Roe Deer (Capreolus capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements
Abstract
1. Introduction
2. Materials and Methods
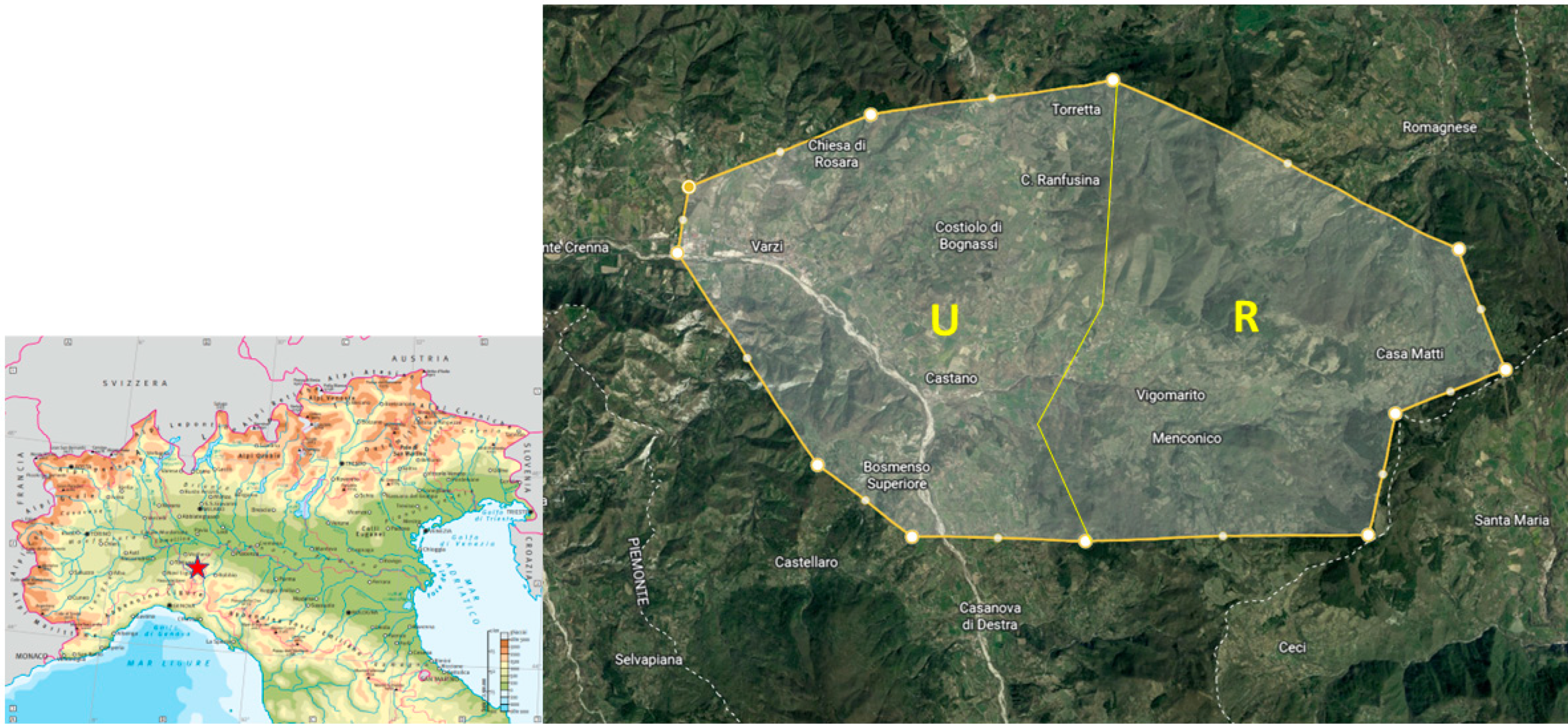
2.1. Sample Collection and Georeferencing
2.2. Trace and Toxic Elements Analysis
2.3. Statistical Analysis
3. Results
3.1. Method Validation
3.2. Average Concentrations of Trace and Toxic Elements
3.3. Average Concentration Comparison between the Rural and Urbanized Area
3.4. Average Concentration Comparison between Age Classes
3.5. Average Concentration Comparison between Sexes
4. Discussion
4.1. Comparison between Rural and Urbanized Areas
4.2. Comparison between the Class of Age
4.3. Comparison between Sexes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FSIN. Global Report on Food Crises 2017; FSIN: Saskatoon, SK, Canada, 2017. [Google Scholar]
- UN-HABITAT. State of the World’s Cities 2008/2009-Harmonious Cities; UN-HABITAT: Nairobi, Kenya, 2012. [Google Scholar]
- Agradi, S.; Gazzonis, A.; Curone, G.; Faustini, M.; Draghi, S.; Brecchia, G.; Vigo, D.; Manfredi, M.; Zanzani, S.; Pulinas, L.; et al. Lactation Characteristics in Alpine and Nera Di Verzasca Goats in Northern Italy: A Statistical Bayesian Approach. Appl. Sci. 2021, 11, 7235. [Google Scholar] [CrossRef]
- Agradi, S.; Menchetti, L.; Curone, G.; Faustini, M.; Vigo, D.; Villa, L.; Zanzani, S.A.; Postoli, R.; Kika, T.S.; Riva, F.; et al. Comparison of Female Verzaschese and Camosciata Delle Alpi Goats’ Hematological Parameters in The Context of Adaptation to Local Environmental Conditions in Semi-Extensive Systems in Italy. Animals 2022, 12, 1703. [Google Scholar] [CrossRef] [PubMed]
- Agradi, S.; Curone, G.; Negroni, D.; Vigo, D.; Brecchia, G.; Bronzo, V.; Panseri, S.; Chiesa, L.M.; Peric, T.; Danes, D.; et al. Determination of Fatty Acids Profile in Original Brown Cows Dairy Products and Relationship with Alpine Pasture Farming System. Animals 2020, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Guénette, J.-D.; Kenworthy, P.; Wheeler, C.; Guénette, J.D. Implications of the War in Ukraine for the Global Economy Equitable Growth, Finance, and Institutons Policy Note Implications of the War in Ukraine for the Global Economy. Available online: https://thedocs.worldbank.org/en/doc/5d903e848db1d1b83e0ec8f744e55570-0350012021/related/Implications-of-the-War-in-Ukraine-for-the-Global-Economy.pdf (accessed on 8 May 2022).
- López-Alonso, M. Trace Minerals and Livestock: Not Too Much Not Too Little. ISRN Vet. Sci. 2012, 2012, 704825. [Google Scholar] [CrossRef]
