Deteriorative Effects of Radiation Injury Combined with Skin Wounding in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Total Body Irradiation
2.3. Skin Wounding
2.4. Thirty-Day Mice Survival
2.5. Body Weight and Wound Measurements
2.6. Blood Collection, Peripheral Blood Cell Count, Serum Preparation, and Tissue Collection
2.7. Quantified Cytokine Array: Serum and Skin Tissue Lysate
2.8. Western Blot Analysis
2.9. Histological Examination of Skin and Sternum
2.10. Statistical Analysis
3. Results
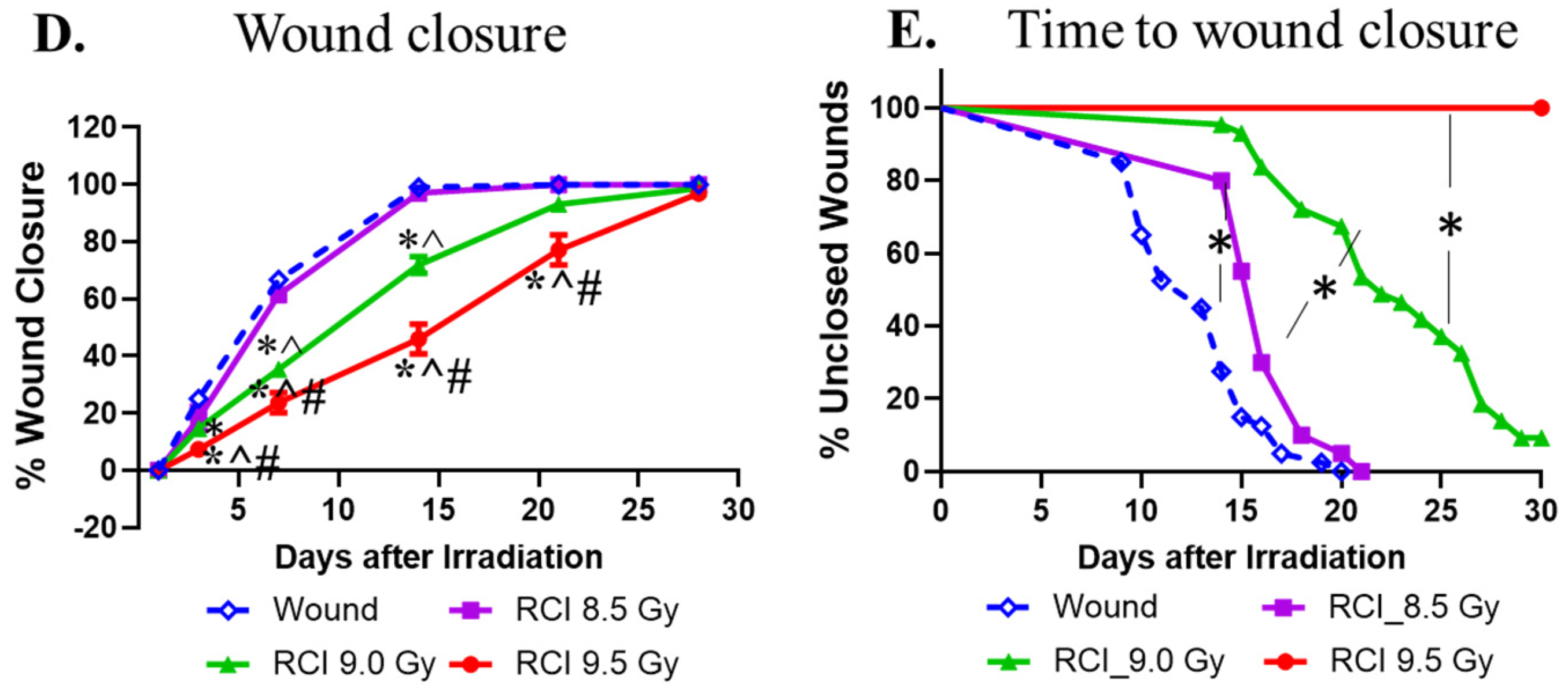
3.1. RCI Results in Increases in 30-Day Lethality, Body Weight Loss, and Delayed Wound Closure in a TBI Dose-Dependent Manner
3.2. RCI Significantly Increases NEU, MONO, and NEU/LYM and Decreases LYM and PLT
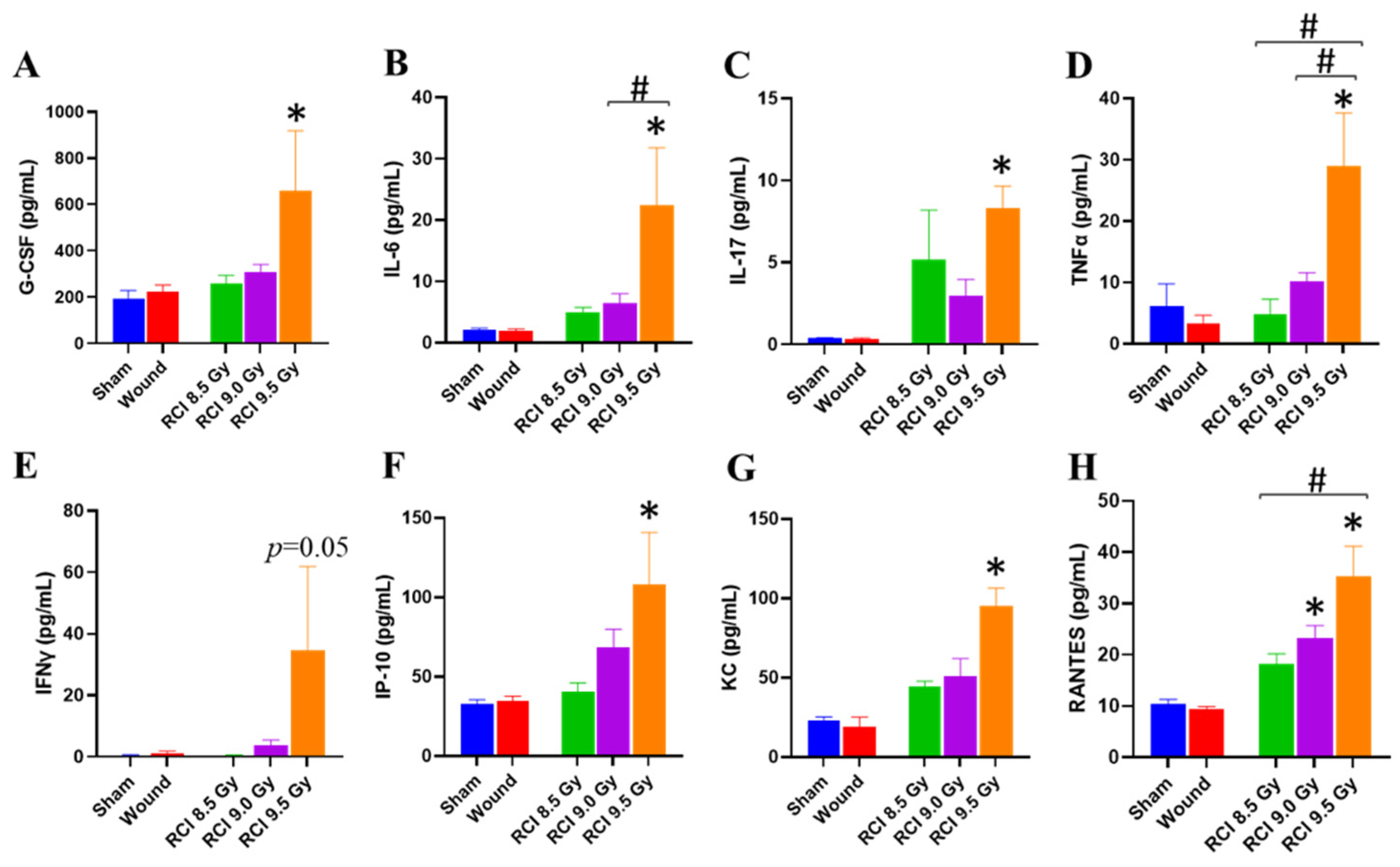
3.3. RCI at 9.5 Gy Induces a Systemic Proinflammatory Response in Mice
3.4. RCI with Doses Ranging from 8.5 Gy to 9.5 Gy Induces Hypocellularity to a Similar Degree of Severity in Mouse Bone Marrow (BM) on Day 30 after RCI
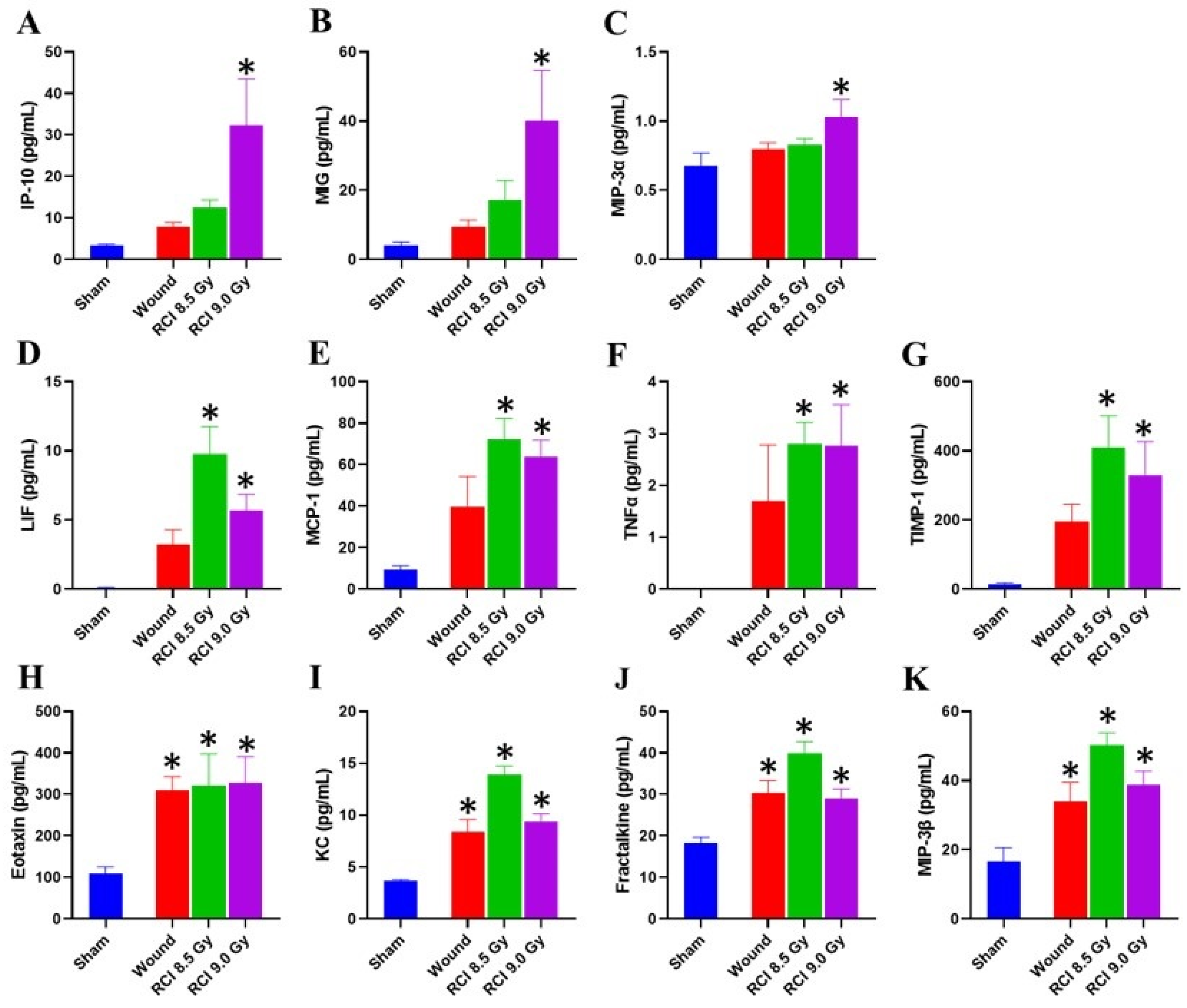
3.5. Chemokine Levels Are Elevated in Skin Wound Sites of RCI Mice Exposed to 9 Gy
3.6. Sublethal RCI and Wounding alone, but Not Lethal RCI, Upregulate VEGF Expression in Skin Wound Sites on Day 30 after TBI
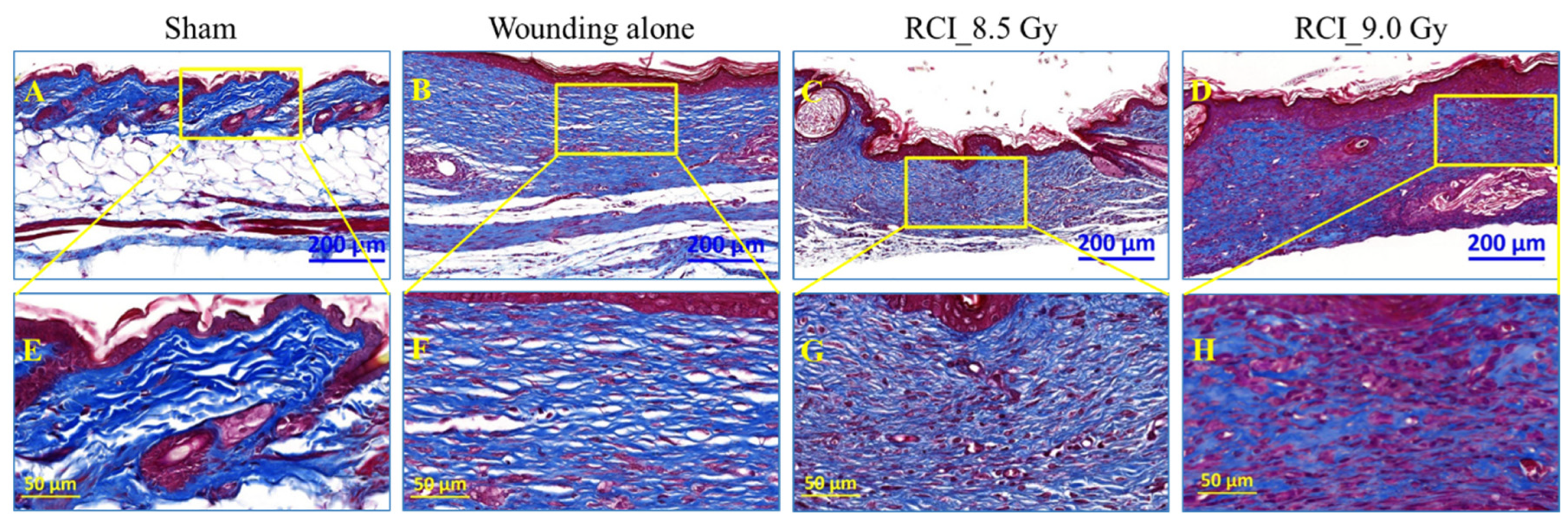
3.7. RCI Mice Display Dysregulated Collagen Homeostasis in Skin Wound Scar
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiCarlo, A.L.; Ramakrishnan, N.; Hatchett, R.J. Radiation combined injury: Overview of NIAID research. Health Phys. 2010, 98, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Garrison, B.R.; Gorbunov, N.V. Radiation combined injury: DNA damage, apoptosis, and autophagy. Adapt. Med. 2010, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R.; Eilertson, K.; Chakraborti, A.; Sharma, S.; Baure, J.; Habdank-Kolaczkowski, J.; Allen, B.; Rosi, S.; Raber, J.; Fike, J.R. Radiation