. Science 2018, 359, 1255–1258. [Google Scholar] [CrossRef]
- Scales, K.L.; Hazen, E.L.; Jacox, M.G.; Castruccio, F.; Maxwell, S.M.; Lewison, R.L.; Bograd, S.J. Fisheries Bycatch Risk to Marine Megafauna Is Intensified in Lagrangian Coherent Structures. Proc. Natl. Acad. Sci. USA 2018, 115, 7362–7367. [Google Scholar] [CrossRef]
- Wallace, B.P.; Stacy, B.A.; Cuevas, E.; Holyoake, C.; Lara, P.H.; Marcondes, A.C.J.; Miller, J.D.; Nijkamp, H.; Pilcher, N.J.; Robinson, I.; et al. Oil Spills and Sea Turtles: Documented Effects and Considerations for Response and Assessment Efforts. Endanger. Species Res. 2020, 41, 17–37. [Google Scholar] [CrossRef]
- Bolten, A.B.; Crowder, L.B.; Dodd, M.G.; Macpherson, S.L.; Musick, J.A.; Schroeder, B.A.; Witherington, B.E.; Long, K.J.; Snover, M.L. Quantifying Multiple Threats to Endangered Species: An Example from Loggerhead Sea Turtles. Front. Ecol. Environ. 2011, 9, 295–301. [Google Scholar] [CrossRef]
- Cuevas, E.; de los ángeles Liceaga-Correa, M.; Uribe-Martínez, A. Ecological Vulnerability of Two Sea Turtle Species in the Gulf of Mexico: An Integrated Spatial Approach. Endanger. Species Res. 2019, 40, 337–356. [Google Scholar] [CrossRef]
- Soto, L.A.; Botello, A.V.; Licea-Durán, S.; Lizárraga-Partida, M.L.; Yáñez-Arancibia, A. The Environmental Legacy of the Ixtoc-I Oil Spill in Campeche Sound, Southwestern Gulf of Mexico. Front. Mar. Sci. 2014, 57. [Google Scholar] [CrossRef]
- (NDC) Navigation Data Center. US Waterway Data–Principal Ports of the United States. 2017. Available online: http://www.navigationdatacenter.us/data/datappor.htm (accessed on 25 December 2022).
- [NMFS] National Marine Fisheries Service. Fisheries of the United States–2015; Lowther, A., Liddel, M., Eds.; US Department of Commerce, National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2016. [Google Scholar]
- ECLAC (Economic Commission for Latin America and the Caribbean). CEPALSTAT. 2020. Available online: https://cepalstat-prod.cepal.org/cepalstat/tabulador/ConsultaIntegrada.asp?idIndicador=3961&idioma=e (accessed on 23 November 2020).
- [INEGI] Instituto Nacional de Estadística y Geografía. Anuario Estadístico y Geográfico Por Entidad Federativa; Instituto Nacional de Estadística y Geografía: Mexico City, Mexico, 2015. [Google Scholar]
- Ashford, M.; Watling, J.I.; Hart, K. One Shell of a Problem: Cumulative Threat Analysis of Male Sea Turtles Indicates High Anthropogenic Threat for Migratory Individuals and Gulf of Mexico Residents. Remote Sens. 2022, 14, 3887. [Google Scholar] [CrossRef]
- Ylitalo, G.M.; Collier, T.K.; Anulacion, B.F.; Juaire, K.; Boyer, R.H.; da Silva, D.A.M.; Keene, J.L.; Stacy, B.A. Determining Oil and Dispersant Exposure in Sea Turtles from the Northern Gulf of Mexico Resulting from the Deepwater Horizon Oil Spill. Endanger. Species Res. 2017, 33, 9–24. [Google Scholar] [CrossRef]
- Wallace, B.P.; Stacy, B.A.; Rissing, M.; Cacela, D.; Garrison, L.P.; Graettinger, G.D.; Holmes, J.V.; McDonald, T.; McLamb, D.; Schroeder, B. Estimating Sea Turtle Exposures to Deepwater Horizon Oil. Endanger. Species Res. 2017, 33. [Google Scholar] [CrossRef]
- Wilcox, C.; Puckridge, M.; Schuyler, Q.A.; Townsend, K.; Hardesty, B.D. A Quantitative Analysis Linking Sea Turtle Mortality and Plastic Debris Ingestion. Sci. Rep. 2018, 8, 12536. [Google Scholar] [CrossRef]