- McDowell, L.R. Minerals in Animal and Human Nutrition, 1st ed.; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Kisnieriene, V.; Lapeikaite, I.; Kisnierienė, V.; Lapeikaitė, I. When Chemistry Meets Biology: The Case of Aluminium-A Review. Chemija 2015, 26, 148–158. [Google Scholar]
- Kharroubi, W.; Ahmed, S.H.; Nury, T.; Andreoletti, P.; Haouas, Z.; Zarrouk, A.; Sakly, R.; Hammami, M.; Lizard, G. Evidence of Hormesis on Human Neuronal SK-N-BE Cells Treated with Sodium Arsenate: Impact at the Mitochondrial Level. Environ. Sci. Pollut. Res. 2016, 23, 8441–8452. [Google Scholar] [CrossRef]
- Bielicka, A.; Bojanowska, I.; Wiśniewski, A. Two Faces of Chromium-Pollutant and Bioelement. Pol. J. Environ. Stud. 2005, 14, 5–10. [Google Scholar]
- Degeratu, C.N.; Mabilleau, G.; Cincu, C.; Chappard, D. Aluminum Inhibits the Growth of Hydroxyapatite Crystals Developed on a Biomimetic Methacrylic Polymer. J. Trace Elem. Med. Biol. 2013, 27, 346–351. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Sullivan, K.; Irons, D.; McKnight, A. Arsenic, Cadmium, Chromium, Lead, Manganese, Mercury, and Selenium in Feathers of Black-Legged Kittiwake (Rissa tridactyla) and Black Oystercatcher (Haematopus bachmani) from Prince William Sound, Alaska. Sci. Total Environ. 2008, 398, 20–25. [Google Scholar] [CrossRef]
- Spahn, S.; Sherry, T. Cadmium and Lead Exposure Associated with Reduced Growth Rates, Poorer Fledging Success of Little Blue Heron Chicks (Egretta caerulea) in South Louisiana Wetlands. Arch. Env. Contam. Toxicol. 1999, 37, 377–384. [Google Scholar] [CrossRef]
- Tataruch, F.; Kierdorf, H. Chapter 20 Mammals as Biomonitors. In Trace Metals and Other Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2003; pp. 737–772. [Google Scholar]
- Tommasi, F.; Thomas, P.J.; Lyons, D.M.; Pagano, G.; Oral, R.; Siciliano, A.; Toscanesi, M.; Guida, M.; Trifuoggi, M. Evaluation of Rare Earth Element-Associated Hormetic Effects in Candidate Fertilizers and Livestock Feed Additives. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, H. German Appropriate Technology Exchange. In Biological Monitoring: Signals from the Environment, 1st ed.; Informatica International, I., Ed.; Vieweg: Madison, WI, USA, 1991; ISBN 3-528-02302-3. [Google Scholar]
- Burbaitė, L.; Csányi, S. Roe Deer Population and Harvest Changes in Europe. Est. J. Ecol. 2009, 58, 169. [Google Scholar] [CrossRef]
- Pandini, W.; Cesaris, C. Home Range and Habitat Use of Roe Deer (Capreolus capreolus) Reared in Captivity and Released in the Wild. Hystrix Ital. J. Mammal. 1997, 9, 45–50. [Google Scholar]
- Fraser, E.E.; Longstaffe, F.J.; Fenton, M.B. Moulting Matters: The Importance of Understanding Moulting Cycles in Bats When Using Fur for Endogenous Marker Analysis. Can. J. Zool. 2013, 91, 533–544. [Google Scholar] [CrossRef]