exposure prior to traumatic brain injury induces responses that differ as a function of animal age. Int. J. Radiat. Biol. 2014, 90, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Jiao, W.; Cary, L.H.; Mog, S.R.; Elliott, T.B.; Pellmar, T.C.; Ledney, G.D. Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat. Res. 2010, 173, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Fukumoto, R. Ciprofloxacin increases survival after ionizing irradiation combined injury: Gamma-H2AX formation, cytokine/chemokine, and red blood cells. Health Phys. 2014, 106, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Ledney, G.D. Skin injuries reduce survival and modulate corticosterone, C-reactive protein, complement component 3, IgM, and prostaglandin E 2 after whole-body reactor-produced mixed field (n + gamma-photons) irradiation. Oxidative Med. Cell. Longev. 2013, 2013, 821541. [Google Scholar] [CrossRef]
- Ledney, G.D.; Elliott, T.B. Combined injury: Factors with potential to impact radiation dose assessments. Health Phys. 2010, 98, 145–152. [Google Scholar] [CrossRef]
- Pellmar, T.C.; Ledney, G. Combined injury: Radiation in combination with trauma, infectious disease, or chemical exposures. In Proceedings of the Human Factors and Medicine Panel Research Task Group 099 “Radiation Bioeffects and Countermeasures”, Bethesda, MD, USA, 21–23 June 2005. [Google Scholar]
- Kiang, J.G.; Garrison, B.R.; Burns, T.M.; Zhai, M.; Dews, I.C.; Ney, P.H.; Cary, L.H.; Fukumoto, R.; Elliott, T.B.; Ledney, G.D. Wound trauma alters ionizing radiation dose assessment. Cell Biosci. 2012, 2, 20. [Google Scholar] [CrossRef]
- Fukumoto, R.; Burns, T.M.; Kiang, J.G. Ciprofloxacin enhances stress erythropoiesis in spleen and increases survival after whole-body irradiation combined with skin-wound trauma. PLoS ONE 2014, 9, e90448. [Google Scholar] [CrossRef]
