Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial
Abstract
:1. Introduction
2. Experimental Materials
2.1. Composition of Grape Pomace
2.2. Cardiometabolic Markers
2.3. Fecal Microbiota
2.4. Fecal Short-Chain Fatty Acids
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Evolution in Cardiometabolic Markers
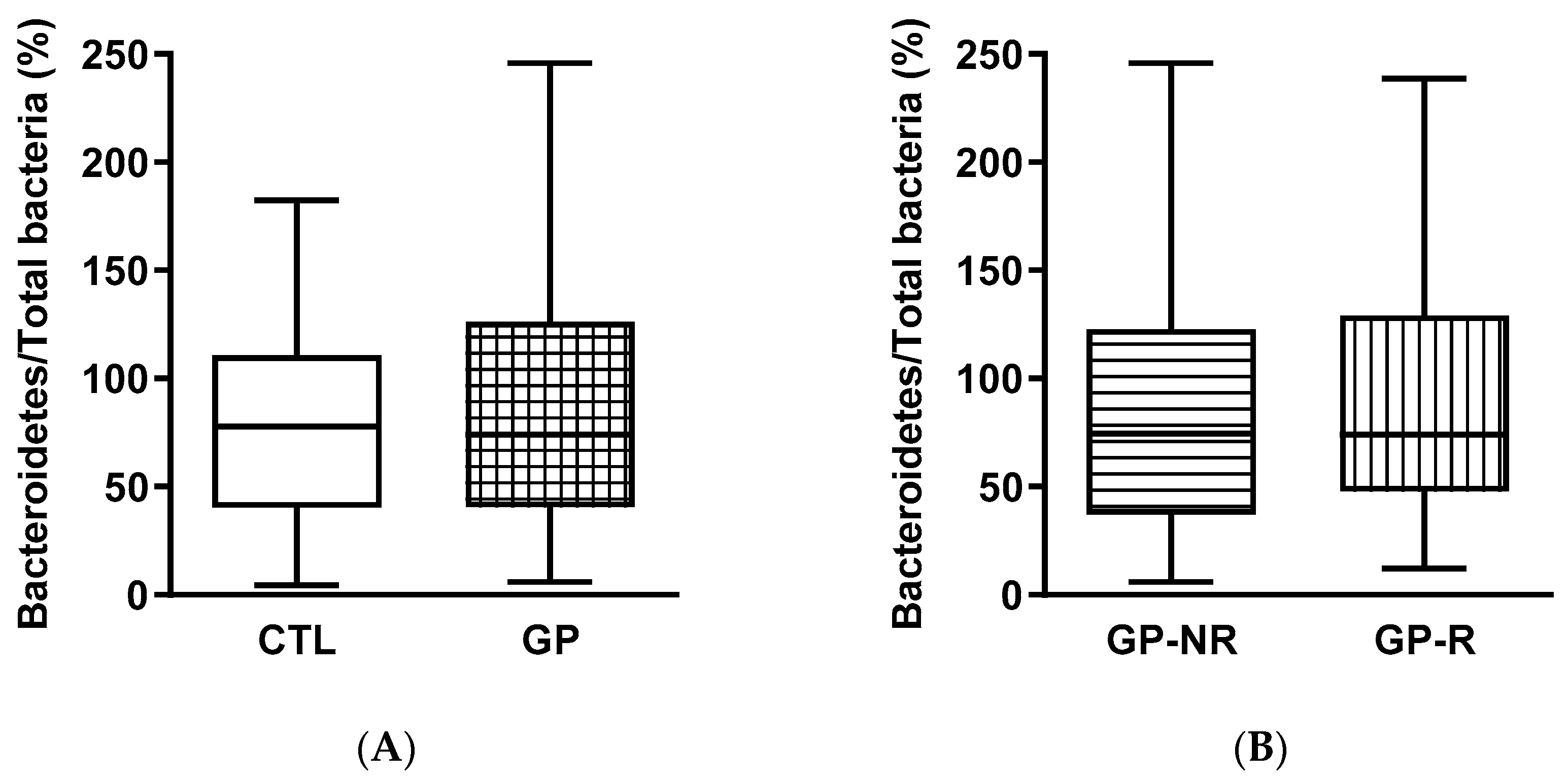
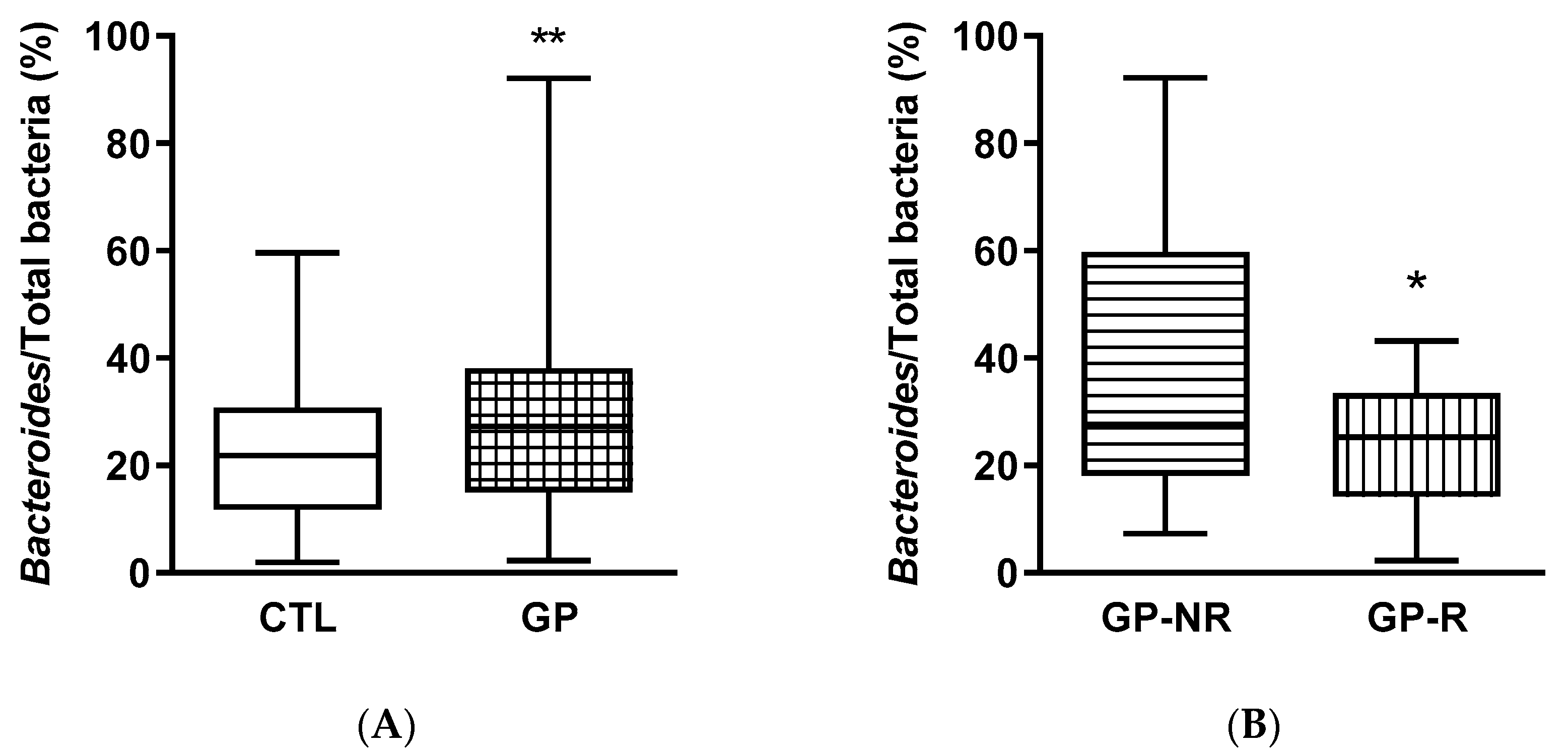
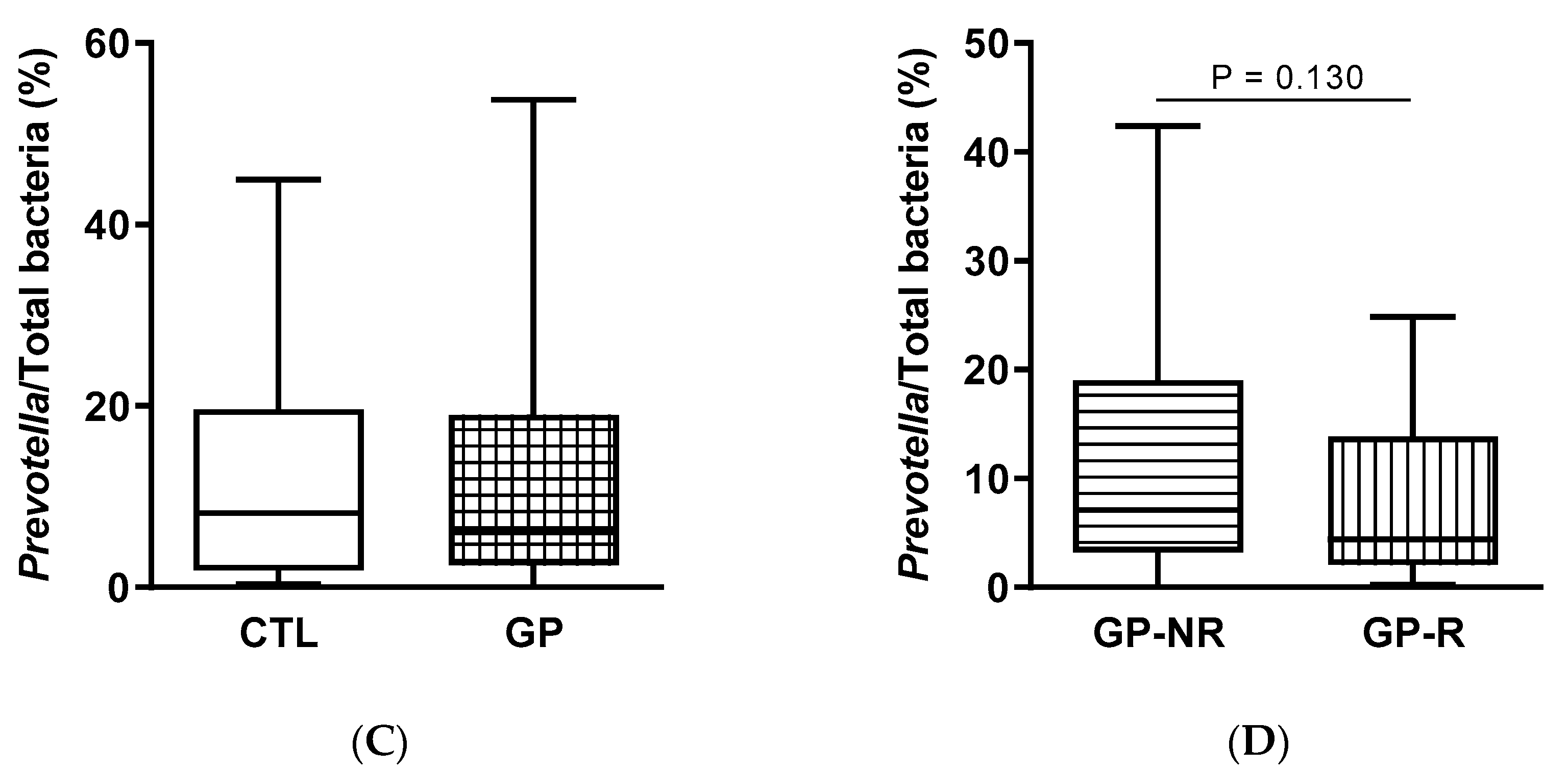
3.3. Fecal Microbiota and SCFAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HOMA-IR | homeostatic model assessment for insulin resistance |
| MetS | metabolic syndrome |
| OGT | oral glucose tolerance test |
| GP | grape pomace |
References
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.M.; Kahn, S.A.; Delmont, T.O.; Shaiber, A.; Esen, O.C.; Hubert, N.A.; Morrison, H.G.; Antonopoulos, D.A.; Rubin, D.T.; Eren, A.M. Tracking microbial colonization in fecal microbiota transplantation experiments via genome-resolved metagenomics. Microbiome 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S. Insulin signaling, resistance, and metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA J. Am. Med. Assoc. 2015, 313, 1973–1974. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Tresserra-Rimbau, A.; Vitelli-Storelli, F.; Domènech, M.; Salas-Salvadó, J.; Martín-Sánchez, V.; Rubín-García, M.; Buil-Cosiales, P.; Corella, D.; Fitó, M.; et al. Dietary polyphenol intake is associated with HDL-cholesterol and a better profile of other components of the metabolic syndrome: A PREDIMED-plus sub-study. Nutrients 2020, 12, 689. [Google Scholar] [CrossRef] [Green Version]