- Rendón-Lugo, A.N.; Santiago, P.; Puente-Lee, I.; León-Paniagua, L. Permeability of Hair to Cadmium, Copper and Lead in Five Species of Terrestrial Mammals and Implications in Biomonitoring. Environ. Monit. Assess. 2017, 189, 640. [Google Scholar] [CrossRef]
- Tobin, D.J. Human hair pigmentation–biological aspects. Int. J. Cosmet. Sci. 2008, 30, 233–257. [Google Scholar] [CrossRef]
- Applicability of Human Hair as a Bioindicator for Trace Elements Exposure. Available online: https://www.researchgate.net/publication/235953428_Applicability_of_Human_Hair_as_a_Bioindicator_for_Trace_Elements_Exposure (accessed on 25 October 2022).
- Cygan-Szczegielniak, D.; Stanek, M.; Stasiak, K.; Roślewska, A.; Janicki, B. The Content of Mineral Elements and Heavy Metals in the Hair of Red Deer (Cervus elaphus L.) from Selected Regions of Poland. Folia Biol. 2018, 66, 133–142. [Google Scholar] [CrossRef]
- Rashed, M.N.; Soltan, M.E. Animal Hair as Biological Indicator for Heavy Metal Pollution in Urban and Rural Areas. Environ. Monit. Assess. 2005, 110, 41–53. [Google Scholar] [CrossRef]
- Osweiler, G.D. Toxicology, 1st ed.; Wiley-Blackwell: New York, NY, USA, 1996. [Google Scholar]
- Squadrone, S.; Robetto, S.; Orusa, R.; Griglione, A.; Falsetti, S.; Paola, B.; Abete, M.C. Wildlife Hair as Bioindicators of Metal Exposure. Biol. Trace Elem. Res. 2022, 200, 5073–5080. [Google Scholar] [CrossRef]
- Squadrone, S.; Abete, M.C.; Rizzi, M.; Monaco, G.; Favaro, L. Bioaccumulation of Trace and Non-Trace Elements in Blood and Fibers of Alpacas (Vicugna pacos) That Graze in Italian Pastures. Water Air Soil Pollut. 2018, 229, 41. [Google Scholar] [CrossRef]
- Loh, Z.H.; Ouwerkerk, D.; Klieve, A.V.; Hungerford, N.L.; Fletcher, M.T. Toxin Degradation by Rumen Microorganisms: A Review. Toxins 2020, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Kośla, T.; Skibniewska, E.; Skibniewski, M. The State of Bioelements in the Hair of Free-Ranging European Bisons from Bialowieza Primeval Forest. Pol. J. Vet. Sci. 2011, 14, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Granados, A.M.; Vaquero, M.P. Silicon, Aluminium, Arsenic and Lithium: Essentiality and Human Health Implications. J. Nutr. Health Aging 2002, 6, 154–162. [Google Scholar] [PubMed]
- Kucharzak, E.; Moryl, A.; Szyoiszynski, K.; Jopek, Z. Influence of Environment on Aluminum Content in Game Animal Tissues. Med. Weter 2005, 61, 1277–1279. [Google Scholar]
- Goldberg, S. Interaction of Aluminum and Iron Oxides and Clay Minerals and Their Effect on Soil Physical Properties: A Review. Commun. Soil Sci. Plant Anal. 1989, 20, 1181–1207. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Yu, S.-D.; Hong, Y.-S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–183. [Google Scholar]