- Kiang, J.G.; Anderson, M.N.; Smith, J.T. Ghrelin therapy mitigates bone marrow injury and splenocytopenia by sustaining circulating G-CSF and KC increases after irradiation combined with wound. Cell Biosci. 2018, 8, 27. [Google Scholar] [CrossRef]
- Kiang, J.G.; Gorbunov, N.V. Bone marrow mesenchymal stem cells increase survival after ionizing irradiation combined with wound trauma: Characterization and therapy. J. Cell Sci. Ther. 2014, 5, 190. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Smith, J.T.; Cannon, G.; Anderson, M.N.; Ho, C.; Zhai, M.; Cui, W.; Xiao, M. Ghrelin, a novel therapy, corrects cytokine and NF-kappaB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci. 2020, 10, 63. [Google Scholar] [CrossRef]
- Kiang, J.G.; Zhai, M.; Bolduc, D.L.; Smith, J.T.; Anderson, M.N.; Ho, C.; Lin, B.; Jiang, S. Combined therapy of Pegylated G-CSF and Alxn4100TPO improves survival and mitigates acute radiation syndrome after whole-body ionizing irradiation alone and followed by wound trauma. Radiat. Res. 2017, 188, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Bolduc, D.L.; Elliott, T.B.; Gorbunov, N.V. Pegylated G-CSF inhibits blood cell depletion, increases platelets, blocks splenomegaly, and improves survival after whole-body ionizing irradiation but not after irradiation combined with burn. Oxidative Med. Cell. Longev. 2014, 2014, 481392. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Elliott, T.B.; Gorbunov, N.V. Ghrelin therapy improves survival after whole-body ionizing irradiation or combined with burn or wound: Amelioration of leukocytopenia, thrombocytopenia, splenomegaly, and bone marrow injury. Oxidative Med. Cell. Longev. 2014, 2014, 215858. [Google Scholar] [CrossRef]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Ho, C.; Gorbunov, N.V.; Elliott, T.B. Thrombopoietin receptor agonist mitigates hematopoietic radiation syndrome and improves survival after whole-body ionizing irradiation followed by wound trauma. Mediat. Inflamm. 2017, 2017, 7582079. [Google Scholar] [CrossRef]