- Chiva-Blanch, G.; Badimón, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Cilleros, D.; Ramos, S.; Goya, L.; Martín, M.A. Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction. Food Chem. Toxicol. 2018, 115, 88–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of alpha-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Blade, C.; Arola, L.; Salvadó, M.-J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef]
- Castell-Auví, A.; Cedo, L.; Pallarés, V.; Blay, M.T.; Pinent, M.; Motilva, M.J.; García-Vallve, S.; Pujadas, G.; Maechler, P.; Ardèvol, A. Procyanidins modify insulinemia by affecting insulin production and degradation. J. Nutr. Biochem. 2012, 23, 1565–1572. [Google Scholar] [CrossRef]
- Chou, E.J.; Keevil, J.G.; Aeschlimann, S.; Wiebe, D.A.; Folts, J.D.; Stein, J.H. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am. J. Cardiol. 2001, 88, 553–555. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Cueva, C.; Silva, M.; Pinillos, I.; Bartolomé, B.; Moreno-Arribas, M.V. Interplay between dietary polyphenols and oral and gut microbiota in the development of colorectal cancer. Nutrients 2020, 12, 625. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, M.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci. Technol. 2020, 99, 634–649. [Google Scholar] [CrossRef]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodríguez-Mateos, A.; de Roos, B.; García-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol. Nutr. Food Res. 2020. [Google Scholar] [CrossRef]