- Vetlényi, E.; Rácz, G. A Réz Élettani Funkciója, a Rézfelhalmozódás És a Rézhiány Kóroktani Szerepe. Orv. Hetil. 2020, 161, 1488–1496. [Google Scholar] [CrossRef]
- Perillo, L.; Arfuso, F.; Piccione, G.; Dara, S.; Tropia, E.; Cascone, G.; Licitra, F.; Monteverde, V. Quantification of Some Heavy Metals in Hair of Dairy Cows Housed in Different Areas from Sicily as a Bioindicator of Environmental Exposure—A Preliminary Study. Animals 2021, 11, 2268. [Google Scholar] [CrossRef]
- Filistowicz, A.; Dobrzański, Z.; Przysiecki, P.; Nowicki, S.; Filistowicz, A. Concentration of Heavy Metals in Hair and Skin of Silver and Red Foxes (Vulpes vulpes). Environ. Monit. Assess. 2011, 182, 477–484. [Google Scholar] [CrossRef]
- Montillo, M.; Caslini, C.; Peric, T.; Prandi, A.; Netto, P.; Tubaro, F.; Pedrotti, L.; Bianchi, A.; Mattiello, S. Analysis of 19 Minerals and Cortisol in Red Deer Hair in Two Different Areas of the Stelvio National Park: A Preliminary Study. Animals 2019, 9, 492. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy Metals Levels in Raw Cow Milk and Health Risk Assessment across the Globe: A Systematic Review. Sci. Total Environ. 2021, 751, 141830. [Google Scholar] [CrossRef]
- Metcheva, R.; Yurukova, L.; Teodorova, S.; Nikolova, E. The Penguin Feathers as Bioindicator of Antarctica Environmental State. Sci. Total Environ. 2006, 362, 259–265. [Google Scholar] [CrossRef]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, Its Adverse Health Effects & Oxidative Stress. Indian J. Med. Res. 2008, 128, 412–425. [Google Scholar]
- Kośla, T.; Małgorzata Skibniewska, E.; Skibniewski, M.; Małgorzata Kołnierzak, M.; Lasocka, I.; Kmieć, H. Hair Concentration of Selenium in European Bison in Relation to Sex and Age, with Regard to Liver and Kidney Se Levels. Folia Biol. 2019, 67, 99–108. [Google Scholar] [CrossRef]
- Faye, B.; Seboussi, R.; Askar, M. Trace Elements and Heavy Metals Status in Arabian Camel. In Impact of Pollution on Animal Products; Springer: Dordrecht, The Netherlands, 2008; pp. 97–106. [Google Scholar]
- Pfister, J.A.; Davis, T.Z.; Hall, J.O. Effect of Selenium Concentration on Feed Preferences by Cattle and Sheep1. J. Anim. Sci. 2013, 91, 5970–5980. [Google Scholar] [CrossRef]