- Hart, K.M.; Iverson, A.R.; Fujisaki, I.; Lamont, M.M.; Bucklin, D.; Shaver, D.J. Sympatry or Syntopy? Investigating Drivers of Distribution and Co-Occurrence for Two Imperiled Sea Turtle Species in Gulf of Mexico Neritic Waters. Ecol. Evol. 2018, 8, 12656–12669. [Google Scholar] [CrossRef]
- Lamont, M.M.; Iverson, A.R. Shared Habitat Use by Juveniles of Three Sea Turtle Species. Mar. Ecol. Prog. Ser. 2018, 606. [Google Scholar] [CrossRef]
- Gallaway, B.J.; Gazey, W.J.; Wibbels, T.; Bevan, E.; Shaver, D.J.; George, J. Evaluation of the Status of the Kemp’s Ridley Sea Turtle after the 2010 Deepwater Horizon Oil Spill. Gulf Mex. Sci. 2016, 33. [Google Scholar] [CrossRef]
- García-Besné, G.; Valdespino, C.; Rendón-von Osten, J. Comparison of Organochlorine Pesticides and PCB Residues among Hawksbill (Eretmochelys imbricata) and Green (Chelonia mydas) Turtles in the Yucatan Peninsula and Their Maternal Transfer. Mar. Pollut. Bull. 2015, 91, 139–148. [Google Scholar] [CrossRef]
- Salvarani, P.I.; Vieira, L.R.; Ku-Peralta, W.; Morgado, F.; Osten, J.R.-V. Oxidative Stress Biomarkers and Organochlorine Pesticides in Nesting Female Hawksbill Turtles Eretmochelys imbricata from Mexican Coast (Punta Xen, Mexico). Environ. Sci. Pollut. Res. 2018, 25, 23809–23816. [Google Scholar] [CrossRef] [PubMed]
- Labastida-Estrada, E.; Machkour-M’Rabet, S.; Díaz-Jaimes, P.; Cedeño-Vázquez, J.R.; Hénaut, Y. Genetic Structure, Origin, and Connectivity between Nesting and Foraging Areas of Hawksbill Turtles of the Yucatan Peninsula: A Study for Conservation and Management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 211–222. [Google Scholar] [CrossRef]
- Tremblay, N.; Arana, A.O.; Jáuregui, M.G.; Rendón-von Osten, J. Relationship between Organochlorine Pesticides and Stress Indicators in Hawksbill Sea Turtle (Eretmochelys Imbricata) Nesting at Punta Xen (Campeche), Southern Gulf of Mexico. Ecotoxicology 2016, 26, 173–183. [Google Scholar] [CrossRef]
- Biddiscombe, S.J.; Smith, E.A.; Hawkes, L.A. A Global Analysis of Anthropogenic Development of Marine Turtle Nesting Beaches. Remote Sens. 2020, 12, 1492. [Google Scholar] [CrossRef]
- Bovery, C.M.; Wyneken, J. Seasonal Variation in Sea Turtle Density and Abundance in the Southeast Florida Current and Surrounding Waters. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Wildermann, N.E.; Sasso, C.R.; Stokes, L.W.; Snodgrass, D.; Fuentes, M.M.P.B. Habitat Use and Behavior of Multiple Species of Marine Turtles at a Foraging Area in the Northeastern Gulf of Mexico. Front. Mar. Sci. 2019, 6, 155. [Google Scholar] [CrossRef]
- Keller, J.M.; Kucklick, J.R.; Harms, C.A.; McClellan-Green, P.D. Organochlorine Contaminants in Sea Turtles: Correlations between Whole Blood and Fat. Environ. Toxicol. Chem. 2004, 23, 726–738. [Google Scholar] [CrossRef]
- Shillinger, G.L.; Palacios, D.M.; Bailey, H.; Bograd, S.J.; Swithenbank, A.M.; Gaspar, P.; Wallace, B.P.; Spotila, J.R.; Paladino, F.V.; Piedra, R.; et al. Persistent Leatherback Turtle Migrations Present Opportunities for Conservation. PLoS Biol. 2008, 6, e171. [Google Scholar] [CrossRef]
