Abstract
Blue light primarily exhibits antimicrobial activity through the activation of endogenous photosensitizers, which leads to the formation of reactive oxygen species that attack components of bacterial cells. Current data show that blue light is innocuous on the skin, but may inflict photo-damage to the eyes. Laboratory measurements indicate that antimicrobial blue light has minimal effects on the sensorial and nutritional properties of foods, although future research using human panels is required to ascertain these findings. Food properties also affect the efficacy of antimicrobial blue light, with attenuation or enhancement of the bactericidal activity observed in the presence of absorptive materials (for example, proteins on meats) or photosensitizers (for example, riboflavin in milk), respectively. Blue light can also be coupled with other treatments, such as polyphenols, essential oils and organic acids. While complete resistance to blue light has not been reported, isolated evidence suggests that bacterial tolerance to blue light may occur over time, especially through gene mutations, although at a slower rate than antibiotic resistance. Future studies can aim at characterizing the amount and type of intracellular photosensitizers across bacterial species and at assessing the oxygen-independent mechanism of blue light—for example, the inactivation of spoilage bacteria in vacuum-packed meats.
1. Introduction
Annually, there are 600 million cases and 420,000 deaths associated with food-borne pathogens, with the majority of the disease burdens (550 million cases and 230,000 deaths yearly) attributed to diarrheal diseases [1]. Bacterial pathogenic agents are major contributors to these diarrheal infections, particularly Salmonella enterica, Camplyobacter spp. and Escherichia coli [1], and can linger in food-processing environments and food products (for example, minimally-processed foods, such as fresh-cut fruits and vegetables or raw seafood). These findings highlight the importance of robust sanitization systems in the food industry.
While heat is a potent germicidal agent, thermal processing of foods may lead to undesirable organoleptic properties and the loss of nutrients. Consumer perception of food safety has also been associated with aversion to chemical hazards, which include food preservatives, pesticides and drug residues [2,3]. Thus, there is a need for non-thermal sanitization technologies that are also free of chemicals.
The emerging non-thermal food-processing technologies include high-pressure processing (HPP) and pulsed electric field (PEF) [4,5,6]. However, the current forms of these technologies are more costly and less energy efficient—and thus less environmentally friendly—than thermal processing. For example, when used to pasteurize orange juice, HPP and PEF were estimated to consume 24–27 times more electricity (kW/year), incur 5–7 times higher total cost (cents/L) and emit 7–9 times more carbon dioxide than thermal pasteurization [7].
Alternatively, light-based technologies, particularly light-emitting diodes (LED), can be used as a cheap and sustainable non-thermal sanitization system [8]. It is known that ultraviolet-C (UV-C; particularly at 254 nm) exhibits bactericidal activities by inducing the formation of pyrimidine dimers in the bacterial genome and thus can be used within the food industry to sanitize food products or the processing environments [9,10]. However, health issues may arise from the use of ultraviolet (UV) radiation in the food industry, especially as constant exposure of workers to UV may increase the risks for skin cancer (for example, basal cell carcinoma, squamous cell carcinoma and malignant melanoma) [11,12]. A study also reported that accidental exposure of two healthcare workers to UV-C germicidal lamps (254 nm) led to bilateral keratoconjunctivitis and face erythema after 12–24 h, followed by other complications to the skin, eyes, nail and hair after 24 months [13].
In this review, we provide discussions on another emerging light-based sanitization technology derived from the blue region of the visible light spectrum, which is less detrimental to mammalian cells than UV [14] and thus allows for a wider application within the food supply chain due to its safety. We focus on studies that assessed the bactericidal efficacy of blue light-mediated technology on surfaces and in different food matrixes. Further, we also discuss the antimicrobial mechanisms of blue light, available technologies, safety aspects, the combination of blue light with other treatments (hurdle technology) and the potential development of bacterial tolerance to blue light. Additionally, a brief discussion on the inactivation of fish pathogenic bacteria (non-human pathogens) is also provided.
2. Pathogenic Bacteria in Food
Food-borne diseases are mainly caused by the consumption of contaminated food or water, with contamination possibly occurring at any point of production or distribution. Globally, the major food-borne pathogenic bacteria include Salmonella spp., Campylobacter spp., enterohemorrhagic E. coli (EHEC), Listeria monocytogenes and Vibrio cholerae [15]—the distribution of these bacteria across the globe, among other food-borne pathogenic agents, is summarized in a report by the World Health Organization [1].