- Sach, F.; Yon, L.; Henley, M.D.; Bedetti, A.; Buss, P.; de Boer, W.F.; Dierenfeld, E.S.; Gardner, A.; Langley-Evans, S.C.; Hamilton, E.; et al. Spatial Geochemistry Influences the Home Range of Elephants. Sci. Total Environ. 2020, 729, 139066. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Stella, C.; Mantia, M.; Pederiva, S.; Giordanengo, G.; Pistone, G.; Abete, M.C. Distribution and Bioaccumulation of Trace Elements and Lanthanides in Apples from Northwestern Italy. J. Trace Elem. Med. Biol. 2020, 62, 126646. [Google Scholar] [CrossRef]
- Husson, O. Redox Potential (Eh) and PH as Drivers of Soil/Plant/Microorganism Systems: A Transdisciplinary Overview Pointing to Integrative Opportunities for Agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Panseri, S.; Bonerba, E.; Nobile, M.; di Cesare, F.; Mosconi, G.; Cecati, F.; Arioli, F.; Tantillo, G.; Chiesa, L. Pesticides and Environmental Contaminants in Organic Honeys According to Their Different Productive Areas toward Food Safety Protection. Foods 2020, 9, 1863. [Google Scholar] [CrossRef]
- Cygan-Szczegielniak, D.; Stanek, M.; Giernatowska, E.; Janicki, B. Impact of Breeding Region and Season on the Content of Some Trace Elements and Heavy Metals in the Hair of Cows. Folia Biol. 2014, 62, 164–170. [Google Scholar] [CrossRef]
- Lazarus, M.; Orct, T.; Blanuša, M.; Vicković, I.; Šoštarić, B. Toxic and Essential Metal Concentrations in Four Tissues of Red Deer (Cervus elaphus) from Baranja, Croatia. Food Addit. Contam. Part A 2008, 25, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Demesko, J.; Markowski, J.; Słaba, M.; Hejduk, J.; Minias, P. Age-Related Patterns in Trace Element Content Vary Between Bone and Teeth of the European Roe Deer (Capreolus capreolus). Arch. Environ. Contam. Toxicol. 2018, 74, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Durkalec, M.; Kolacz, R.; Opalinski, S.; Nawrocka, A. Zmudzki Bioaccumulation of Lead, Cadmium and Mercury in Roe Deer and Wild Boars from Areas with Different Levels of Toxic Metal Pollution. Int. J. Environ. Res. 2015, 9, 205–212. [Google Scholar]
- García, M.H.D.M.; Hernández Moreno, D.; Soler Rodríguez, F.; Beceiro, A.L.; Álvarez, L.E.F.; López, M.P. Sex- and Age-Dependent Accumulation of Heavy Metals (Cd, Pb and Zn) in Liver, Kidney and Muscle of Roe Deer (Capreolus capreolus) from NW Spain. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2011, 46, 109–116. [Google Scholar] [CrossRef]
- Cygan-Szczegielniak, D.; Stasiak, K. Effects of Age and Sex on the Content of Heavy Metals in the Hair, Liver and the Longissimus Lumborum Muscle of Roe Deer Capreolus capreolus L. Environ. Sci. Pollut. Res. 2022, 29, 10782–10790. [Google Scholar] [CrossRef] [PubMed]
- French, A.S.; Shaw, D.; Gibb, S.W.; Taggart, M.A. Geochemical Landscapes as Drivers of Trace and Toxic Element Profiles in Wild Red Deer (Cervus elaphus). Sci. Total Environ. 2017, 601–602, 1606–1618. [Google Scholar] [CrossRef]
