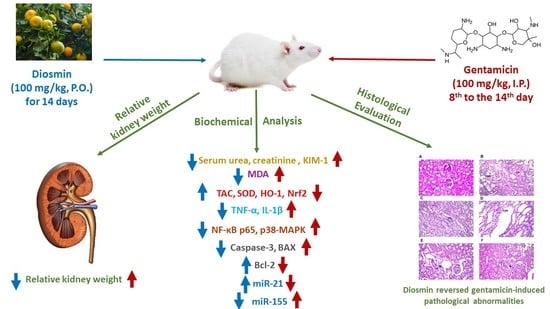
Diosmin Mitigates Gentamicin-Induced Nephrotoxicity in Rats: Insights on miR-21 and -155 Expression, Nrf2/HO-1 and p38-MAPK/NF-κB Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals
2.3. Experiment Design
2.4. Methods
2.4.1. Estimation of Relative Kidney Weight
2.4.2. Measurement of Total Protein
2.4.3. Biochemical Investigation
2.4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.5. Western Blotting
2.4.6. Quantitative Real-Time RT-PCR
2.4.7. Histopathological Examinations
2.5. Statistical Analysis
3. Results
3.1. Measurement of Relative Kidney Weight
3.2. Estimation of Renal Function Markers
3.3. Determination of Renal Oxidative Stress Markers
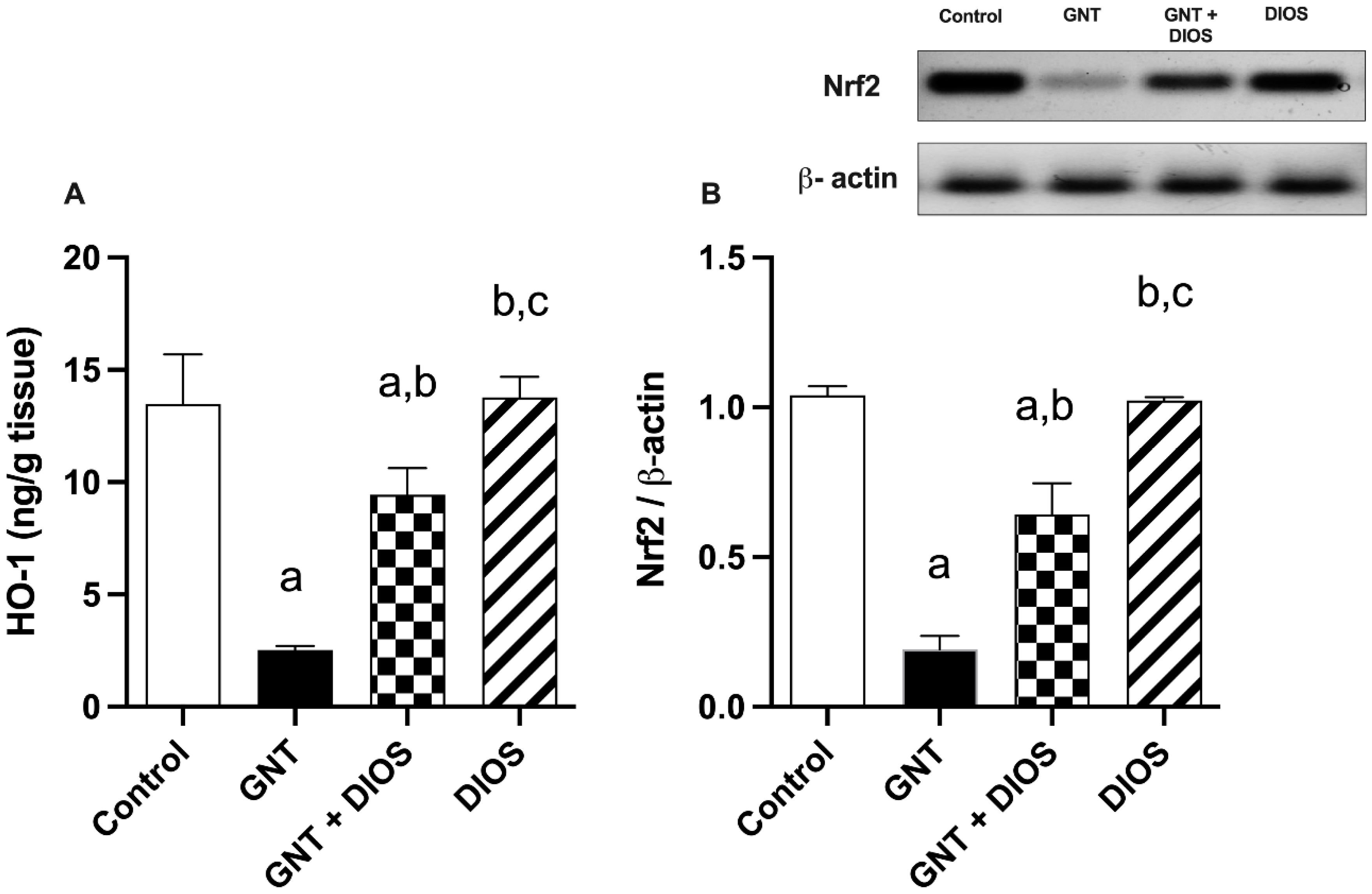
3.4. Assessment of Nrf2 and HO-1 Expression in the Kidney
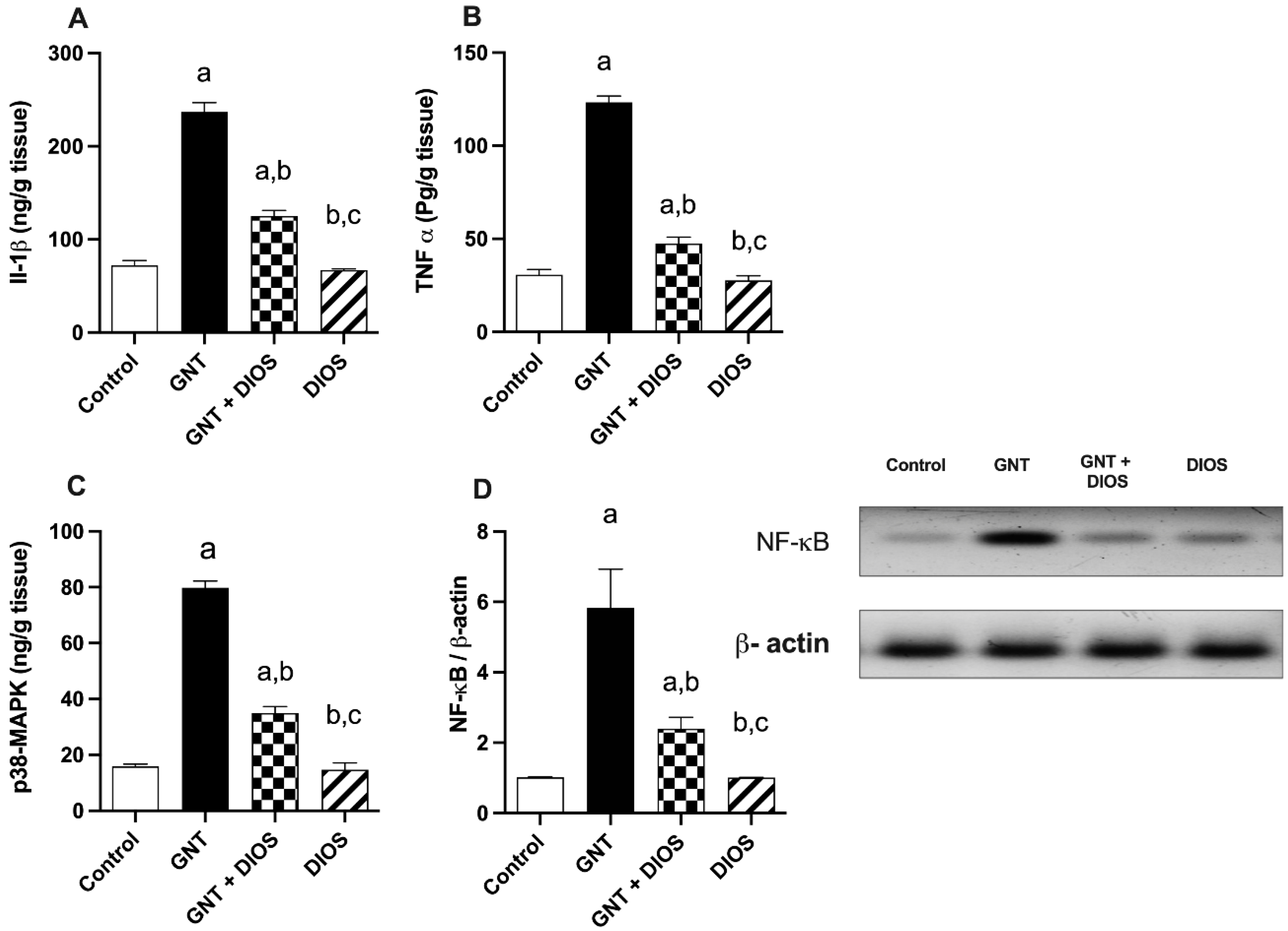
3.5. Evaluation of Inflammatory Markers, p38-MAPK and NF-κB p65 Levels
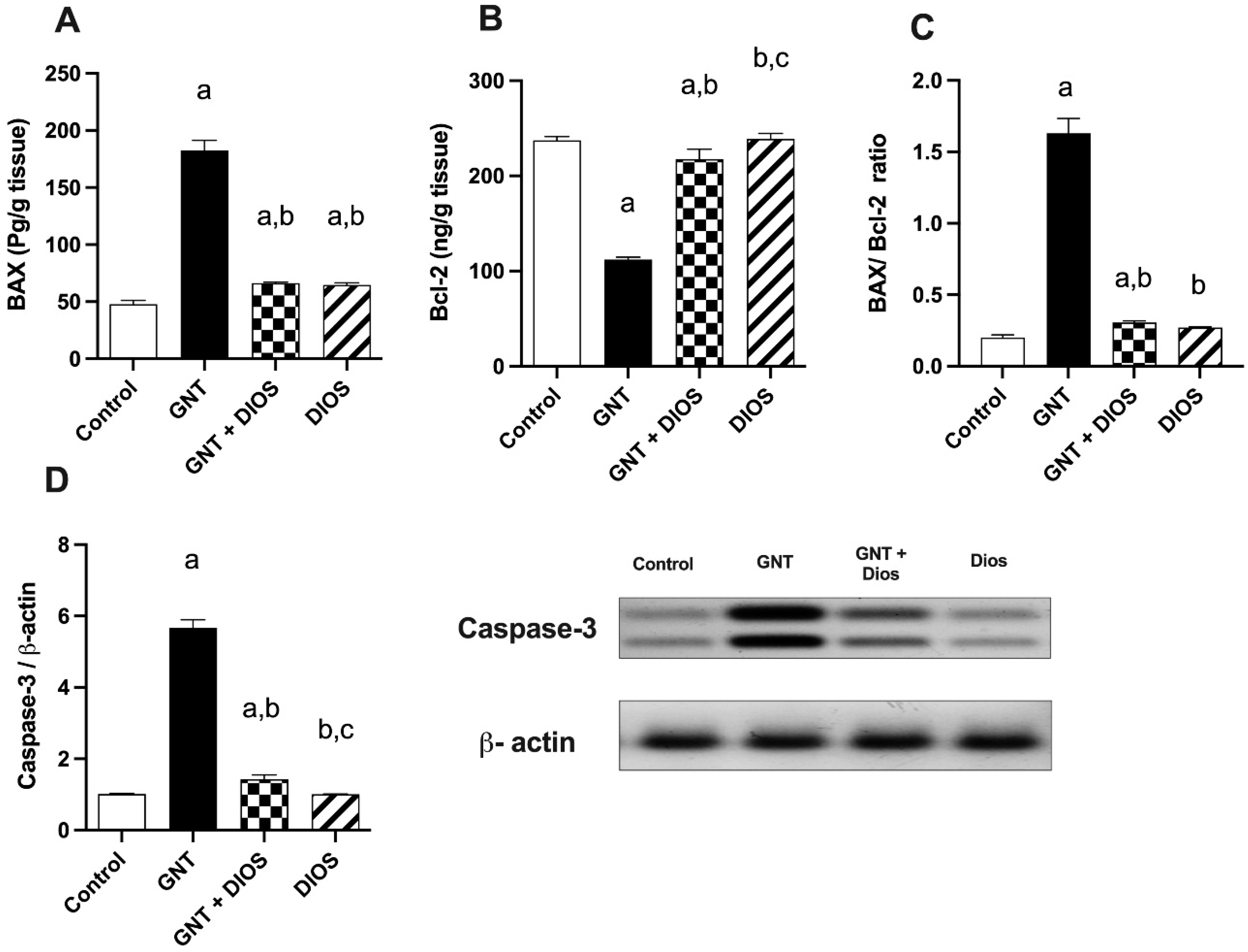
3.6. Estimation of Apoptotic Markers
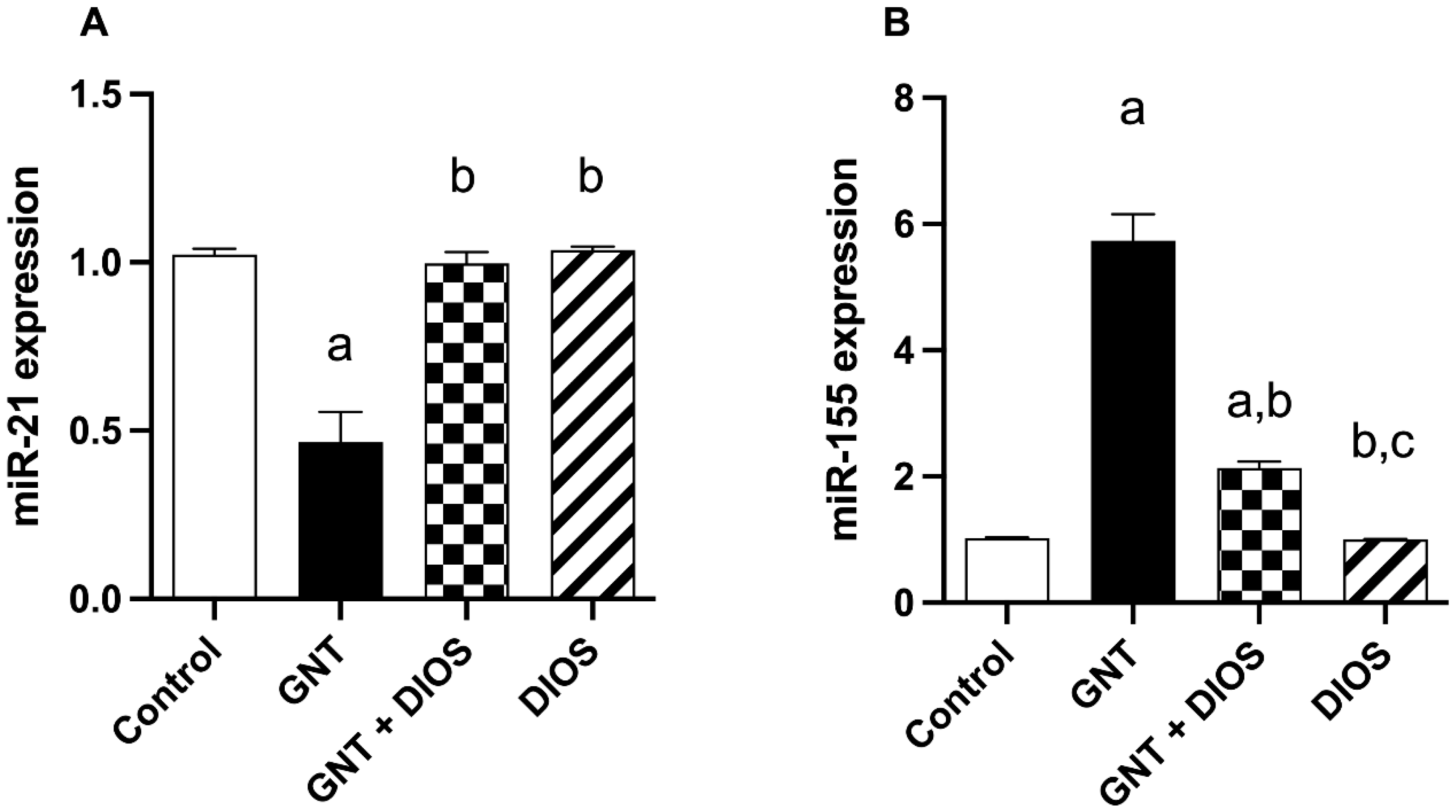
3.7. Effect of Diosmin on microRNAs Expression
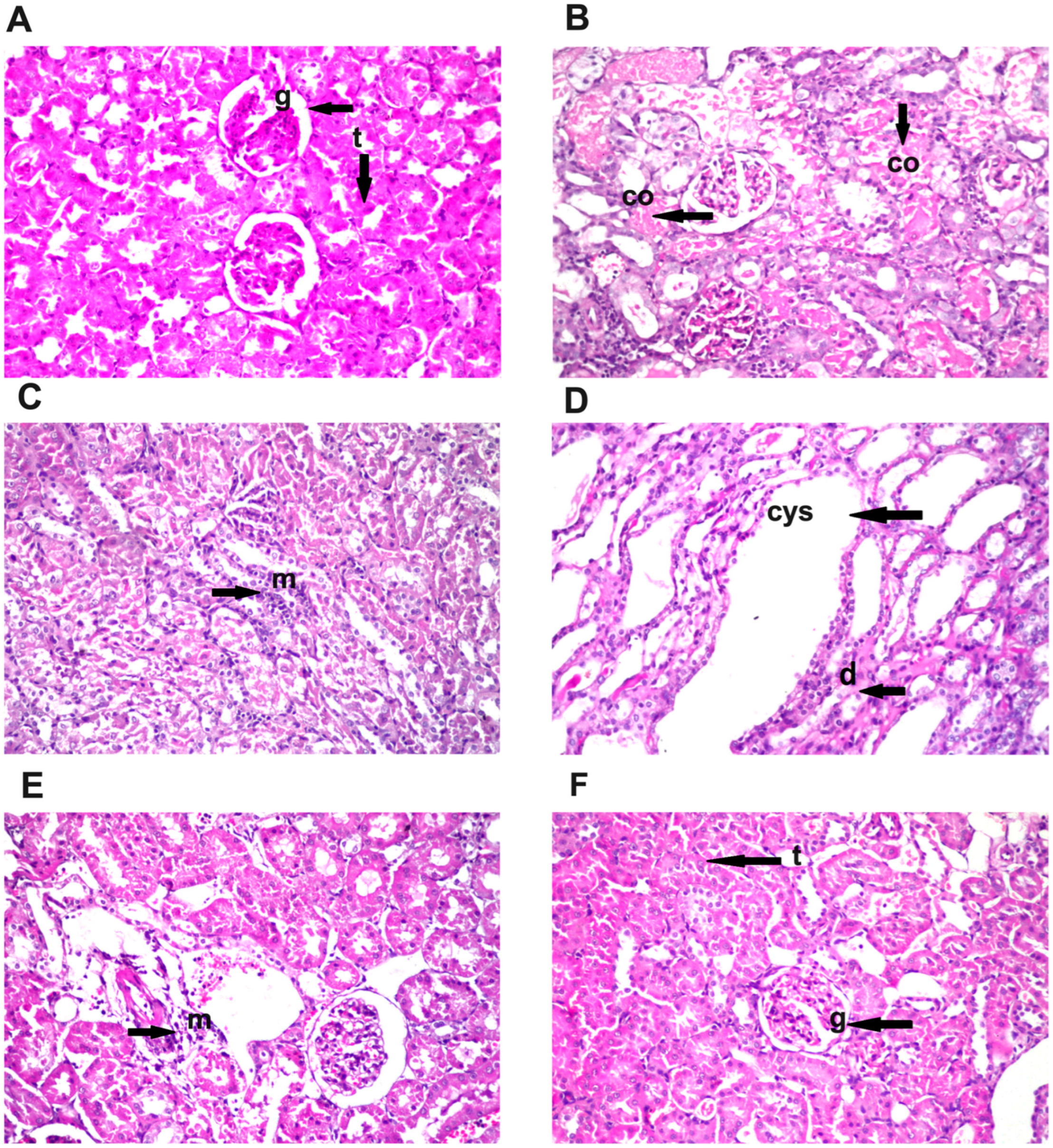
3.8. Effect of Diosmin Supplementation on Kidney Histology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balakumar, P.; Rohilla, A.; Thangathirupathi, A. Gentamicin-Induced Nephrotoxicity: Do We Have a Promising Therapeutic Approach to Blunt It? Pharmacol. Res. 2010, 62, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, N.; Veljkovic, S.; Mihailovic, D.; Stoiljkovic, M.; Radenkovic, M.; Rankovic, G.; Randjelovic, P. Protective Effects of Pentoxifylline Treatment on Gentamicin-Induced Nephrotoxicity in Rats. Ren. Fail. 2009, 31, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Medić, B.; Stojanović, M.; Rovčanin, B.; Kekić, D.; Škodrić, S.R.; Jovanović, G.B.; Vujović, K.S.; Divac, N.; Stojanović, R.; Radenković, M.; et al. Pioglitazone Attenuates Kidney Injury in an Experimental Model of Gentamicin-Induced Nephrotoxicity in Rats. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Udupa, V.; Prakash, V. Gentamicin Induced Acute Renal Damage and Its Evaluation Using Urinary Biomarkers in Rats. Toxicol. Rep. 2019, 6, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Juan, S.H.; Chen, C.H.; Hsu, Y.H.; Hou, C.C.; Chen, T.H.; Lin, H.; Chu, Y.L.; Sue, Y.M. Tetramethylpyrazine Protects Rat Renal Tubular Cell Apoptosis Induced by Gentamicin. Nephrol. Dial. Transplant. 2007, 22, 732–739. [Google Scholar] [CrossRef]
- Lee, I.C.; Kim, S.H.; Lee, S.M.; Baek, H.S.; Moon, C.; Kim, S.H.; Park, S.C.; Kim, H.C.; Kim, J.C. Melatonin Attenuates Gentamicin-Induced Nephrotoxicity and Oxidative Stress in Rats. Arch. Toxicol. 2012, 86, 1527–1536. [Google Scholar] [CrossRef]
- Jaikumkao, K.; Pongchaidecha, A.; Thongnak, L.O.; Wanchai, K.; Arjinajarn, P.; Chatsudthipong, V.; Chattipakorn, N.; Lungkaphin, A. Amelioration of Renal Inflammation, Endoplasmic Reticulum Stress and Apoptosis Underlies the Protective Effect of Low Dosage of Atorvastatin in Gentamicin-Induced Nephrotoxicity. PLoS ONE 2016, 11, e0164528. [Google Scholar] [CrossRef]