In food-processing environments, Gram-negative bacteria are pre-dominant, particularly Pseudomonas spp., Enterobacteriaceae (especially Serratia spp.) and Acinetobacter spp. Among Gram-positive bacteria, lactic acid bacteria (LAB), Staphylococcus spp. and Bacillus spp. are the most commonly identified residential bacteria. While some of these tend to be innocuous, several pathogenic strains, such as Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus cereus, are also known to linger on surfaces, especially due to their ability to form spores or biofilms [16].
The main pathogenic bacteria associated with dairy products are L. monocytogenes, Salmonella spp., S. aureus, Cronobacter spp. and Shiga toxin-producing E. coli (STEC) [17,18]. Dairy farm environments are a common habitat for L. monocytogenes, Salmonella spp. and STEC [19,20,21,22,23,24,25], whereas S. aureus is less prevalent in the environments and its transmissions are more likely to occur through contaminated animals (for example, those that have mastitis) [26,27]. There are no conclusive data on the natural environments of Cronobacter spp., especially Cronobacter sakazakii that is commonly associated with contaminated infant formulas [28,29], albeit these bacteria have been associated with plants [30,31,32] and animal feed [27,33]. The prevalence and type of dairy-associated pathogenic bacteria may also vary with animal source of the milk and geographical location [34,35]. Based upon these data, pathogens are mainly transferred to dairy products or processing environments from farm environments (soil, animal feed, etc.). For instance, two outbreaks (STEC O26:H11 in Italy and L. monocytogenes in Canada) were associated with contaminated dairy processing plants (cheese and milk plants) [36,37]—one study found that contaminated cheeses from a dairy plant had been distributed to approximately 300 retailers, which caused extensive cross-contamination [36]. Contamination at the retail level has also been reported, with L. monocytogenes, Salmonella spp., Shigella spp. and E. coli O157:H7 found in cheeses and raw milk [38,39,40].
Outbreaks related to farm-based meat products, such as beef and pork, are primarily caused by Salmonella spp. and EHEC (particularly E. coli O157:H7) [41,42,43]. Other bacteria have also been identified as causes of meat product-related outbreaks, namely L. monocytogenes, S. aureus, Campylobacter spp., Clostridium spp. and B. cereus [41,42,44]. While poultry products may also carry all of these pathogens, previous outbreaks were mostly caused by Salmonella spp., Campylobacter spp. or Clostridium perfringens [45,46,47,48,49,50,51]. In food-processing plants, fecal matters (for example, in hides or poultry skin) and aerosols generated during processing (for example, dehiding or evisceration processes) could facilitate the spread of pathogenic bacteria [44,52]. Several studies have also reported on the prevalence of pathogenic bacteria, including E. coli O157:H7, Salmonella spp., Shigella spp. and antibiotic-resistant S. aureus, in retail shops across countries—these bacteria were found on the products (raw or cooked beef, mutton, pork, chicken and turkey) and in the environments [39,53,54,55,56].
In seafood, the major pathogenic bacteria include Vibrio spp., Salmonella spp., L. monocytogenes, Campylobacter spp., EHEC, Clostridium spp. and Shigella spp., which could cause diseases ranging from mild gastroenteritis to life-threatening infections [57,58,59,60]. Vibrio spp. is ubiquitous in aquatic ecosystems, with infections in humans commonly associated with Vibrio parahaemolyticus, Vibrio vulnificus and V. cholerae [57,61,62]. Other bacteria, such as Salmonella spp. and E. coli, may also proliferate in bodies of water, particularly when contaminated with sewage effluents [57,63]. Cross-contamination during food production is the primary route of transmission for L. monocytogenes within the seafood industry and thus presents a major concern due to its ability to persist in the environment and to multiply during refrigeration [64]. Similarly, a study identified the contamination of a cutting board by V. parahaemolyticus from raw squid as a cause of a gastroenteritis outbreak at a food bazaar in South Korea [65]. Further, there is a heightened health risk in consuming raw seafood, as evident from previous outbreaks associated with uncooked (or undercooked) fish, oysters, abalone or sea squirt [66,67,68,69].