- Ledney, G.D.; Elliott, T.B.; Moore, M.M. Modulations of Mortality by Tissue Trauma and Sepsis in Mice after Radiation Injury; Williams & Wilkins: Baltimore, MD, USA, 1992. [Google Scholar]
- Swift, J.M.; Smith, J.T.; Kiang, J.G. Ciprofloxacin therapy results in mitigation of ATP Loss after irradiation combined with wound trauma: Preservation of pyruvate dehydrogenase and inhibition of pyruvate dehydrogenase kinase 1. Radiat. Res. 2015, 183, 684–692. [Google Scholar] [CrossRef]
- Wang, L.; Zhai, M.; Lin, B.; Cui, W.; Hull, L.; Li, X.; Anderson, M.N.; Smith, J.T.; Umali, M.V.; Jiang, S.; et al. PEG-G-CSF and L-citrulline combination therapy for mitigating skin wound combined radiation injury in a mouse model. Radiat. Res. 2021, 196, 113–127. [Google Scholar] [CrossRef]
- Chua, H.L.; Plett, P.A.; Sampson, C.H.; Katz, B.P.; Carnathan, G.W.; MacVittie, T.J.; Lenden, K.; Orschell, C.M. Survival efficacy of the PEGylated G-CSFs Maxy-G34 and neulasta in a mouse model of lethal H-ARS, and residual bone marrow damage in treated survivors. Health Phys. 2014, 106, 21–38. [Google Scholar] [CrossRef]
- Farese, A.M.; Cohen, M.V.; Stead, R.B.; Jackson, W., 3rd; Macvittie, T.J. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat. Res. 2012, 178, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hankey, K.G.; Farese, A.M.; Blaauw, E.C.; Gibbs, A.M.; Smith, C.P.; Katz, B.P.; Tong, Y.; Prado, K.L.; MacVittie, T.J. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat. Res. 2015, 183, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and wound healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Vegesna, V.; Withers, H.R.; Holly, F.E.; McBride, W.H. The effect of local and systemic irradiation on impairment of wound healing in mice. Radiat. Res. 1993, 135, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.E.; Burns, A.L.; Gray, K.L.; DiPietro, L.A. Age-related alterations in the inflammatory response to dermal injury. J. Investig. Dermatol. 2001, 117, 1027–1035. [Google Scholar] [CrossRef]
- Koch, A.; Gulani, J.; King, G.; Hieber, K.; Chappell, M.; Ossetrova, N. Establishment of early endpoints in mouse total-body irradiation model. PLoS ONE 2016, 11, e0161079. [Google Scholar] [CrossRef]
- Li, X.; Cui, W.; Hull, L.; Smith, J.T.; Kiang, J.G.; Xiao, M. Effects of low-to-moderate doses of gamma radiation on mouse hematopoietic system. Radiat. Res. 2018, 190, 612–622. [Google Scholar] [CrossRef]
- Schiappacassi, M.; Lovisa, S.; Lovat, F.; Fabris, L.; Colombatti, A.; Belletti, B.; Baldassarre, G. Role of T198 modification in the regulation of p27(Kip1) protein stability and function. PLoS ONE 2011, 6, e17673. [Google Scholar] [CrossRef][Green Version]
- Hosoya, A.; Nakamura, H.; Ninomiya, T.; Yoshiba, K.; Yoshiba, N.; Nakaya, H.; Wakitani, S.; Yamada, H.; Kasahara, E.; Ozawa, H. Immunohistochemical localization of alpha-Smooth muscle actin during rat molar tooth development. J. Histochem. Cytochem. 2006, 54, 1371–1378. [Google Scholar] [CrossRef]
- Di Maggio, F.M.; Minafra, L.; Forte, G.I.; Cammarata, F.P.; Lio, D.; Messa, C.; Gilardi, M.C.; Bravata, V. Portrait of inflammatory response to ionizing radiation treatment. J. Inflamm. 2015, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in radiobiological responses: A review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- De Filippo, K.; Henderson, R.B.; Laschinger, M.; Hogg, N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 2008, 180, 4308–4315. [Google Scholar] [CrossRef]
