Growth Potential of Listeria monocytogenes on Refrigerated Spinach and Rocket Leaves in Modified Atmosphere Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of L. monocytogenes for Inoculation Experiments
2.2. Preparation of the Polypropylene Bags and RTE Leafy Vegetables and Subsequent Inoculation and Storage
2.3. Sampling of the Leafy RTE Vegetable Packs and Analysis
2.4. Total Bacteria Count
2.5. Product pH and Water Activity
2.6. Statistical Analysis
3. Results
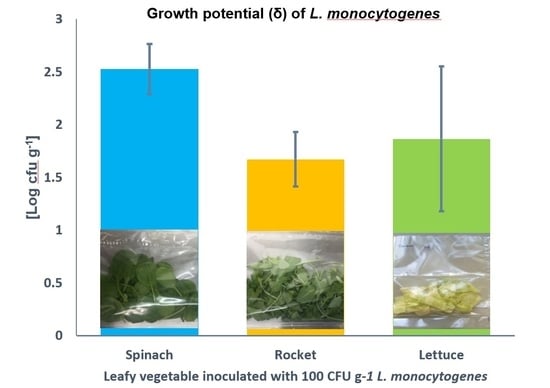
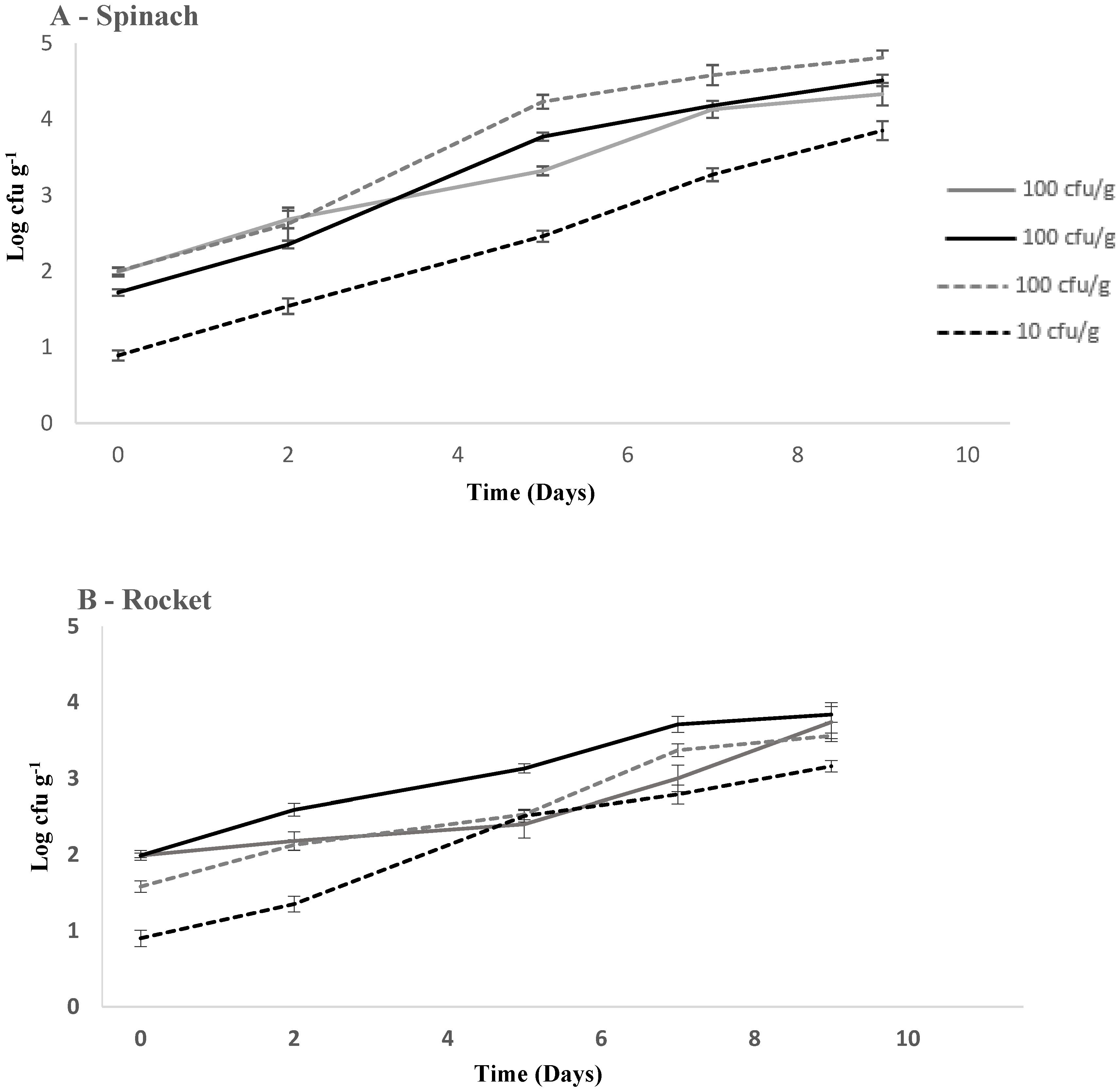
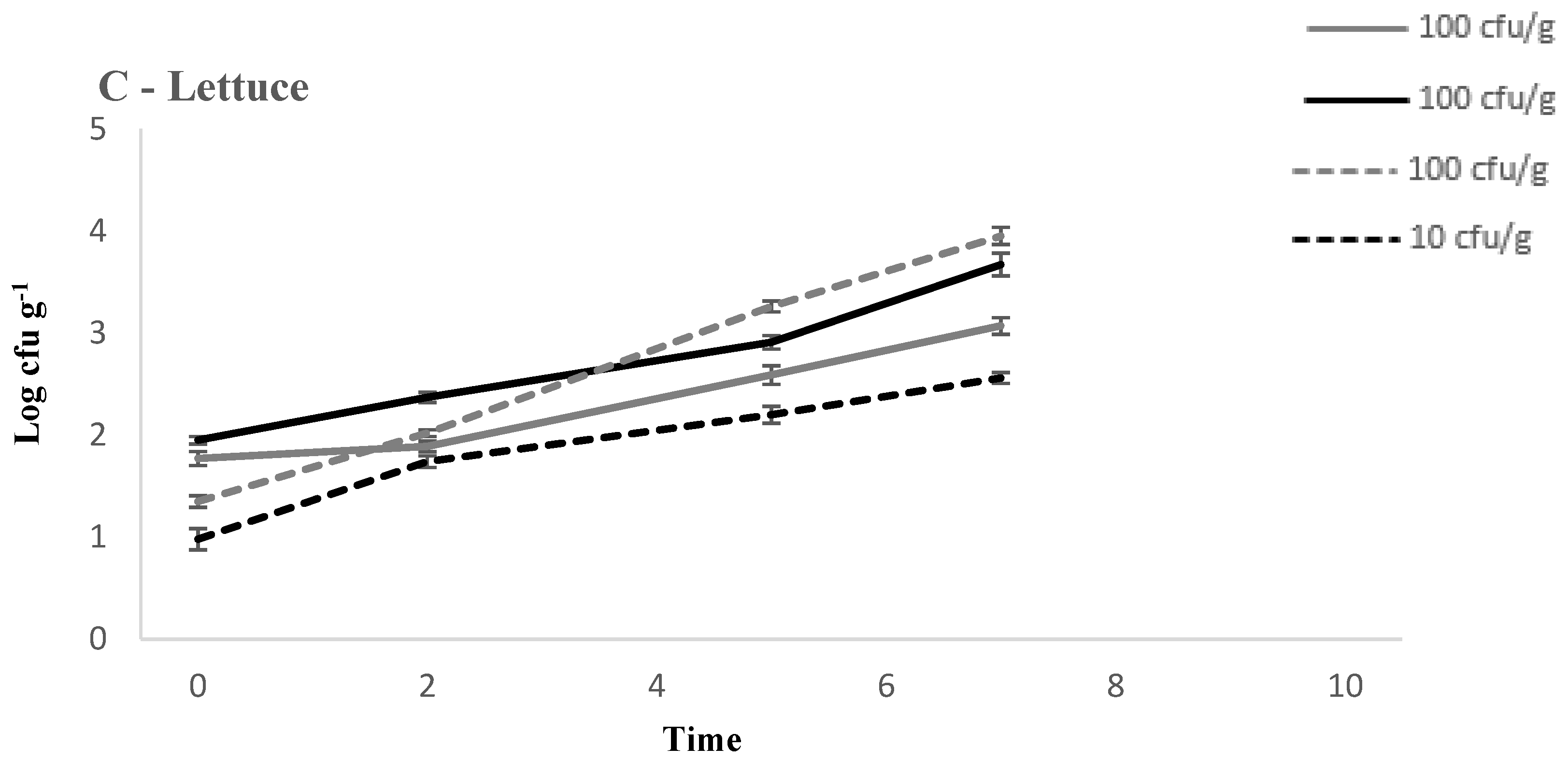
3.1. Comparison of Growth of L. monocytogenes on RTE Leafy Vegetables Iceberg Lettuce, Spinach, and Rocket over 7 Days
3.2. Comparison of Growth of L. monocytogenes on RTE Leafy Vegetables Spinach and Rocket over 9 Days
3.3. Total Bacteria Count
3.4. Product pH, Water Activity, and Atmosphere
3.5. Visual Appearance of Spinach and Rocket
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- FAO (FAOSTAT—Food and Agriculture Organization of the United Nations), 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 19 August 2019).
- Mir, S.; Shah, M.; Mir, M.; Dar, B.; Greiner, R.; Roohinejad, S. Microbiological contamination of ready-to-eat vegetable salads in developing countries and potential solutions in the supply chain to control microbial pathogens. Food Control 2018, 85, 235–244. [Google Scholar] [CrossRef]
- Santos, J.; Herrero, M.; Mendiola, J.; Oliva-Teles, M.; Ibáñez, E.; Delerue-Matos, C.; Oliveira, M. Assessment of nutritional and metabolic profiles of pea shoots: The new ready-to-eat baby-leaf vegetable. Food Res. Int. 2014, 58, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Eurostat 2017. Available online: https://ec.europa.eu/eurostat/web/agriculture/data/database (accessed on 21 August 2019).