| Parameters | Assigned Value |
|---|---|
| Plasma gas flow rate | 15 L/min |
| Argon carrier flow rate | 0.5 L/min |
| Sample flow rate | 1.51 L/min |
| The speed of peristaltic pump | 100 rpm |
| RF Power | 1150 W |
| Elements | Wave Length (nm) |
|---|---|
| Aluminium (Al) | 167.070 |
| Arsenic (As) | 189.042 |
| Cadmium (Cd) | 228.802 |
| Chromium (Cr) | 267.716 |
| Copper (Cu) | 324.754 |
| Iron (Fe) | 259.940 |
| Magnesium (Mg) | 285.213 |
| Manganese (Mn) | 257.610 |
| Nickel (Ni) | 341.476 |
| Lead (Pb) | 220.353 |
| Selenium (Se) | 196.090 |
| Zinc (Zn) | 206.200 |
| Elements | Quality Control (QC) | LOD * | LOQ * | Expected Concentration | Measured Concentration (n = 3) (ppm) | Precision (RSD%) |
|---|---|---|---|---|---|---|
| Cr | QC-1 QC-2 | 0.003 | 0.008 | 0.500 1.000 | 0.500 1.000 | 1.632 0.816 |
| Cu | QC-1 QC-2 | 0.002 | 0.006 | 0.500 1.000 | 0.510 1.010 | 1.227 0.538 |
| Fe | QC-1 QC-2 | 0.003 | 0.004 | 0.500 1.000 | 0.490 0.990 | 5.772 0.846 |
| Al | QC-1 QC-2 | 0.001 | 0.003 | 2.500 5.000 | 2.420 5.000 | 0.168 0.144 |
| Ni | QC-1 QC-2 QC-3 QC-4 | 0.000 | 0.002 | 0.050 0.100 0.500 1.000 | 0.050 0.100 0.490 0.990 | 2.817 1.694 0.838 0.124 |
| Zn | QC-1 QC-2 | 0.003 | 0.009 | 0.500 1.000 | 0.540 1.000 | 0.756 0.343 |
| Se | QC-1 QC-2 | 0.000 | 0.001 | 0.050 0.100 | 0.050 0.100 | 1.632 0.816 |
| Mn | QC-1 QC-2 | 0.000 | 0.000 | 0.050 0.100 | 0.050 0.100 | 4.320 1.414 |
| As | QC-1 QC-2 QC-3 QC-4 | 0.000 | 0.001 | 0.050 0.100 0.500 1.000 | 0.050 0.100 0.490 0.960 | 5.684 0.974 4.248 1.274 |
| Mg | QC-1 QC-2 | 0.000 | 0.001 | 0.500 1.000 | 0.490 1.010 | 0.419 1.011 |
| Pd | QC-1 QC-2 | 0.000 | 0.004 | 0.500 1.000 | 0.490 1.010 | 3.332 0.451 |
| Cd | QC-1 QC-2 | 0.008 | 0.014 | 0.500 1.000 | 0.490 1.004 | 0.419 1.044 |
| Concentration (mg·kg−1) | |||
|---|---|---|---|
| Element | Mean ± SD | Median | Minimum-Maximum |
| Cr | 1.61 ± 1.04 | 1.31 | 0.60–6.58 |
| Cu | 7.49 ± 2.09 | 6.95 | 4.71–12.21 |
| Mn | 3.10 ± 2.40 | 2.46 | 0.64–9.71 |
| Se | 1.61 ± 1.23 | 1.58 | 0.09–6.15 |
| As | 1.97 ± 1.69 | 1.42 | 0.05–6.80 |
| Cd | 0.08 ± 0.16 | 0.05 | 0.00–0.99 |
| Ni | 0.82 ± 1.13 | 0.54 | 0.00–6.78 |
| Pb | 1.39 ± 1.63 | 0.99 | 0.01–9.42 |
| Al | 140 ± 280 | 60 | 20–1800 |
| Fe | 160 ± 144 | 110 | 30–670 |
| Mg | 230 ± 120 | 170 | 110–680 |
| Zn | 70 ± 10 | 70 | 50–90 |
| Element | Urbanized | Rural | |
|---|---|---|---|
| Cu | mean ± SD | 7.81 ± 2.04 | 7.18 ± 1.48 |
| median | 7.84 | 6.22 | |
| minimum–maximum | 4.71–12.21 | 4.91–11.40 | |
| Fe ** | mean ± SD | 220 ± 170 | 90 ± 60 |
| median | 170 | 60 | |
| minimum–maximum | 30–670 | 50–200 | |
| Mg * | mean ± SD | 260 ± 140 | 200 ± 100 |