Fruits and vegetables may harbor a myriad of pathogenic bacteria, such as Shigella spp., B. cereus, Campylobacter spp., Yersenia enterocolitica and Clostridium botulinum, albeit previous outbreaks were mostly associated with STEC (particularly E. coli O157:H7), Salmonella spp. and L. monocytogenes [70,71,72]. Irrigation water that comes from contaminated sources is a major reservoir for these pathogens [73,74,75] and may occasionally carry Vibrio spp., for example, two studies identified Vibrio spp. on vegetables irrigated with untreated water from streams [76] and wastewater [77]. Other sources of contamination include pre-harvest factors, such as compost, insects, soil and wildlife animals, along with harvesting equipment or post-harvest vectors, including human (during packing), transport vehicles and processing equipment [70,71]. Pathogenic bacteria have also been detected in different horticultural products at the retail level across the globe: L. monocytogenes and E. coli isolated from frozen fruits or vegetables (England) [78]; L. monocytogenes, S. enterica or E. coli from ready-to-eat raw vegetables (UK, Malaysia or Nigeria) [79,80,81]; and Salmonella enterica subsp. enterica serovar Typhimurium, C. perfringens, Campylobacter spp. or L. monocytogenes from fresh produce (Mexico, Canada or New Zealand) [82,83,84]. In addition, a meta-analysis of 53 studies identified 453 cumulative incidences of STEC, L. monocytogenes and Salmonella spp. in fruits/vegetables from retail establishments across Europe between the years 2001 and 2017, with L. monocytogenes dominating in vegetables and STEC in fruits [85].
Bacteria may form biofilms to resist physical, mechanical and/or chemical stresses, including chemical disinfectants used in food-processing environments. For instance, several pathogenic staphylococci isolated from food or food equipment, namely Staphylococcus capitis, Staphylococcus cohini, Staphylococcus saprophyticus and Staphylococcus epidermidis, had shown abilities to form biofilms on polystyrene and stainless steel [86]. The stability of these biofilms against disinfectants (benzalkonium chloride) or denaturation enzymes (dispersin B, proteinase K or trypsin) is dictated by their compositions, which are determined by the presence of genes encoding either cell wall anchored proteins (CWA) or polysaccharide intracellular adhesin (PIA) [87]. This finding presents an alternative mechanism of biofilm formation in Staphylococcus spp., which is predominantly attributed to the presence of ica operon (icaADBC locus and icaR regulatory gene) that encodes PIA [88]—for example, icaA gene was found to be correlated to strong biofilm formation in food-related staphylococci isolates [86]. Other major biofilm-forming pathogenic bacteria include L. monocytogenes (poultry, red meat, seafood and dairy), Salmonella spp. (poultry, red meat, seafood and horticulture), E. coli O157:H7 (red meat and horticulture), B. cereus (dairy, seafood and horticulture), Vibrio spp. (seafood) and Campylobacter spp. (poultry) [89,90,91,92].
In addition, biofilms are composed of bacterial aggregates enclosed in extracellular polymeric matrix, which constitutes polysaccharides, proteins, lipids and exogenous deoxyribonucleic acids (DNA), and can function as a platform for physical/social interactions (for example, microbial consortia) that enhance gene transfers [93]. Several bacteria, such as B. cereus and E. coli O157:H7, also form multispecies biofilms to enhance their survival in food-processing lines [94,95]. The formation of biofilms is also dependent on bacterial structures that are responsible for initial surface attachment, such as flagella and/or fimbriae (for example, curli) in L. monocytogenes [96,97], S. Typhimurium [98,99], E. coli O157:H7 [100] and V. cholerae [101].
3. Antimicrobial Blue Light
3.1. Mechanism
Bactericidal effects of blue light are mostly attributed to the wavelength range of 400 to 450 nm [102], although several reports have demonstrated the antimicrobial efficacy of blue light at longer wavelengths (460, 465 or 470 nm) [103,104,105,106]. Blue light-mediated inactivation of bacteria is associated with the generation of reactive oxygen species (ROS) when the light is absorbed by endogenous photosensitizers, which can be found in different types of bacteria (Gram positive and Gram negative; aerobic and anaerobic) [107]. Given that these photosensitizers, such as protoporphyrin, coproporphyrin and uroporphyrin, are intermediate species in the heme biosynthesis, it is likely that they are accumulated in the cytoplasmic matrix [108,109], although their precise locations within the bacterial cell are not fully understood.