- Yu, H.; Chen, B.; Ren, Q. Baicalin Relieves Hypoxia-Aroused H9c2 Cell Apoptosis by Activating Nrf2/HO-1-Mediated HIF1α/BNIP3 Pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3657–3663. [Google Scholar] [CrossRef]
- He, L.; Peng, X.; Zhu, J.; Liu, G.; Chen, X.; Tang, C.; Liu, H.; Liu, F.; Peng, Y. Protective Effects of Curcumin on Acute Gentamicin-Induced Nephrotoxicity in Rats. Can. J. Physiol. Pharmacol. 2015, 93, 275–282. [Google Scholar] [CrossRef]
- Subramanian, P.; Anandan, R.; Jayapalan, J.J.; Hashim, O.H. Hesperidin Protects Gentamicin-Induced Nephrotoxicity via Nrf2/HO-1 Signaling and Inhibits Inflammation Mediated by NF-ΚB in Rats. J. Funct. Foods 2015, 13, 89–99. [Google Scholar] [CrossRef]
- Nassan, M.A.; Soliman, M.M.; Aldhahrani, A.; Althobaiti, F.; Alkhedaide, A.Q. Ameliorative Impacts of Glycyrrhiza Glabra Root Extract against Nephrotoxicity Induced by Gentamicin in Mice. Food Sci. Nutr. 2021, 9, 3405–3413. [Google Scholar] [CrossRef] [PubMed]
- Volpini, R.A.; Balbi, A.P.C.; Costa, R.S.; Coimbra, T.M. Increased Expression of P38 Mitogen-Activated Protein Kinase Is Related to the Acute Renal Lesions Induced by Gentamicin. Braz. J. Med. Biol. Res. 2006, 39, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E.; Cekmen, M.; Ilbey, Y.O.; Simsek, A.; Polat, E.C.; Somay, A. Atorvastatin Prevents Gentamicin-Induced Renal Damage in Rats through the Inhibition of P38-MAPK and NF-KB Pathways. Ren. Fail. 2009, 31, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Luo, N.; Wang, L.; Zhao, Z.; Bu, H.; Xu, G.; Yan, Y.; Che, X.; Jiao, Z.; Zhao, T.; et al. Hydrogen Sulfide Ameliorates Chronic Renal Failure in Rats by Inhibiting Apoptosis and Inflammation through ROS/MAPK and NF-ΚB Signaling Pathways. Sci. Rep. 2017, 7, 455. [Google Scholar] [CrossRef]
- Ahmed, H.I.; Mohamed, E.A. Candesartan and Epigallocatechin-3-Gallate Ameliorate Gentamicin-Induced Renal Damage in Rats through P38-MAPK and NF-ΚB Pathways. J. Biochem. Mol. Toxicol. 2019, 33, e22254. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Jones, T.F.; Bekele, S.; O’Dwyer, M.J.; Prowle, J.R. MicroRNAs in Acute Kidney Injury. Nephron 2018, 140, 124–128. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, A.; Sarkar, D.; Langthasa, M.; Rahman, M.; Bari, M.; Singha, R.K.S.; Malakar, A.K.; Chakraborty, S. Interplay between MiRNAs and Human Diseases. J. Cell. Physiol. 2018, 233, 2007–2018. [Google Scholar] [CrossRef]
- Huang, W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017, 1617, 57–67. [Google Scholar] [CrossRef]
- Suo, A.; Lan, Z.; Lu, C.; Zhao, Z.; Pu, D.; Wu, X.; Jiang, B.; Zhou, N.; Ding, H.; Zhou, D.; et al. Characterizing MicroRNAs and Their Targets in Different Organs of Camellia Sinensis Var. Assamica. Genomics 2021, 113, 159–170. [Google Scholar] [CrossRef]