- González-Tejedor, G.; Garre, A.; Esnoz, A.; Artés-Hernández, F.; Fernández, P. Effect of storage conditions in the response of Listeria monocytogenes in a fresh purple vegetable smoothie compared with an acidified TSB medium. Food Microbiol. 2018, 72, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Thienhirun, S.; Chung, S. Consumer attitudes and preferences toward cross-cultural ready-to-eat (RTE) food. J. Food Prod. Mark. 2017, 24, 56–79. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.; Selma, M.; Tudela, J.; Abad, J.; Gil, M. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Critzer, F.; Doyle, M. Microbial ecology of foodborne pathogens associated with produce. Curr. Opin. Biotechnol. 2010, 21, 125–130. [Google Scholar] [CrossRef]
- EFSA and ECDC—European Food Safety Authority, and European Centre for Disease Prevention and Control. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.; Bodero, M.; Riveros, G.; Lapierre, L.; Gaggero, A.; Vidal, R.; Vidal, M. Molecular epidemiology and genetic diversity of Listeria monocytogenes isolates from a wide variety of ready-to-eat foods and their relationship to clinical strains from listeriosis outbreaks in Chile. Front. Microbiol. 2015, 6, 384. [Google Scholar] [CrossRef] [Green Version]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2017, 16, 32–46. [Google Scholar] [CrossRef]
- Pereira, S.; Alves, Â.; Ferreira, V.; Teixeira, P. The Impact of Environmental Stresses in the Virulence Traits of Listeria monocytogenes Relevant to Food Safety. List. Monocytogenes 2018. [Google Scholar] [CrossRef] [Green Version]
- Francis, G.; O’Beirne, D. Effects of vegetable type, package atmosphere and storage temperature on growth and survival of Escherichia coli O157:H7 and Listeria monocytogenes. J. Ind. Microbiol. Biotechnol. 2001, 27, 111–116. [Google Scholar] [CrossRef] [PubMed]
- McManamon, O.; Scollard, J.; Schmalenberger, A. Inoculation density is affecting growth conditions of Listeria monocytogenes on fresh cut lettuce. World J. Microbiol. Biotechnol. 2017, 33, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, M.; Kent, D.; Stephan, R.; Guldimann, C. Growth potential of Listeria monocytogenes in twelve different types of RTE salads: Impact of food matrix, storage temperature and storage time. Int. J. Food Microbiol. 2019, 296, 83–92. [Google Scholar] [CrossRef] [PubMed]