- Witt, M.; McGowan, A.; Blumenthal, J.; Broderick, A.; Gore, S.; Wheatley, D.; White, J.; Godley, B. Inferring Vertical and Horizontal Movements of Juvenile Marine Turtles from Time-Depth Recorders. Aquat. Biol. 2010, 8, 169–177. [Google Scholar] [CrossRef]
- Witt, M.J.; Bonguno, E.A.; Broderick, A.C.; Coyne, M.S.; Formia, A.; Gibudi, A.; Mounguengui, G.A.M.; Moussounda, C.; Nsafou, M.; Nougessono, S.; et al. Tracking Leatherback Turtles from the World’s Largest Rookery: Assessing Threats across the South Atlantic. Proc. R. Soc. B Biol. Sci. 2011, 278, 2338–2347. [Google Scholar] [CrossRef]
- Nivière, M.; Chambault, P.; Pérez, T.; Etienne, D.; Bonola, M.; Martin, J.; Barnérias, C.; Védie, F.; Mailles, J.; Dumont-Dayot, É.; et al. Identification of Marine Key Areas across the Caribbean to Ensure the Conservation of the Critically Endangered Hawksbill Turtle. Biol. Conserv. 2018, 223, 170–180. [Google Scholar] [CrossRef]
- Guzmán-Hernández, V.; García-Alvarado, P.A. Informe Técnico 2009 Del Programa de Conservación de Tortugas Marinas En Laguna de Términos, Campeche, México. Contiene Inf. 2010, 1. [Google Scholar]
- Shillinger, G.L.; Swithenbank, A.M.; Bograd, S.J.; Bailey, H.; Castelton, M.R.; Wallace, B.P.; Spotila, J.R.; Paladino, F.V.; Piedra, R.; Block, B.A. Identification of High-Use Internesting Habitats for Eastern Pacific Leatherback Turtles: Role of the Environment and Implications for Conservation. Endanger. Species Res. 2010, 10. [Google Scholar] [CrossRef][Green Version]
- Lazar, B.; Maslov, L.; Romanić, S.H.; Gračan, R.; Krauthacker, B.; Holcer, D.; Tvrtković, N. Accumulation of Organochlorine Contaminants in Loggerhead Sea Turtles, Caretta caretta, from the Eastern Adriatic Sea. Chemosphere 2011, 82, 121–129. [Google Scholar] [CrossRef]
- Camacho, M.; Boada, L.D.; Orós, J.; López, P.; Zumbado, M.; Almeida-González, M.; Luzardo, O.P. Comparative Study of Organohalogen Contamination between Two Populations of Eastern Atlantic Loggerhead Sea Turtles (Caretta caretta). Bull. Environ. Contam. Toxicol. 2013, 91, 678–683. [Google Scholar] [CrossRef]
- Alava, J.J.; Keller, J.M.; Kucklick, J.R.; Wyneken, J.; Crowder, L.; Scott, G.I. Loggerhead Sea Turtle (Caretta caretta) Egg Yolk Concentrations of Persistent Organic Pollutants and Lipid Increase during the Last Stage of Embryonic Development. Sci. Total Environ. 2006, 367, 170–181. [Google Scholar] [CrossRef]
- Barraza, A.D.; Komoroske, L.M.; Allen, C.D.; Eguchi, T.; Gossett, R.; Holland, E.; Lawson, D.D.; LeRoux, R.A.; Lorenzi, V.; Seminoff, J.A.; et al. Persistent Organic Pollutants in Green Sea Turtles (Chelonia mydas) Inhabiting Two Urbanized Southern California Habitats. Mar. Pollut. Bull. 2020, 153, 110979. [Google Scholar] [CrossRef]
- Keller, J.M.; Kucklick, J.R.; McClellan-Green, P.D. Organochlorine Contaminants in Loggerhead Sea Turtle Blood: Extraction Techniques and Distribution among Plasma and Red Blood Cells. Arch. Environ. Contam. Toxicol. 2004, 46, 254–264. [Google Scholar] [CrossRef]