- Wei, Q.; Mi, Q.S.; Dong, Z. The Regulation and Function of Micrornas in Kidney Diseases. IUBMB Life 2013, 65, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kwan, B.C.H.; Lai, F.M.M.; Chow, K.M.; Li, P.K.T.; Szeto, C.C. Elevated Levels of MiR-146a and MiR-155 in Kidney Biopsy and Urine from Patients with IgA Nephropathy. Dis. Markers 2011, 30, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Saikumar, J.; Hoffmann, D.; Kim, T.M.; Gonzalez, V.R.; Zhang, Q.; Goering, P.L.; Brown, R.P.; Bijol, V.; Park, P.J.; Waikar, S.S.; et al. Expression, Circulation, and Excretion Profile of MicroRNA-21, -155, and -18a Following Acute Kidney Injury. Toxicol. Sci. 2012, 129, 256–267. [Google Scholar] [CrossRef]
- Glowacki, F.; Savary, G.; Gnemmi, V.; Buob, D.; van der Hauwaert, C.; Lo-Guidice, J.M.; Bouyé, S.; Hazzan, M.; Pottier, N.; Perrais, M.; et al. Increased Circulating MiR-21 Levels Are Associated with Kidney Fibrosis. PLoS ONE 2013, 8, e58014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hasni, S.A.; Perez, P.; Tandon, M.; Jang, S.I.; Zheng, C.; Kopp, J.B.; Austin, H.; Balow, J.E.; Alevizos, I.; et al. MiR-150 Promotes Renal Fibrosis in Lupus Nephritis by Downregulating SOCS1. J. Am. Soc. Nephrol. 2013, 24, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, R.; Peng, H.; Liu, H.; Wen, L.; Wu, T.; Yi, H.; Li, A.; Zhang, Z. MiR-451 Suppresses the NF-KappaB-Mediated Proinflammatory Molecules Expression through Inhibiting LMP7 in Diabetic Nephropathy. Mol. Cell. Endocrinol. 2016, 433, 75–86. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and Pharmacological Activities of the Natural Health-Benefiting Compound Diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef]
- Rehman, M.U.; Tahir, M.; Quaiyoom Khan, A.; Khan, R.; Lateef, A.; Hamiza, O.O.; Ali, F.; Sultana, S. Diosmin Protects against Trichloroethylene-Induced Renal Injury in Wistar Rats: Plausible Role of P53, Bax and Caspases. Br. J. Nutr. 2013, 110, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.E.M.; Bakr, A.G.; Abo-youssef, A.M.; Azouz, A.A.; Hemeida, R.A.M. Targeting Keap-1/Nrf-2 Pathway and Cytoglobin as a Potential Protective Mechanism of Diosmin and Pentoxifylline against Cholestatic Liver Cirrhosis. Life Sci. 2018, 207, 50–60. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Azouz, A.A.; Bakr, A.G.; Abo-youssef, A.M.; Hemeida, R.A.M. Hepatoprotective Effects of Diosmin and/or Sildenafil against Cholestatic Liver Cirrhosis: The Role of Keap-1/Nrf-2 and P38-MAPK/NF-ΚB/INOS Signaling Pathway. Food Chem. Toxicol. 2018, 120, 294–304. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, M.H.; Cheng, J.T.; Wu, M.C. Antihyperglycaemic Action of Diosmin, a Citrus Flavonoid, Is Induced through Endogenous β-Endorphin in Type I-like Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2017, 44, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-Induced Senescence, Apoptosis and Autophagy in Breast Cancer Cells of Different P53 Status and ERK Activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Villalobos, A.I.; González-Trujano, M.E.; Pellicer, F.; Alvarado-Vásquez, N.; López-Muñoz, F.J. Central and Peripheral Anti-Hyperalgesic Effects of Diosmin in a Neuropathic Pain Model in Rats. Biomed. Pharmacother. 2018, 97, 310–320. [Google Scholar] [CrossRef]
- El-Fawal, R.; el Fayoumi, H.M.; Mahmoud, M.F. Diosmin and Crocin Alleviate Nephropathy in Metabolic Syndrome Rat Model: Effect on Oxidative Stress and Low Grade Inflammation. Biomed. Pharmacother. 2018, 102, 930–937. [Google Scholar] [CrossRef]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary Plants for the Prevention and Management of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef]
- Schlottfeldt, F.D.S.; Fernandes, S.M.; Martins, D.M.; Cordeiro, P.; da Fonseca, C.D.; Watanabe, M.; Vattimo, M.D.F.F. Prevention of Amphotericin B Nephrotoxicity through Use of Phytotherapeutic Medication. Rev. Da Esc. De Enferm. 2015, 49, 73–78. [Google Scholar] [CrossRef]
- Ahmed, S.; Mundhe, N.; Borgohain, M.; Chowdhury, L.; Kwatra, M.; Bolshette, N.; Ahmed, A.; Lahkar, M. Diosmin Modulates the NF-KB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation 2016, 39, 1783–1797. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Khalifa, H.A.; Abushouk, A.I.; Dkhil, M.A.; Al-Quraishy, S.A. Diosmin Attenuates Methotrexate-Induced Hepatic, Renal, and Cardiac Injury: A Biochemical and Histopathological Study in Mice. Oxid. Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Sayed, A.M.; El-Bahrawy, A.H.; Omar, Z.M.M.; Hassanein, E.H.M. Targeting KEAP1/Nrf2, AKT, and PPAR-γ Signals as a Potential Protective Mechanism of Diosmin against Gentamicin-Induced Nephrotoxicity. Life Sci. 2021, 275, 119349. [Google Scholar] [CrossRef]
- Ali, N.; AlAsmari, A.F.; Imam, F.; Ahmed, M.Z.; Alqahtani, F.; Alharbi, M.; AlSwayyed, M.; AlAsmari, F.; Alasmari, M.; Alshammari, A.; et al. Protective effect of diosmin against doxorubicin-induced nephrotoxicity. Saudi J. Biol. Sci. 2021, 28, 4375–4383. [Google Scholar] [CrossRef]
- El-Agroudy, N.N.; El-Naga, R.N.; El-Razeq, R.A.; El-Demerdash, E. Forskolin, a Hedgehog Signalling Inhibitor, Attenuates Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Br. J. Pharmacol. 2016, 173, 3248–3260. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Abo-Youssef, A.M.; Khalaf, M.M.; Abo-Saif, A.A.; Saleh, I.G.; Abdelghany, T.M. Vitamin E Mitigates Cisplatin-Induced Nephrotoxicity Due to Reversal of Oxidative/Nitrosative Stress, Suppression of Inflammation and Reduction of Total Renal Platinum Accumulation. J. Biochem. Mol. Toxicol. 2017, 31, e21833-9. [Google Scholar] [CrossRef]