| median | 220 | 160 | |
| minimum–maximum | 140–680 | 110–490 | |
| Mn ** | mean ± SD | 4.35 ± 2.79 | 1.91 ± 1.05 |
| median | 3.58 | 1.75 | |
| minimum–maximum | 1.12–9.71 | 0.64–4.21 | |
| Se | mean ± SD | 1.70 ± 1.42 | 1.52 ± 1.06 |
| median | 1.58 | 1.58 | |
| minimum–maximum | 0.09–6.15 | 0.22–3.15 | |
| Zn | mean ± SD | 70 ± 10 | 70 ± 10 |
| median | 70 | 60 | |
| minimum–maximum | 60–90 | 50–90 | |
| Al * | mean ± SD | 220 ± 390 | 70 ± 60 |
| median | 90 | 50 | |
| minimum–maximum | 30–1800 | 20–240 | |
| As | mean ± SD | 2.26 ± 2.05 | 1.69 ± 1.24 |
| median | 1.54 | 1.34 | |
| minimum–maximum | 0.10–6.79 | 0.05–4.59 | |
| Cd | mean ± SD | 0.10 ± 0.22 | 0.06 ± 0.05 |
| median | 0.05 | 0.04 | |
| minimum–maximum | 0.00–0.99 | 0.00–0.17 | |
| Cr *** | mean ± SD | 2.01 ± 1.33 | 1.23 ± 0.43 |
| median | 1.60 | 1.18 | |
| minimum–maximum | 0.94–6.58 | 2.22 | |
| Ni | mean ± SD | 0.68 ± 0.58 | 0.96 ± 1.49 |
| median | 0.60 | 0.53 | |
| minimum–maximum | 0.00–2.29 | 0.00–6.78 | |
| Pb * | mean ± SD | 1.99 ± 2.09 | 0.83 ± 0.70 |
| median | 1.81 | 0.60 | |
| minimum–maximum | 0.01–9.42 | 0.05–2.25 |
| Element | Aged < 36 months | Aged ≥ 36 months | |
|---|---|---|---|
| Cu | mean ± SD | 6.96 ± 1.61 | 7.90 ± 2.35 |
| median | 6.16 | 7.93 | |
| minimum–maximum | 5.25–11.40 | 4.71–12.21 | |
| Fe | mean ± SD | 120 ± 80 | 180 ± 170 |
| median | 110 | 120 | |
| minimum–maximum | 30–330 | 30–670 | |
| Mg * | mean ± SD | 180 ± 70 | 260 ± 140 |
| median | 160 | 210 | |
| minimum–maximum | 110–360 | 120–680 | |
| Mn | mean ± SD | 2.53 ± 1.57 | 3.54 ± 2.76 |
| median | 2.32 | 2.65 | |
| minimum–maximum | 0.91–6.87 | 0.64–9.71 | |
| Se | mean ± SD | 1.20 ± 0.85 | 1.93 ± 1.40 |
| median | 1.16 | 1.99 | |
| minimum–maximum | 0.09–2.67 | 0.22–6.15 | |
| Zn | mean ± SD | 70 ± 10 | 70 ± 110 |
| median | 60 | 70 | |
| minimum–maximum | 60–90 | 50–90 | |
| Al | mean ± SD | 86 ± 68 | 190 ± 170 |
| median | 60 | 70 | |
| minimum–maximum | 20–240 | 20–180 | |
| As | mean ± SD | 1.93 ± 1.33 | 2.00 ± 1.96 |
| median | 1.42 | 1.33 | |
| minimum–maximum | 0.22–4.24 | 0.05–6.80 | |
| Cd | mean ± SD | 0.067 ± 0.05 | 0.087 ± 0.20 |
| median | 0.057 | 0.03 | |
| minimum–maximum | 0.00–0.17 | 0.00–0.99 | |
| Cr ** | mean ± SD | 1.17 ± 0.36 | 1.96 ± 1.26 |
| median | 1.12 | 1.63 | |
| minimum–maximum | 0.06–1.86 | 0.81–6.58 | |
| Ni | mean ± SD | 0.63 ± 0.57 | 0.97 ± 1.42 |
| median | 0.57 | 0.43 | |
| minimum–maximum | 0.00–2.30 | 0.00–6.78 | |
| Pb | mean ± SD | 1.08 ± 0.86 | 1.63 ± 2.02 |
| median | 1.86 | 1.10 | |
| minimum–maximum | 0.05–2.74 | 0.01–9.42 |
| Element | Female | Male | |
|---|---|---|---|