- Ragland, J.M.; Arendt, M.D.; Kucklick, J.R.; Keller, J.M. Persistent Organic Pollutants in Blood Plasma of Satellite-tracked Adult Male Loggerhead Sea Turtles (Caretta Caretta). Environ. Toxicol. Chem. 2011, 30, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.C.; Hendriks, A.J.; Ragas, A.M.J.; Vermeiren, P. Internal and Maternal Distribution of Persistent Organic Pollutants in Sea Turtle Tissues: A Meta-Analysis. Environ. Sci. Technol. 2021, 55, 10012–10024. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.L.; Schlenk, D. Accumulation Patterns, Biotransformation Enzymes, and in Vitro Biotransformation of Polychlorinated Biphenyls in Several Species of Sea Turtle. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, S9. [Google Scholar] [CrossRef]
- Monagas, P.; Orós, J.; Araña, J.; González-Díaz, O.M. Organochlorine Pesticide Levels in Loggerhead Turtles (Caretta caretta) Stranded in the Canary Islands, Spain. Mar. Pollut. Bull. 2008, 56, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.G.; Jacobson, E.R.; Bresette, M.J.; Singewald, D.A.; Scarpino, R.A.; Bolten, A.B. Reference Intervals and Relationships between Health Status, Carapace Length, Body Mass, and Water Temperature and Concentrations of Plasma Total Protein and Protein Electrophoretogram Fractions in Atlantic Loggerhead Sea Turtles and Green Turtles. J. Am. Vet. Med. Assoc. 2010, 237, 561–567. [Google Scholar] [CrossRef]
- Anderson, E.T.; Minter, L.J.; Clarke, E.O.; Mroch, R.M.; Beasley, J.F.; Harms, C.A. The Effects of Feeding on Hematological and Plasma Biochemical Profiles in Green (Chelonia mydas) and Kemp’s Ridley (Lepidochelys kempii) Sea Turtles. Vet. Med. Int. 2011, 2011, 890829. [Google Scholar] [CrossRef]
- Espinoza-Romo, B.A.; Sainz-Hernández, J.C.; Ley-Quiñónez, C.P.; Hart, C.E.; Leal-Moreno, R.; Aguirre, A.A.; Zavala-Norzagaray, A.A. Blood Biochemistry of Olive Ridley (Lepidochelys olivacea) Sea Turtles Foraging in Northern Sinaloa, Mexico. PLoS ONE 2018, 13, e0199825. [Google Scholar] [CrossRef]
- Stacy, N.I.; Bjorndal, K.A.; Perrault, J.R.; Martins, H.R.; Bolten, A.B. Blood Analytes of Oceanic-Juvenile Loggerhead Sea Turtles (Caretta caretta) from Azorean Waters: Reference Intervals, Size-Relevant Correlations and Comparisons to Neritic Loggerheads from Western Atlantic Coastal Waters. Conserv. Physiol. 2018, 6, coy006. [Google Scholar] [CrossRef]
- Fleming, K.A.; Perrault, J.R.; Stacy, N.I.; Coppenrath, C.M.; Gainsbury, A.M. Heat, Health and Hatchlings: Associations of in Situ Nest Temperatures with Morphological and Physiological Characteristics of Loggerhead Sea Turtle Hatchlings from Florida. Conserv. Physiol. 2020, 8, coaa046. [Google Scholar] [CrossRef]
- Page-Karjian, A.; Chabot, R.; Stacy, N.I.; Morgan, A.S.; Valverde, R.A.; Stewart, S.; Coppenrath, C.M.; Manire, C.A.; Herbst, L.H.; Gregory, C.R.; et al. Comprehensive Health Assessment of Green Turtles Chelonia mydas Nesting in Southeastern Florida, USA. Endanger. Species Res. 2020, 42, 21–35. [Google Scholar] [CrossRef]
- Keller, J.M.; Ngai, L.; McNeill, J.B.; Wood, L.D.; Stewart, K.R.; O’Connell, S.G.; Kucklick, J.R. Perfluoroalkyl Contaminants in Plasma of Five Sea Turtle Species: Comparisons in Concentration and Potential Health Risks. Environ. Toxicol. Chem. 2012, 31, 1223–1230. [Google Scholar] [CrossRef]
- Moorcroft, P.R.; Lewis, M.A. Mechanistic Home Range Analysis; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Broderick, A.C.; Coyne, M.S.; Fuller, W.J.; Glen, F.; Godley, B.J. Fidelity and Over-Wintering of Sea Turtles. Proc. R. Soc. B Biol. Sci. 2007, 274, 1533–1539. [Google Scholar] [CrossRef]