- Jung, D.; Biggs, H.; Erikson, J.; Ledyard, P.U. New Colorimetric Reaction for End Point, Continuous Flow, and Kinetic Measurement of Urea. Clin. Chem. 1975, 21, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhang, S.X.; Mott, R.; Knapp, R.R.; Cao, W.; Lau, K.; Ma, J.X. Salutary Effect of Pigment Epithelium-Derived Factor in Diabetic Nephropathy: Evidence for Antifibrogenic Activities. Diabetes 2006, 55, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Nishikimi, M.; Roa, N.A.; Yogi, K. Determination of Superoxide Dismutase in Tissue Homogenate. Biochem. Bioph. Res. Common. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the Measurement of Antioxidant Activity in Human Fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gamble, M. The Hematoxylins and Eosin. In Theory and Practice of Histological Techniques; Bancroft, J., Gamble, M., Eds.; Churchill Livingstone Elsevier: Phiadelphia, PA, USA, 2008; pp. 121–134. [Google Scholar]
- Perazella, M.A. Pharmacology behind Common Drug Nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908. [Google Scholar] [CrossRef]
- Randjelović, P.; Veljković, S.; Stojiljković, N.; Sokolović, D.; Ilić, I. Gentamicin Nephrotoxicity in Animals: Current Knowledge and Future Perspectives. EXCLI J. 2017, 16, 388–399. [Google Scholar]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood Kidney Injury Molecule-1 Is a Biomarker of Acute and Chronic Kidney Injury and Predicts Progression to ESRD in Type I Diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Naimi, M.S. Renoprotective Effect of Irbesartan in a Rat Model of Gentamicin-Induced Nephrotoxicity: Role of Oxidative Stress. J. Lab. Physicians 2019, 11, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Igwebuike, C.; Yaglom, J.; Huiting, L.; Feng, H.; Campbell, J.D.; Wang, Z.; Havasi, A.; Pimentel, D.; Sherman, M.Y.; Borkan, S.C. Cross Organelle Stress Response Disruption Promotes Gentamicin-Induced Proteotoxicity. Cell Death Dis. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bulboacă, A.E.; Porfire, A.; Bolboacă, S.D.; Nicula, C.A.; Feștilă, D.G.; Roman, A.; Râjnoveanu, R.M.; Râjnoveanu, A.; Dogaru, G.; Boarescu, P.M.; et al. Protective Effects of Liposomal Curcumin on Oxidative Stress/Antioxidant Imbalance, Metalloproteinases 2 and -9, Histological Changes and Renal Function in Experimental Nephrotoxicity Induced by Gentamicin. Antioxidants 2021, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.S.; Jiang, W.P.; Chen, C.C.; Lee, L.Y.; Li, P.Y.; Huang, W.C.; Liao, J.C.; Chen, H.Y.; Huang, S.S.; Huang, G.J. Cordyceps Cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-κB/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxid. Med. Cell. Longev. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Cao, L.; Zhi, D.; Han, J.; Kumar Sah, S.; Xie, Y. Combinational Effect of Curcumin and Metformin against Gentamicin-Induced Nephrotoxicity: Involvement of Antioxidative, Anti-Inflammatory and Antiapoptotic Pathway. J. Food Biochem. 2019, 43, e12836. [Google Scholar] [CrossRef]