| Cu * | mean ± SD | 8.32 ± 2.16 | 6.85 ± 1.83 |
| median | 8.39 | 6.12 | |
| minimum–maximum | 4.83–12.04 | 4.71–12.21 | |
| Fe * | mean ± SD | 160 ± 80 | 150 ± 180 |
| median | 170 | 60 | |
| minimum–maximum | 50–370 | 30–670 | |
| Mg * | mean ± SD | 280 ± 150 | 180 ± 70 |
| median | 270 | 170 | |
| minimum–maximum | 130–680 | 110–410 | |
| Mn | mean ± SD | 2.86 ± 2.04 | 3.29 ± 2.68 |
| median | 2.55 | 2.25 | |
| minimum–maximum | 0.79–9.71 | 0.64–9.21 | |
| Se | mean ± SD | 1.98 ± 1.35 | 1.33 ± 1.08 |
| median | 1.58 | 0.74 | |
| minimum–maximum | 0.30–6.15 | 0.09–3.06 | |
| Zn | mean ± SD | 70 ± 10 | 70 ± 10 |
| median | 70 | 60 | |
| minimum–maximum | 50–90 | 50–90 | |
| Al | mean ± SD | 210 ± 410 | 90 ± 80 |
| median | 90 | 60 | |
| minimum–maximum | 30–1800 | 20–330 | |
| As | mean ± SD | 1.45 ± 1.31 | 2.37 ± 1.86 |
| median | 1.13 | 1.63 | |
| minimum–maximum | 0.05–4.58 | 0.10–6.80 | |
| Cd * | mean ± SD | 0.09 ± 0.24 | 0.07 ± 0.05 |
| median | 0.03 | 0.06 | |
| minimum–maximum | 0.00–0.99 | 0.00–0.17 | |
| Cr *** | mean ± SD | 2.13 ± 1.33 | 1.21 ± 0.49 |
| median | 1.86 | 1.13 | |
| minimum–maximum | 1.00–6.58 | 0.60–2.55 | |
| Ni | mean ± SD | 1.01 ± 1.62 | 0.68 ± 0.52 |
| median | 0.51 | 0.56 | |
| minimum–maximum | 0.00–6.78 | 0.00–2.30 | |
| Pb | mean ± SD | 1.80 ± 2.26 | 1.07 ± 0.81 |
| median | 1.23 | 0.95 | |
| minimum–maximum | 0.01–9.42 | 0.05–2.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draghi, S.; Agradi, S.; Riva, F.; Tarhan, D.; Bilgiç, B.; Dokuzeylül, B.; Ercan, A.M.; Or, M.E.; Brecchia, G.; Vigo, D.; et al. Roe Deer (Capreolus capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements. Toxics 2023, 11, 49. https://doi.org/10.3390/toxics11010049
Draghi S, Agradi S, Riva F, Tarhan D, Bilgiç B, Dokuzeylül B, Ercan AM, Or ME, Brecchia G, Vigo D, et al. Roe Deer (Capreolus capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements. Toxics. 2023; 11(1):49. https://doi.org/10.3390/toxics11010049
Chicago/Turabian StyleDraghi, Susanna, Stella Agradi, Federica Riva, Duygu Tarhan, Bengü Bilgiç, Banu Dokuzeylül, Alev Meltem Ercan, Mehmet Erman Or, Gabriele Brecchia, Daniele Vigo, and et al. 2023. "Roe Deer (Capreolus capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements" Toxics 11, no. 1: 49. https://doi.org/10.3390/toxics11010049
APA StyleDraghi, S., Agradi, S., Riva, F., Tarhan, D., Bilgiç, B., Dokuzeylül, B., Ercan, A. M., Or, M. E., Brecchia, G., Vigo, D., Arioli, F., Di Cesare, F., & Curone, G. (2023). Roe Deer (Capreolus capreolus) Hair as a Bioindicator for the Environmental Presence of Toxic and Trace Elements. Toxics, 11(1), 49. https://doi.org/10.3390/toxics11010049