- von Luettichau, I.; Nelson, P.J.; Pattison, J.M.; van de Rijn, M.; Huie, P.; Warnke, R.; Wiedermann, C.J.; Stahl, R.A.; Sibley, R.K.; Krensky, A.M. RANTES chemokine expression in diseased and normal human tissues. Cytokine 1996, 8, 89–98. [Google Scholar] [CrossRef]
- Kiang, J.G.; Zhai, M.; Lin, B.; Smith, J.T.; Anderson, M.N.; Jiang, S. Co-therapy of Pegylated G-CSF and ghrelin for enhancing survival after exposure to lethal radiation. Front. Pharmacol. 2021, 12, 628018. [Google Scholar] [CrossRef]
- Horowitz, M.C.; Berry, R.; Holtrup, B.; Sebo, Z.; Nelson, T.; Fretz, J.A.; Lindskog, D.; Kaplan, J.L.; Ables, G.; Rodeheffer, M.S.; et al. Bone marrow adipocytes. Adipocyte 2017, 6, 193–204. [Google Scholar] [CrossRef]
- Chandra, A.; Lagnado, A.B.; Farr, J.N.; Schleusner, M.; Monroe, D.G.; Saul, D.; Passos, J.F.; Khosla, S.; Pignolo, R.J. Bone marrow adiposity in models of radiation- and aging-related bone loss is dependent on cellular senescence. J. Bone Miner. Res. 2022, 37, 997–1011. [Google Scholar] [CrossRef]
- Dekker, H.; Schulten, E.; Lichters, I.; van Ruijven, L.; van Essen, H.W.; Blom, G.J.; Bloemena, E.; Ten Bruggenkate, C.M.; Kullaa, A.M.; Bravenboer, N. Osteocyte apoptosis, bone marrow adiposity, and fibrosis in the irradiated human mandible. Adv. Radiat. Oncol. 2022, 7, 100951. [Google Scholar] [CrossRef]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Lokmic, Z.; Musyoka, J.; Hewitson, T.D.; Darby, I.A. Hypoxia and hypoxia signaling in tissue repair and fibrosis. Int. Rev. Cell Mol. Biol. 2012, 296, 139–185. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; van de Water, L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef]
- Alfaro, M.P.; Deskins, D.L.; Wallus, M.; DasGupta, J.; Davidson, J.M.; Nanney, L.B.; Guney, M.A.; Gannon, M.; Young, P.P. A physiological role for connective tissue growth factor in early wound healing. Lab. Investig. 2013, 93, 81–95. [Google Scholar] [CrossRef]