- Putman, N. Marine Migrations. Curr. Biol. 2018, 28, R972–R976. [Google Scholar] [CrossRef]
- Guirlet, E.; Das, K.; Thomé, J.P.; Girondot, M. Maternal Transfer of Chlorinated Contaminants in the Leatherback Turtles, Dermochelys coriacea, Nesting in French Guiana. Chemosphere 2010, 79, 720–726. [Google Scholar] [CrossRef]
- Storelli, M.M.; Zizzo, N. Occurrence of Organochlorine Contaminants (PCBs, PCDDs and PCDFs) and Pathologic Findings in Loggerhead Sea Turtles, Caretta caretta, from the Adriatic Sea (Mediterranean Sea). Sci. Total Environ. 2014, 472, 855–861. [Google Scholar] [CrossRef]
- Ikonomopoulou, M.P.; Olszowy, H.; Francis, R.; Ibrahim, K.; Whittier, J. Accumulation of Trace Metals in the Embryos and Hatchlings of Chelonia mydas from Peninsular Malaysia Incubated at Different Temperatures. Sci. Total Environ. 2013, 450, 301–306. [Google Scholar] [CrossRef]
- De Andrés, E.; Gómara, B.; González-Paredes, D.; Ruiz-Martín, J.; Marco, A. Persistent Organic Pollutant Levels in Eggs of Leatherback Turtles (Dermochelys coriacea) Point to a Decrease in Hatching Success. Chemosphere 2016, 146, 354–361. [Google Scholar] [CrossRef]
- Stewart, K.R.; Keller, J.M.; Templeton, R.; Kucklick, J.R.; Johnson, C. Monitoring Persistent Organic Pollutants in Leatherback Turtles (Dermochelys coriacea) Confirms Maternal Transfer. Mar. Pollut. Bull. 2011, 62, 1396–1409. [Google Scholar] [CrossRef]
- Escanero-F, G.; Vigilante, S.; Gómez, G.; Frazier, J.; Vázquez, R.; Galicia, E.; Durán, R.; Capurro, L. Informe Anual Del Programa de Protección y Estudio de Las Tortugas Marinas En Isla Aguada-Sabancuy, Campeche, Temporada 1990. In Memorias del IV Taller Regional sobre Programas de Conservación de Tortugas Marinas en la Península de Yucatán; Frazier, J., Ed.; Universidad Autónoma de Yucatán: Mérida, Mexico, 1993; pp. 77–90. ISBN 968-6843-23-X. Available online: https://www.academia.edu/86327550/Memorias_del_IV_Taller_Regional_sobre_Programas_de_Conservaci%C3%B3n_de_Tortugas_Marinas_en_la_Pen%C3%ADnsula_de_Yucat%C3%A1n?f_ri=250538 (accessed on 25 December 2022).
- Salvarani, P.I.; von Osten, J.R.; Morgado, F. Plasma Biochemistry Values in Wild Female Hawksbill Turtle (Eretmochelys imbricata) during Nesting in Mexican Coast. Brazilian J. Vet. Res. Anim. Sci. 2019, 55, e134727. [Google Scholar] [CrossRef]
- Owens, D.W.; Ruiz, G.J. New Methods of Obtaining Blood and Cerebrospinal Fluid from Marine Turtles. Herpetologica 1980, 36, 17–20. [Google Scholar]
- Zhang, H.S.; Wang, Z.P.; Lu, B.; Zhu, C.; Wu, G.H.; Walter, V. Occurrence of Organochlorine Pollutants in the Eggs and Dropping-Amended Soil of Antarctic Large Animals and Its Ecological Significance. Sci. China Ser. D Earth Sci. 2007, 50, 1086–1096. [Google Scholar] [CrossRef]
- Zar, J.H. Biological Analysis; Prentice Hall: Englewood Cliffs, NJ, USA, 1996. [Google Scholar]
- Muñoz, C.C.; Vermeiren, P. Profiles of Environmental Contaminants in Hawksbill Turtle Egg Yolks Reflect Local to Distant Pollution Sources among Nesting Beaches in the Yucatán Peninsula, Mexico. Mar. Environ. Res. 2018, 135, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Abreu-Grobois, F.A.; Guzmán-Hernández, V.; Liceaga-Correa, M.A.; Van Dam, R.P. Post-Nesting Migratory Movements of Hawksbill Turtles Eretmochelys Imbricata in Waters Adjacent to the Yucatan Peninsula, Mexico. Endanger. Species Res. 2008, 10, 123–133. [Google Scholar] [CrossRef]