- EURL—European Union Reference Laboratory 2019. Lm Technical Guidance Document for Conducting Shelf-Life Studies on Listeria monocytogenes in Ready-to-Eat Foods. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en.pdf (accessed on 15 January 2020).
- Ziegler, M.; Rüegg, S.; Stephan, R.; Guldimann, C. Growth potential of Listeria monocytogenes in six different RTE fruit products: Impact of food matrix, storage temperature and shelf life. Ital. J. Food Saf. 2018, 7, 7581. [Google Scholar] [CrossRef]
- Denis, N.; Zhang, H.; Leroux, A.; Trudel, R.; Bietlot, H. Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control 2016, 67, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Cordano, A.; Jacquet, C. Listeria monocytogenes isolated from vegetable salads sold at supermarkets in Santiago, Chile: Prevalence and strain characterization. Int. J. Food Microbiol. 2009, 132, 176–179. [Google Scholar] [CrossRef]
- Kramarenko, T.; Roasto, M.; Meremäe, K.; Kuningas, M.; Põltsama, P.; Elias, T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control 2013, 30, 24–29. [Google Scholar] [CrossRef]
- Sant’Ana, A.; Barbosa, M.; Destro, M.; Landgraf, M.; Franco, B. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready-to-eat vegetables stored at variable temperature conditions during shelf-life. Int. J. Food Microbiol. 2012, 157, 52–58. [Google Scholar] [CrossRef]
- Lokerse, R.; Maslowska-Corker, K.; van de Wardt, L.; Wijtzes, T. Growth capacity of Listeria monocytogenes in ingredients of ready-to-eat salads. Food Control 2016, 60, 338–345. [Google Scholar] [CrossRef]
- Söderqvist, K.; Lambertz, S.; Vågsholm, I.; Fernström, L.; Alsanius, B.; Mogren, L.; Boqvist, S. Fate of Listeria monocytogenes, pathogenic Yersinia enterocolitica, and Escherichia coli O157:H7gfp+in ready-to-eat salad during cold storage: What is the risk to consumers? J. Food Prot. 2017, 80, 204–212. [Google Scholar] [CrossRef]
- McManamon, O.; Kaupper, T.; Scollard, J.; Schmalenberger, A. Nisin application delays growth of Listeria monocytogenes on fresh-cut iceberg lettuce in modified atmosphere packaging, while the bacterial community structure changes within one week of storage. Postharvest Biol. Technol. 2019, 147, 185–195. [Google Scholar] [CrossRef]