- Servais, H.; Ortiz, A.; Devuyst, O.; Denamur, S.; Tulkens, P.M.; Mingeot-Leclercq, M.P. Renal Cell Apoptosis Induced by Nephrotoxic Drugs: Cellular and Molecular Mechanisms and Potential Approaches to Modulation. Apoptosis 2008, 13, 11–32. [Google Scholar] [CrossRef]
- El Gamal, A.A.; Alsaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta Vulgaris L.) Extract Ameliorates Gentamicin-Induced Nephrotoxicity Associated Oxidative Stress, Inflammation, and Apoptosis in Rodent Model. Mediat. Inflamm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Sahu, B.D.; Tatireddy, S.; Koneru, M.; Borkar, R.M.; Kumar, J.M.; Kuncha, M.; Srinivas, R.; Shyam Sunder, R.; Sistla, R. Naringin Ameliorates Gentamicin-Induced Nephrotoxicity and Associated Mitochondrial Dysfunction, Apoptosis and Inflammation in Rats: Possible Mechanism of Nephroprotection. Toxicol. Appl. Pharmacol. 2014, 277, 8–20. [Google Scholar] [CrossRef]
- Shalkami, A.S.; Hassan, M.I.A.; Bakr, A.G. Anti-Inflammatory, Antioxidant and Anti-Apoptotic Activity of Diosmin in Acetic Acid-Induced Ulcerative Colitis. Hum. Exp. Toxicol. 2018, 37, 78–86. [Google Scholar] [CrossRef]
- Mahgoub, S.; Sallam, A.O.; Sarhan, H.K.A.; Ammar, A.A.A.; Soror, S.H. Role of Diosmin in protection against the oxidative stress induced damage by gamma-radiation in Wistar albino rats. Regul. Toxicol. Pharmacol. 2020, 113, 1–11. [Google Scholar] [CrossRef]
- Kumar, D.; Singla, S.K.; Puri, V.; Puri, S. The Restrained Expression of NF-KB in Renal Tissue Ameliorates Folic Acid Induced Acute Kidney Injury in Mice. PLoS ONE 2015, 10, e115947. [Google Scholar] [CrossRef] [PubMed]
- Sherif, I.O.; Al-Mutabagani, L.A.; Alnakhli, A.M.; Sobh, M.A.; Mohammed, H.E. Renoprotective Effects of Angiotensin Receptor Blocker and Stem Cells in Acute Kidney Injury: Involvement of Inflammatory and Apoptotic Markers. Exp. Biol. Med. 2015, 240, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahmad, S.F.; Attia, S.M.; Alsaad, A.M.S.; Bakheet, S.A. Sinapic Acid Ameliorate Cadmium-Induced Nephrotoxicity: In Vivo Possible Involvement of Oxidative Stress, Apoptosis, and Inflammation via NF-ΚB Downregulation. Environ. Toxicol. Pharmacol. 2017, 51, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Ali, F.E.M.; Kozman, M.R.; Abd El-Ghafar, O.A.M. Umbelliferone Attenuates Gentamicin-Induced Renal Toxicity by Suppression of TLR-4/NF-ΚB-P65/NLRP-3 and JAK1/STAT-3 Signaling Pathways. Environ. Sci. Pollut. Res. 2021, 28, 11558–11571. [Google Scholar] [CrossRef] [PubMed]
- Babaeenezhad, E.; Hadipour Moradi, F.; Rahimi Monfared, S.; Fattahi, M.D.; Nasri, M.; Amini, A.; Dezfoulian, O.; Ahmadvand, H. D-Limonene Alleviates Acute Kidney Injury Following Gentamicin Administration in Rats: Role of NF- κ B Pathway, Mitochondrial Apoptosis, Oxidative Stress, and PCNA. Oxid. Med. Cell. Longev. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Gutiérrez-Escolano, A.; Santacruz-Vázquez, E.; Gómez-Pérez, F. Dysregulated MicroRNAs Involved in Contrast-Induced Acute Kidney Injury in Rat and Human. Ren. Fail. 2015, 37, 1498–1506. [Google Scholar] [CrossRef]