- Clyde-Brockway, C.E.; Heidemeyer, M.; Paladino, F.V.; Flaherty, E.A. Diet and Foraging Niche Flexibility in Green and Hawksbill Turtles. Mar. Biol. 2022, 169, 108. [Google Scholar] [CrossRef]
- Sánchez-Sarmiento, A.M.; Rossi, S.; Vilca, F.Z.; Thijl Vanstreels, R.E.; Monteiro, S.H.; Vale, L.A.S.; Dos Santos, R.G.; Marigo, J.; Bertozzi, C.P.; Grisi Filho, J.H.H.; et al. Organochlorine Pesticides in Green Sea Turtles (Chelonia Mydas) with and without Fibropapillomatosis Caught at Three Feeding Areas off Brazil. J. Mar. Biol. Assoc. 2017, 97, 215–223. [Google Scholar] [CrossRef]
- Keller, J.M. 11 Exposure to and Effects of Persistent Organic Pollutants. Biol. Sea Turt. 2013, 3, 285. [Google Scholar]
- Van de Merwe, J.P.; Hodge, M.; Whittier, J.M.; Ibrahim, K.; Lee, S.Y. Persistent Organic Pollutants in the Green Sea Turtle Chelonia mydas: Nesting Population Variation, Maternal Transfer, and Effects on Development. Mar. Ecol. Prog. Ser. 2010, 403, 269–278. [Google Scholar] [CrossRef]
- Ehsanpour, M.; Ahmadi, M.R.; Bahri, A.H.; Afkhami, M.; Reich, K.J. Plasma Biochemistry Values in Wild Female Hawksbill Turtles (Eretmochelys imbricata), during Nesting and Foraging Seasons in Qeshm Island, Persian Gulf. Comp. Clin. Path. 2015, 24, 561–566. [Google Scholar] [CrossRef]
- Guillette, L.J., Jr.; Crain, D.A.; Rooney, A.A.; Pickford, D.B. Organization versus Activation: The Role of Endocrine-Disrupting Contaminants (EDCs) during Embryonic Development in Wildlife. Environ. Health Perspect. 1995, 103 (Suppl. 7), 157–164. [Google Scholar]
- Longnecker, M.P.; Klebanoff, M.A.; Zhou, H.; Brock, J.W. Association between Maternal Serum Concentration of the DDT Metabolite DDE and Preterm and Small-for-Gestational-Age Babies at Birth. Lancet 2001, 358, 110–114. [Google Scholar] [CrossRef]
- Schwacke, L.H.; Voit, E.O.; Hansen, L.J.; Wells, R.S.; Mitchum, G.B.; Hohn, A.A.; Fair, P.A. Probabilistic Risk Assessment of Reproductive Effects of Polychlorinated Biphenyls on Bottlenose Dolphins (Tursiops Truncatus) from the Southeast United States Coast. Environ. Toxicol. Chem. 2002, 21, 2752–2764. [Google Scholar] [CrossRef] [PubMed]
- Guillette, L.J., Jr.; Gross, T.S.; Masson, G.R.; Matter, J.M.; Percival, H.F.; Woodward, A.R. Developmental Abnormalities of the Gonad and Abnormal Sex Hormone Concentrations in Juvenile Alligators from Contaminated and Control Lakes in Florida. Environ. Health Perspect. 1994, 102, 680. [Google Scholar] [CrossRef] [PubMed]
- Brasfield, S.M.; Bradham, K.; Wells, J.B.; Talent, L.G.; Lanno, R.P.; Janz, D.M. Development of a Terrestrial Vertebrate Model for Assessing Bioavailability of Cadmium in the Fence Lizard (Sceloporus Undulatus) and in Ovo Effects on Hatchling Size and Thyroid Function. Chemosphere 2004, 54, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, M.; Nel, R.; Bouwman, H. First Report of Metallic Elements in Loggerhead and Leatherback Turtle Eggs from the Indian Ocean. Chemosphere 2018, 197, 716–728. [Google Scholar] [CrossRef]