- Scollard, J.; McManamon, O.; Schmalenberger, A. Inhibition of Listeria monocytogenes growth on fresh-cut produce with thyme essential oil and essential oil compound verbenone. Postharvest Biol. Technol. 2016, 120, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Hunt, K.; Blanc, M.; Álvarez-Ordóñez, A.; Jordan, K. Challenge studies to determine the ability of foods to support the growth of Listeria monocytogenes. Pathogens 2018, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Omac, B.; Moreira, R.; Castell-Perez, E. Quantifying growth of cold-adapted Listeria monocytogenes and Listeria innocua on fresh spinach leaves at refrigeration temperatures. J. Food Eng. 2018, 224, 17–26. [Google Scholar] [CrossRef]
- Boyacioglu, O.; Sulakvelidze, A.; Sharma, M.; Goktepe, I. Effect of a bacteriophage cocktail in combination with modified atmosphere packaging in controlling Listeria monocytogenes on fresh-cut spinach. Ir. J. Agric. Food Res. 2016, 55, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Beaufort, A.; Bergis, H.; Lardeux, A.-L.; Lombard, B.; Polet, M.; Botteldoorn, N.; Papageorgiou, G.; Andersen, J.K.; Boel, J.; Hickey, B.; et al. Technical Guidance Document for Conducting Shelf-Life Studies on Listeria monocytogenes in Ready-to-Eat Foods; EURL Lm Technical Guidance Document; European Union Reference Laboratory for Listeria Monocytogenes, 2019; pp. 1–47. Available online: https://eurl-listeria.anses.fr/en/system/files/LIS-Cr-201909D2.pdf (accessed on 10 January 2020).
- Castro-Ibáñez, I.; Gil, M.; Allende, A. Ready-to-eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT Food Sci Technol 2017, 85, 284–292. [Google Scholar] [CrossRef]
- Miceli, A.; Settanni, L. Influence of agronomic practices and pre-harvest conditions on the attachment and development of Listeria monocytogenes in vegetables. Ann. Microbiol. 2019, 69, 185–199. [Google Scholar] [CrossRef]
- Bardsley, C.; Boyer, R.; Rideout, S.; Strawn, L. Survival of Listeria monocytogenes on the surface of basil, cilantro, dill, and parsley plants. Food Control 2019, 95, 90–94. [Google Scholar] [CrossRef]