- García-Villalba, R.; Vissenaekens, H.; Pitart, J.; Romo-Vaquero, M.; Espín, J.C.; Grootaert, C.; Selma, M.V.; Raes, K.; Smagghe, G.; Possemiers, S.; et al. Gastrointestinal simulation model TWIN-SHIME shows differences between human urolithin-metabotypes in gut microbiota composition, pomegranate polyphenol metabolism, and transport along the intestinal tract. J. Agric. Food Chem. 2017, 65, 5480–5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Jiménez, J.; Arranz, S.; Saura-Calixto, F. Proanthocyanidin content in foods is largely underestimated in the literature data: An approach to quantification of the missing proanthocyanidins. Food Res. Int. 2009, 42, 1381–1388. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity-A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: A pilot study. Clin. Nutr. 2019, 38, 2727–2734. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Esteban-Fernández, A.; de Llano, D.G.; Sanz-Buenhombre, M.; Guadarrana, A.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Gómez, L.M.; Bermejo, M.L.G.; et al. Supplementation with grape pomace in healthy women: Changes in biochemical parameters, gut microbiota and related metabolic biomarkers. J. Funct. Foods 2018, 45, 34–46. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Zapatera, B.; Gallego-Narbón, A.; Vaquero, M.P.; Saura-Calixto, F.; Pérez-Jiménez, J. A 6-week supplementation with grape pomace to subjects at cardiometabolic risk ameliorates insulin sensitivity, without affecting other metabolic syndrome markers. Food Funct. 2018, 9, 6010–6019. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goni, I.; Saura-Calixto, F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008, 24, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; D’Acuna, S.; Pérez, D.; Dicenta, S.; Echeverria, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorraquín, I.; Sánchez-Hernández, E.; Ayuda-Durán, B.; Silva, M.; Gonáalez-Paramás, A.M.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Current and future experimental approaches in the study of grape and wine polyphenols interacting gut microbiota. J. Sci. Food Agric. 2020, 100, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome. A joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Hartman, A.L.; Lough, D.M.; Barupal, D.K.; Fiehn, O.; Fishbein, T.; Zasloff, M.; Eisen, J.A. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 17187–17192. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.A.; Ragab, S.H.; Elbaky, A.A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef]
- Haakensen, M.; Dobson, C.M.; Deneer, H.; Ziola, B. Real-time PCR detection of bacteria belonging to the Firmicutes Phylum. Int. J. Food Microbiol. 2008, 125, 236–241. [Google Scholar] [CrossRef]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [Green Version]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Clifford, M.N. Advances in polyphenol research: A journal of agricultural and food chemistry virtual issue. J. Agric. Food Chem. 2017, 65, 8093–8095. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, W.; Tao, H.; Zhang, Y.; Liu, L.; Liu, Z.; Qiu, B.; Xu, T. Effect of industrial trans-fatty acids-enriched diet on gut microbiota of C57BL/6 mice. Eur. J. Nutr. 2019, 58, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Consolandi, C.; Turroni, S.; Emmi, G.; Severgnini, M.; Fiori, J.; Peano, C.; Biagi, E.; Grassi, A.; Rampelli, S.; Silvestri, E.; et al. Behcet’s syndrome patients exhibit specific microbiome signature. Autoimmun. Rev. 2015, 14, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Cilleros, D.; Ramos, S.; López-Oliva, M.E.; Escrivá, F.; Álvarez, C.; Fernández-Millán, E.; Martín, M.A. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, J.; Jackson, M.I.; Zhao, F.-Q.; Yan, L.; Combs, G.F., Jr. Fatty liver accompanies an increase in Lactobacillus species in the hind gut of C57BL/6 mice fed a high-fat diet. J. Nutr. 2013, 143, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Casanova-Martí, A.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardèvol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female ratst. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gómez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona-Díaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Wexler, A.G.; Goodman, A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [Green Version]
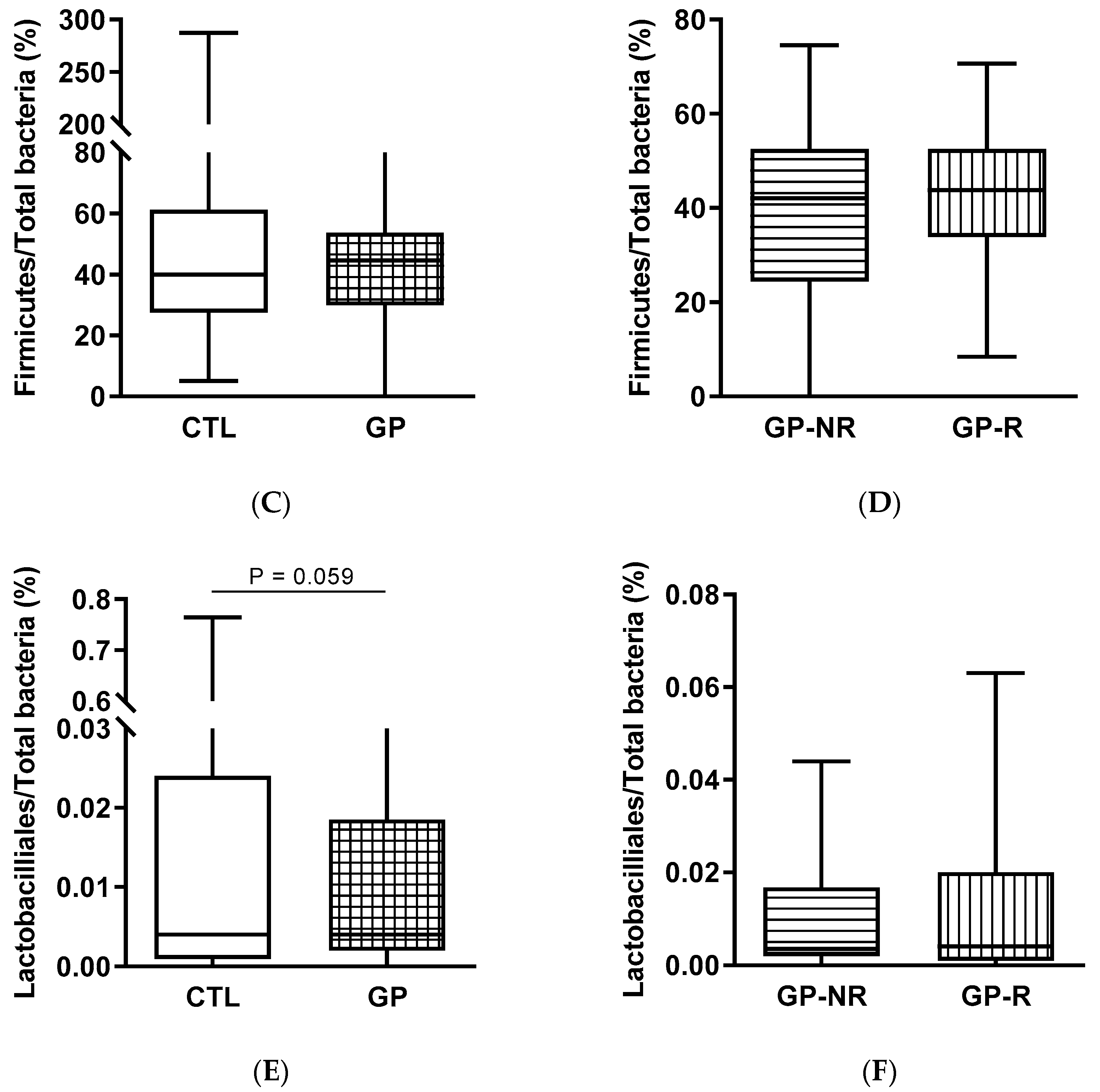
| Target Bacteria | Annealing Temperature (°C) | Sequences (5′-3′) | Positive Control | Reference |
|---|---|---|---|---|
| Total Bacteria | 65 | F: ACT CCT ACG GGA GGC AGC AGT | (a) | [35] |
| R: ATT ACC GCG GCT GCT GGC | ||||
| Bacteroidetes | 62 | F: ACG CTA GCT ACA GGC TTA A | Bacteroides fragilis | [36] |
| R: ACG CTA CTT GGC TGG TTC A | ||||
| Firmicutes | 52 | F: CTG ATG GAG CAA CGC CGC GT | Ruminococcus productus | [37] |
| R: ACA CYT AGY ACT CAT CGT TT | ||||