- Russell, R.W.; Gobas, F.A.P.C.; Haffner, G.D. Maternal Transfer and in Ovo Exposure of Organochlorines in Oviparous Organisms: A Model and Field Verification. Environ. Sci. Technol. 1999, 33, 416–420. [Google Scholar] [CrossRef]
- Seminoff, J.A.; Shanker, K. Marine Turtles and IUCN Red Listing: A Review of the Process, the Pitfalls, and Novel Assessment Approaches. J. Exp. Mar. Bio. Ecol. 2008, 356, 52–68. [Google Scholar] [CrossRef]
- Marsh, H.; Dennis, A.; Hines, H.; Kutt, A.; McDonald, K.; Weber, E.; Williams, S.; Winter, J. Optimizing Allocation of Management Resources for Wildlife. Conserv. Biol. 2007, 21, 387–399. [Google Scholar] [CrossRef]
- Amorocho, D.; Leslie, A.; Fish, M.; Sanjurjo, E.; Amoros, S.; Avila, I.C.; Douthwaite, K. Marine Turtle Action Plan. In WWF Latin America and The Caribbean: 2015–2020; Amorocho, D., Dereix, C.A., Eds.; WWF-Colombia: Cali, Colombia, 2016; 122p, ISBN 978-958-8915-28-9. [Google Scholar]
- Sandbrook, C.; Adams, W.M.; Büscher, B.; Vira, B. Social Research and Biodiversity Conservation. Conserv. Biol. 2013, 27, 1487–1490. [Google Scholar] [CrossRef]
- Mortimer, J.A.; Donnelly, M. Eretmochelys imbricata. IUCN Red List Threat. Species 2008, 2008, e.T8005A12881238. [Google Scholar]


| Parameters | First Year Nesting Season | Second Year Nesting Season | Mann-Whitney U Test | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Min | Max | Mean ± SEM | Min | Max | U | p * | |
| CCL (cm) | 89.953 ± 1.155 | 76 | 101 | 89.233 ± 1.205 | 75.5 | 100.0 | 407 | 0.671 |
| CCW (cm) | 77.881 ± 1.652 | 39 | 90 | 79.550 ± 1.142 | 70.0 | 93.5 | 393.5 | 0.577 |
| Total Eggs | 133.56 ± 3.882 | 83 | 178 | 136.14 ± 5.391 | 87.0 | 194.0 | 416 | 0.765 |
| Weight of Whole Egg (g) | 30.632 ± 0.479 | 25 | 36.2 | 30.680 ± 0.483 | 25.8 | 37.8 | 420.5 | 0.826 |
| Weight Content (g) | 28.413 ± 0.463 | 23.1 | 33.2 | 27.917 ± 0.462 | 23.3 | 35.1 | 387.5 | 0.471 |
| Shell Mass (g) | 2.382 ± 0.068 | 1.8 | 3.3 | 2.763 ± 0.108 | 2.10 | 4.60 | 261 | 0.008 |
| Blood a,1 | Mann–Whitney U Test | Eggs a | Mann–Whitney U Test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OCPs | N | First Year Nesting Season | N Second Year Nesting Season | U | p * | N First Year Nesting Season | N Second Year Nesting Season | U | p * |
| ΣHCHs | 29 | 0.204 ± 0.062 | 30 1.948 ± 0.930 | 314 | 0.060 | 29 0.521 ± 0.066 | 30 3.429 ± 1.711 | 319.5 | 0.071 |
| ΣDienes | 29 | 0.779 ± 0.091 | 30 0.882 ± 0.449 | 273.5 | 0.009 | 29 0.342 ± 0.089 | 30 2.567 ± 1.522 | 302.5 | 0.045 |
| ΣChlordanes | 29 | 0.129 ± 0.058 | 30 0.468 ± 0.211 | 401 | 0.577 | 29 0.221 ± 0.056 | 30 1.488 ± 0.840 | 308 | 0.048 |
| ΣDDTs | 29 | 0.290 ± 0.095 | 30 1.593 ± 0.631 | 311 | 0.047 | 29 0.203 ± 0.066 | 30 2.072 ± 1.177 | 299.5 | 0.034 |
| ΣHeptachlors | 29 | 0.057 ± 0.016 | 30 0.767 ± 0.417 | 418 | 0.792 | 29 0.110 ± 0.029 | 30 1.100 ± 0.603 | 365 | 0.258 |
| ΣEndosulfans | 29 | 0.149 ± 0.051 | 30 1.166 ± 0.545 | 408 | 0.680 | 29 0.208 ± 0.066 | 30 1.681 ± 0.999 | 334.5 | 0.111 |
| Methoxychlor | 29 | 0.080 ± 0.023 | 30 0.566 ± 0.244 | 307.5 | 0.041 | 29 0.079 ± 0.027 | 30 0.677 ± 0.425 | 366 | 0.228 |
| TOTAL | 1.690 ± 0.373 | 7.395 ± 3.378 | 1.688 ± 0.361 | 13.016 ± 7.229 | |||||