- Omac, B.; Moreira, R.; Castillo, A.; Castell-Perez, E. Growth of Listeria monocytogenes and Listeria innocua on fresh baby spinach leaves: Effect of storage temperature and natural microflora. Postharvest Biol. Technol. 2015, 100, 41–51. [Google Scholar] [CrossRef]
- Valentin-Bon, I.; Jacobson, A.; Monday, S.; Feng, P. Microbiological quality of bagged cut spinach and lettuce mixes. Appl. Environ. Microbiol. 2007, 74, 1240–1242. [Google Scholar] [CrossRef] [Green Version]
- Allende, A.; Luo, Y.; McEvoy, J.; Artés, F.; Wang, C. Microbial and quality changes in minimally processed baby spinach leaves stored under super atmospheric oxygen and modified atmosphere conditions. Postharvest Biol. Technol. 2004, 33, 51–59. [Google Scholar] [CrossRef]
- Kou, L.; Luo, Y.; Park, E.; Turner, E.; Barczak, A.; Jurick, W. Temperature abuse timing affects the rate of quality deterioration of commercially packaged ready-to-eat baby spinach. Part I: Sensory analysis and selected quality attributes. Postharvest Biol. Technol. 2014, 91, 96–103. [Google Scholar] [CrossRef]
- Koukounaras, A.; Siomos, A.; Sfakiotakis, E. Postharvest CO2 and ethylene production and quality of rocket (Eruca sativa Mill.) leaves as affected by leaf age and storage temperature. Postharvest Biol. Technol. 2007, 46, 167–173. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Pla, M.; Oltra, S.; Esteban, M.; Andreu, S.; Palop, A. Comparison of Primary Models to Predict Microbial Growth by the Plate Count and Absorbance Methods. BioMed Res. Int. 2015, 2015, 365025. [Google Scholar] [CrossRef] [PubMed]
- Guzel, M.; Moreira, R.; Omac, B.; Castell-Perez, M. Quantifying the effectiveness of washing treatments on the microbial quality of fresh-cut romaine lettuce and cantaloupe. LWT 2017, 86, 270–276. [Google Scholar] [CrossRef]
| Product | Batch | Inoculation Density [cfu g−1] | Day 0 Median Value (Log10 cfu g−1) | Day 7 Median Value (Log10 cfu g−1) | Growth Potential (δ) (Log10 cfu g−1) |
|---|---|---|---|---|---|
| Spinach | 1 | 100 | 1.98 | 4.14 | 2.16 |
| Spinach | 2 | 100 | 1.73 | 4.19 | 2.46 |