| Lactobacillales | 60 | F: AGC AGT AGG GAA TCT TCC A | Lactobacillus acidophylus | [38] |
| R: CAC CGC TAC ACA TGG AG | ||||
| Bacteroides | 60 | F: GGT TCT GAG AGG AGG TCC C | Bacteroides fragilis | [39] |
| R: GCT GCC TCC CGT AGG AGT | ||||
| Prevotella | 60 | F: CAG CAG CCG CGG TAA TA | Prevotella copri | [39] |
| R: GGC ATC CAT CGT TTA CCG T |
| Parameter | Pre-GP Supplementation | Post-GP Supplementation (Variation, %) | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| Whole Sample (n = 49) | Non-Responders (n = 26) | Responders (n = 23) | |||||
| Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. | ||
| Body mass index (kg/m2) | 31 | 1 | −0.2 | 0.3 | −0.1 | 0.2 | 0.618 |
| Abdominal perimeter (cm) | 103 | 2 | 0.2 | 0.4 | −0.5 | 0.6 | 0.591 |
| Total body fat (%) | 31 | 1 | 0.1 | 0.8 | −1.7 | 0.9 | 0.249 |
| Abdominal fat (%) | 103 | 2 | −0.3 | 1.1 | 0.1 | 0.8 | 0.880 |
| Systolic blood pressure (mm Hg) | 120 | 17 | −2 | 3 | 2 | 2 | 0.322 |
| Diastolic blood pressure (mm Hg) | 84 | 12 | −1 | 3 | 2 | 2 | 0.809 |
| Glucose (mg/dL) | 98 | 2 | 7 | 2 | 1 | 2 | 0.540 |
| Insulin (µU/mL) | 8.9 | 1.9 | 82 | 10 | −40 | 4 | <0.00001 |
| HOMA-IR | 2.1 | 0.4 | 100 | 10 | −40 | 4 | <0.00001 |
| Triglycerides (mg/dL) | 147 | 21 | 22 | 8 | −2 | 5 | 0.595 |
| Total cholesterol (mg/dL) | 341 | 48 | −3 | 2 | −5 | 2 | 0.353 |
| HDL cholesterol (mg/dL) | 47 | 7 | 0.0 | 2 | 4 | 2 | 0.200 |
| LDL cholesterol (mg/dL) | 121 | 17 | −6 | 2 | −8 | 2 | 0.873 |
| Fibrinogen (mg/dL) | 340 | 50 | −0.4 | 2 | −4 | 2 | 0.567 |
| Plasma uric acid (mg/dL) | 6 | 1 | 3 | 2 | −0.3 | 1 | 0.548 |