| Eggs/Blood Ratio | Between Years (a) | Between Tissues (b) | ||||
|---|---|---|---|---|---|---|
| OCPs | First Year Nesting Season a | Second Year Nesting Season a | F | p * | F | p ** |
| ΣHCHs | 5.838 ± 1.877 | 41.132 ± 36.380 | 254.5 | 0.007 | −2.811 | 0.005 |
| ΣDienes | 0.571 ± 0.170 | 47.587 ± 29.849 | 212.5 | 0.000 | −2.381 | 0.017 |
| ΣChlordanes | 1.302 ± 0.603 | 12.458 ± 10.115 | 301.5 | 0.045 | −1.721 | 0.085 |
| ΣDDTs | 1.817 ± 0.598 | 9.736 ± 6.717 | 310 | 0.049 | −0.654 | 0.513 |
| ΣHeptachlors | 2.328 ± 0.829 | 3.668 ± 1.874 | 334 | 0.069 | −0.872 | 0.383 |
| ΣEndosulfans | 2.843 ± 1.153 | 2.851 ± 2.246 | 354 | 0.157 | −1.038 | 0.299 |
| Methoxychlor | 1.591 ± 0.667 | 3.694 ± 2.684 | 349.5 | 0.064 | −0.698 | 0.485 |
| TOTAL | 2.050 ± 0.5286 | 33.8608 ± 21.8481 | ||||
| OCPs | CCL (cm) | CCW (cm) | Total Eggs | Weight of Whole Egg (g) | Weight Content (g) | Shell (g) |
|---|---|---|---|---|---|---|
| ΣHCHs | 0.022 | 0.036 | 0.119 | 0.159 | 0.110 | 0.270 * |
| ΣDienes | −0.060 | −0.028 | 0.001 | −0.099 | −0.114 | 0.271 * |
| ΣChlordanes | −0.130 | −0.106 | 0.017 | −0.030 | −0.062 | 0.279 * |
| ΣDDTs | −0.026 | −0.226 | 0.096 | −0.270 * | −0.267 * | 0.188 |
| ΣHeptachlors | −0.114 | −0.098 | 0.040 | −0.006 | −0.026 | 0.205 |
| ΣEndosulfans | −0.114 | −0.096 | 0.043 | −0.220 | −0.225 | 0.265 * |
| Methoxychlor | −0.149 | −0.147 | 0.017 | −0.150 | −0.163 | 0.297 * |
| Number of Offspring | Hatching Success | ||
|---|---|---|---|
| OCPs in Blood | ΣHCHs | 0.051 | 0.011 |
| ΣDienes | −0.369 * | −0.349 | |
| ΣChlordanes | 0.299 | −0.130 | |
| ΣDDTs | 0.283 | −0.315 | |
| ΣHeptachlors | 0.269 | −0.234 | |
| ΣEndosulfans | 0.234 | −0.426 * | |
| Methoxychlor | 0.293 | −0.350 * | |
| OCPs in Eggs | ΣHCHs | −0.218 | −0.364 * |
| ΣDienes | 0.031 | 0.112 | |
| ΣChlordanes | 0.236 | 0.271 | |
| ΣDDTs | 0.175 | 0.028 | |
| ΣHeptachlors | 0.121 | 0.070 | |
| ΣEndosulfans | 0.144 | 0.013 | |
| Methoxychlor | 0.042 | 0.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvarani, P.I.; Vieira, L.R.; Rendón-von Osten, J.; Morgado, F. Hawksbill Sea Turtle (Eretmochelys imbricata) Blood and Eggs Organochlorine Pesticides Concentrations and Embryonic Development in a Nesting Area (Yucatan Peninsula, Mexico). Toxics 2023, 11, 50. https://doi.org/10.3390/toxics11010050
Salvarani PI, Vieira LR, Rendón-von Osten J, Morgado F. Hawksbill Sea Turtle (Eretmochelys imbricata) Blood and Eggs Organochlorine Pesticides Concentrations and Embryonic Development in a Nesting Area (Yucatan Peninsula, Mexico). Toxics. 2023; 11(1):50. https://doi.org/10.3390/toxics11010050
Chicago/Turabian StyleSalvarani, Patricia I., Luis R. Vieira, Jaime Rendón-von Osten, and Fernando Morgado. 2023. "Hawksbill Sea Turtle (Eretmochelys imbricata) Blood and Eggs Organochlorine Pesticides Concentrations and Embryonic Development in a Nesting Area (Yucatan Peninsula, Mexico)" Toxics 11, no. 1: 50. https://doi.org/10.3390/toxics11010050
APA StyleSalvarani, P. I., Vieira, L. R., Rendón-von Osten, J., & Morgado, F. (2023). Hawksbill Sea Turtle (Eretmochelys imbricata) Blood and Eggs Organochlorine Pesticides Concentrations and Embryonic Development in a Nesting Area (Yucatan Peninsula, Mexico). Toxics, 11(1), 50. https://doi.org/10.3390/toxics11010050