- Liu, X.J.; Hong, Q.; Wang, Z.; Yu, Y.Y.; Zou, X.; Xu, L.H. MicroRNA-34a Suppresses Autophagy in Tubular Epithelial Cells in Acute Kidney Injury. Am. J. Nephrol. 2015, 42, 168–175. [Google Scholar] [CrossRef]
- Li, Y.F.; Jing, Y.; Hao, J.; Frankfort, N.C.; Zhou, X.; Shen, B.; Liu, X.; Wang, L.; Li, R. MicroRNA-21 in the Pathogenesis of Acute Kidney Injury. Protein Cell 2013, 4, 813–819. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, W.; Xi, X.; Zou, C.; Ye, Z. MicroRNA-21 Attenuates Renal Ischemia Reperfusion Injury via Targeting Caspase Signaling in Mice. Am. J. Nephrol. 2014, 40, 215–223. [Google Scholar] [CrossRef]
- Godwin, J.G.; Ge, X.; Stephan, K.; Jurisch, A.; Tullius, S.G.; Iacomini, J. Identification of a MicroRNA Signature of Renal Ischemia Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2010, 107, 14339–14344. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kriegel, A.J.; Liu, Y.; Usa, K.; Mladinov, D.; Liu, H.; Fang, Y.; Ding, X.; Liang, M. Delayed Ischemic Preconditioning Contributes to Renal Protection by Upregulation of MiR-21. Kidney Int. 2012, 82, 1167–1175. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, L. Upregulation of MiR-21 by Ghrelin Ameliorates Ischemia/Reperfusion-Induced Acute Kidney Injury by Inhibiting Inflammation and Cell Apoptosis. DNA Cell Biol. 2016, 35, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J.; Palsson-Mcdermott, E.; Hennessy, E.J.; Martin, C.; O’Leary, J.J.; Ruan, Q.; Johnson, D.S.; Chen, Y.; O’Neill, L.A.J. Negative Regulation of TLR4 via Targeting of the Proinflammatory Tumor Suppressor PDCD4 by the MicroRNA MiR-21. Nat. Immunol. 2010, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Yang, G.X.; Kenny, T.P.; Kawata, K.; Zhang, W.; Huang, W.; Leung, P.S.C.; Lian, Z.X.; Okazaki, K.; Ansari, A.A.; et al. Overexpression of MicroRNA-21 Is Associated with Elevated pro-Inflammatory Cytokines in Dominant-Negative TGF-β Receptor Type II Mouse. J. Autoimmun. 2013, 41, 111–119. [Google Scholar] [CrossRef]
- Wang, H.; Peng, W.; Shen, X.; Huang, Y.; Ouyang, X.; Dai, Y. Circulating Levels of Inflammation-Associated Mir-155 and Endothelial-Enriched Mir-126 in Patients with End-Stage Renal Disease. Braz. J. Med. Biol. Res. 2012, 45, 1308–1314. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, X.; Yao, Y.; Li, H.; Fan, Y.; Zhang, Y.; Zhao, C.; Wang, L.; Ma, M.; Lei, Z.; et al. MiR-155 Regulates the Proliferation and Invasion of Clear Cell Renal Cell Carcinoma Cells by Targeting E2F2. Oncotarget 2016, 7, 20324–20337. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Poursadegh Zonouzi, A.; Ghanbarian, H.; Ghojazadeh, M.; Samadi, N.; Omidi, Y.; Ardalan, M. Differential Expression of Circulating MiR-21, MiR-142-3p and MiR-155 in Renal Transplant Recipients with Impaired Graft Function. Int. Urol. Nephrol. 2017, 49, 1681–1689. [Google Scholar] [CrossRef]