| Urine uric acid (mg/g creatinine) | 490 | 70 | −5 | 7 | 5 | 6 | 0.318 |
| Compound | CTL | GP | p-Value | GP-NR | GP-R | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |||
| Acetic acid | 120 | 20 | 120 | 10 | 0.602 | 110 | 20 | 118 | 20 | 0.879 |
| Propionic acid | 55 | 8 | 56 | 7 | 0.972 | 60 | 10 | 49 | 9 | 0.438 |
| Isobutyric acid | 6.9 | 0.5 | 6.9 | 0.7 | 0.940 | 6.8 | 0.6 | 7.1 | 1.4 | 0.852 |
| Butyric acid | 38 | 5 | 31.6 | 4.1 | 0.352 | 31 | 6 | 32 | 6 | 0.943 |
| Isovaleric acid | 7.1 | 0.5 | 5.7 | 0.4 | 0.023 * | 6.0 | 0.6 | 5.2 | 0.6 | 0.344 |
| Valeric acid | 6.8 | 0.8 | 5.8 | 0.7 | 0.435 | 6.1 | 1.0 | 5.6 | 1.1 | 0.731 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Romero, S.; Martínez-Maqueda, D.; Hereu, M.; Amézqueta, S.; Torres, J.L.; Pérez-Jiménez, J. Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial. Foods 2020, 9, 1279. https://doi.org/10.3390/foods9091279
Ramos-Romero S, Martínez-Maqueda D, Hereu M, Amézqueta S, Torres JL, Pérez-Jiménez J. Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial. Foods. 2020; 9(9):1279. https://doi.org/10.3390/foods9091279
Chicago/Turabian StyleRamos-Romero, Sara, Daniel Martínez-Maqueda, Mercè Hereu, Susana Amézqueta, Josep Lluís Torres, and Jara Pérez-Jiménez. 2020. "Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial" Foods 9, no. 9: 1279. https://doi.org/10.3390/foods9091279
APA StyleRamos-Romero, S., Martínez-Maqueda, D., Hereu, M., Amézqueta, S., Torres, J. L., & Pérez-Jiménez, J. (2020). Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial. Foods, 9(9), 1279. https://doi.org/10.3390/foods9091279