- Chen, C.C.; Lau, L.F. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 2009, 41, 771–783. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Zawaski, J.A.; Yates, C.R.; Miller, D.D.; Kaffes, C.C.; Sabek, O.M.; Afshar, S.F.; Young, D.A.; Yang, Y.; Gaber, M.W. Radiation combined injury models to study the effects of interventions and wound biomechanics. Radiat. Res. 2014, 182, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Fathke, C.; Wilson, L.; Hutter, J.; Kapoor, V.; Smith, A.; Hocking, A.; Isik, F. Contribution of bone marrow-derived cells to skin: Collagen deposition and wound repair. Stem Cells 2004, 22, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Fitridge, R.; Thompson, M. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; The University of Adelaide, Barr Smith Press: Adelaide, Australia, 2011. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lin, B.; Zhai, M.; Cui, W.; Hull, L.; Zizzo, A.; Li, X.; Kiang, J.G.; Xiao, M. Deteriorative Effects of Radiation Injury Combined with Skin Wounding in a Mouse Model. Toxics 2022, 10, 785. https://doi.org/10.3390/toxics10120785
Wang L, Lin B, Zhai M, Cui W, Hull L, Zizzo A, Li X, Kiang JG, Xiao M. Deteriorative Effects of Radiation Injury Combined with Skin Wounding in a Mouse Model. Toxics. 2022; 10(12):785. https://doi.org/10.3390/toxics10120785
Chicago/Turabian StyleWang, Li, Bin Lin, Min Zhai, Wanchang Cui, Lisa Hull, Alex Zizzo, Xianghong Li, Juliann G. Kiang, and Mang Xiao. 2022. "Deteriorative Effects of Radiation Injury Combined with Skin Wounding in a Mouse Model" Toxics 10, no. 12: 785. https://doi.org/10.3390/toxics10120785
APA StyleWang, L., Lin, B., Zhai, M., Cui, W., Hull, L., Zizzo, A., Li, X., Kiang, J. G., & Xiao, M. (2022). Deteriorative Effects of Radiation Injury Combined with Skin Wounding in a Mouse Model. Toxics, 10(12), 785. https://doi.org/10.3390/toxics10120785