- Chen, S.; Shan, J.; Niu, W.; Lin, F.; Liu, S.; Wu, P.; Sun, L.; Lu, W.; Jiang, G. Micro RNA-155 Inhibitor as a Potential Therapeutic Strategy for the Treatment of Acute Kidney Injury (AKI): A Nanomedicine Perspective. RSC Adv. 2018, 8, 15890–15896. [Google Scholar] [CrossRef]
- Mohamed, D.I.; Khairy, E.; Saad, S.S.T.; Habib, E.K.; Hamouda, M.A. Potential Protective Effects of Dapagliflozin in Gentamicin Induced Nephrotoxicity Rat Model via Modulation of Apoptosis Associated MiRNAs. Gene 2019, 707, 198–204. [Google Scholar] [CrossRef]
| Groups | Relative Kidney Weight % |
|---|---|
| Control | 0.369 ± 0.026 |
| GNT | 0.481 ± 0.085 a |
| GNT + DIOS | 0.406 ± 0.021 b |
| DIOS | 0.322 ± 0.034 b,c |
| Groups | BUN | SCr | KIM-1 |
|---|---|---|---|
| Control | 32.57 2.421 | 0.175 0.034 | 107.80 3.794 |
| GNT | 120.40 5.328 a | 1.417 0.339 a | 332.50 28.120 a |
| GNT + DIOS | 61.52 7.413 a,b | 0.416 0.018 b | 164.50 5.526 a,b |
| DIOS | 40.67 5.901 b,c | 0.194 0.020 b | 104.50 6.237 b,c |
| Groups | MDA | TAC | SOD |
|---|---|---|---|
| Control | 44.50 4.472 | 79.58 8.695 | 42.68 4.377 |
| GNT | 190.2 13.040 a | 31.12 4.481 a | 15.51 1.350 a |
| GNT + DIOS | 84.88 9.566 a,b | 51.20 3.913 a,b | 33.45 2.931 a,b |
| DIOS | 50.91 9.671 b,c | 70.82 2.971 b,c | 44.37 3.258 b,c |
| Groups | Tubular Coagulative Necrosis | Perivascular Edema and Inflammatory Cells Infiltration | Tubular Degeneration | Focal Inflammatory Cells Infiltration |
|---|---|---|---|---|
| Control | - | - | - | - |
| GNT | +++ | + | ++ | ++ |
| GNT+ DIOS | - | - | - | + |
| DIOS | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, R.I.; Aboutaleb, A.S.; Younis, N.S.; Ahmed, H.I. Diosmin Mitigates Gentamicin-Induced Nephrotoxicity in Rats: Insights on miR-21 and -155 Expression, Nrf2/HO-1 and p38-MAPK/NF-κB Pathways. Toxics 2023, 11, 48. https://doi.org/10.3390/toxics11010048
Nadeem RI, Aboutaleb AS, Younis NS, Ahmed HI. Diosmin Mitigates Gentamicin-Induced Nephrotoxicity in Rats: Insights on miR-21 and -155 Expression, Nrf2/HO-1 and p38-MAPK/NF-κB Pathways. Toxics. 2023; 11(1):48. https://doi.org/10.3390/toxics11010048
Chicago/Turabian StyleNadeem, Rania I., Amany S. Aboutaleb, Nancy S. Younis, and Hebatalla I. Ahmed. 2023. "Diosmin Mitigates Gentamicin-Induced Nephrotoxicity in Rats: Insights on miR-21 and -155 Expression, Nrf2/HO-1 and p38-MAPK/NF-κB Pathways" Toxics 11, no. 1: 48. https://doi.org/10.3390/toxics11010048
APA StyleNadeem, R. I., Aboutaleb, A. S., Younis, N. S., & Ahmed, H. I. (2023). Diosmin Mitigates Gentamicin-Induced Nephrotoxicity in Rats: Insights on miR-21 and -155 Expression, Nrf2/HO-1 and p38-MAPK/NF-κB Pathways. Toxics, 11(1), 48. https://doi.org/10.3390/toxics11010048