| Spinach | 3 | 100 | 2.02 | 4.60 | 2.58 |
| Spinach | 4 | 10 | 0.88 | 3.27 | 2.39 |
| Rocket | 1 | 100 | 2.00 | 3.08 | 1.08 |
| Rocket | 2 | 100 | 1.61 | 3.37 | 1.76 |
| Rocket | 3 | 100 | 1.99 | 3.69 | 1.70 |
| Rocket | 4 | 10 | 0.92 | 2.74 | 1.82 |
| Lettuce | 1 | 100 | 1.79 | 3.07 | 1.28 |
| Lettuce | 2 | 100 | 1.96 | 3.65 | 1.69 |
| Lettuce | 3 | 100 | 1.34 | 3.96 | 2.62 |
| Lettuce | 4 | 10 | 0.97 | 2.55 | 1.58 |
| Product | Batch | Inoculation Density [cfu g−1] | Day 0 Median Value (Log10 cfu g−1) | Day 9 Median Value (Log10 cfu g−1) | Growth Potential (δ) (Log10 cfu g−1) |
|---|---|---|---|---|---|
| Spinach | 1 | 100 | 1.98 | 4.34 | 2.36 |
| Spinach | 2 | 100 | 1.73 | 4.51 | 2.78 |
| Spinach | 3 | 100 | 2.02 | 4.85 | 2.83 |
| Spinach | 4 | 10 | 0.88 | 3.78 | 2.90 |
| Rocket | 1 | 100 | 2.00 | 3.67 | 1.67 |
| Rocket | 2 | 100 | 1.61 | 3.55 | 1.94 |
| Rocket | 3 | 100 | 1.99 | 3.86 | 1.87 |
| Rocket | 4 | 10 | 0.92 | 3.13 | 2.21 |
| Day 0 | Day 2 | Day 5 | Day 7 | Day 9 | |
|---|---|---|---|---|---|
| Spinach 100 cfu g−1 | 9.2 ± 0.8 | 8.7 ± 0.8 | 8.2 ± 0.4 | 7.2 ± 0.6 | 7.1 ± 0.6 |
| Rocket 100 cfu g−1 | 8.8 ± 0.9 | 8.7 ± 1.1 | 6.3 ± 0.5 | 6.5 ± 0.6 | 5.6 ± 0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culliney, P.; Schmalenberger, A. Growth Potential of Listeria monocytogenes on Refrigerated Spinach and Rocket Leaves in Modified Atmosphere Packaging. Foods 2020, 9, 1211. https://doi.org/10.3390/foods9091211
Culliney P, Schmalenberger A. Growth Potential of Listeria monocytogenes on Refrigerated Spinach and Rocket Leaves in Modified Atmosphere Packaging. Foods. 2020; 9(9):1211. https://doi.org/10.3390/foods9091211
Chicago/Turabian StyleCulliney, Paul, and Achim Schmalenberger. 2020. "Growth Potential of Listeria monocytogenes on Refrigerated Spinach and Rocket Leaves in Modified Atmosphere Packaging" Foods 9, no. 9: 1211. https://doi.org/10.3390/foods9091211
APA StyleCulliney, P., & Schmalenberger, A. (2020). Growth Potential of Listeria monocytogenes on Refrigerated Spinach and Rocket Leaves in Modified Atmosphere Packaging. Foods, 9(9), 1211. https://doi.org/10.3390/foods9091211