The blue light-mediated photosensitization process is dependent on the presence of oxygen and mainly induces cytotoxicity (apoptosis or necrosis) through oxidative stresses caused by singlet oxygen species (1O2) [110]. Upon illumination, photosensitizers at a ground state (lowest energy level) are converted into their excited singlet state (short-lived) or triplet state (long-lived), which, in the presence oxygen, can undergo two types of energy transfer: (1) type I that produces toxic oxygen species, such as hydrogen peroxide (H2O2), superoxide or hydroxyl radicals; (2) type II that generates 1O2 [111]. Subsequently, these ROS can induce damages to different parts of the bacterial cells, including the cell membrane, cell wall and genome (Figure 1).
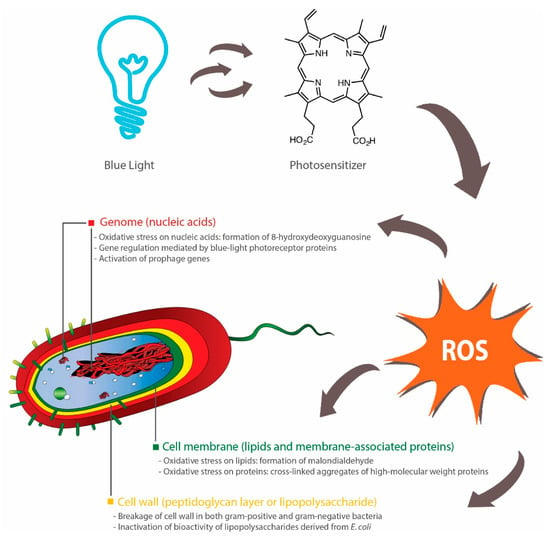
Figure 1.
Bactericidal activities of blue light rely on activation of endogenous photosensitizers, such as porphyrins, which subsequently induces the production of reactive oxygen species (ROS). These ROS inflict oxidative damages to nucleic acids [116], lipids [113,115] and proteins [118]. Inhibition of biofilm formation can also occur through blue light-regulated transcriptional pathways [123,124,125] or bacterial inactivation through the activation of prophage genes [126]. Breakages of cell walls [120,121] and inactivation of lipopolysaccharides (outer membrane of Gram-negative bacteria) [122] have been reported, although the precise effects of antimicrobial blue light on peptidoglycan and lipopolysaccharide are not fully elucidated.
An increase in blue light-induced membrane permeability was observed across several studies [112,113,114,115], although the precise mechanism is not fully elucidated. A study found that blue light illumination (405 nm) did not affect the lipid membrane of Salmonella spp.—there was an absence of malondialdehyde, which is a product of lipid peroxidation [116]. In contrast, two studies demonstrated that blue light inactivation (415 nm) of methicillin-resistant S. aureus (MRSA) or C. sakazakii involved lipid peroxidation, as determined by the detection of malondialdehyde and reduction in post-treatment unsaturated fatty acids (C16:1 in both bacteria, C20:1 and C20:4 in MRSA, and C18:1 and C18:2 in C. sakazakii) [113,115]. Further, while one study observed the presence of blue light-induced oxidation of guanine residues in the bacterial DNA of Salmonella spp. (presence of 8-hydroxydeoxyguanosine) [116], others reported no DNA breakage in blue light-treated (405 nm) E. coli O157:H7, Shigella sonnei and S. Typhimurium [112]. These discrepancies are potentially due to the fact that the type and amount of endogenous photosensitizers vary across different bacterial species, although further investigations are needed to explain the different susceptibilities of bacteria to blue light [117].
In addition to lipids and nucleic acids, blue light can also attack proteins, carbohydrates (polysaccharide) and peptidoglycan (polymers of amino acids and sugars in bacterial cell walls). Blue light treatments, in the presence of exogenous cationic photosensitizers, induced the loss of cell membrane-associated proteins in S. aureus [118] and the reduction of 81% in the polysaccharide content within P. aeruginosa biofilms [119]. In two studies, images taken by transmission electron microscopy revealed blue light-induced breakages of bacterial cell walls in MRSA [120] and Acinetobacter baumannii [121]. Further, E. coli lipopolysaccharide coated on titanium disc was inactivated upon illumination by blue light (405 nm), as evident from the reduced activities of mouse macrophages post-treatment [122]. However, the current literature lacks data on the effect of blue light on lipopolysaccharide (endotoxin) contained within intact outer membranes of Gram-negative bacteria and thus it is a subject of future studies.
Blue light can act as a transcriptional regulator in bacteria [127,128], especially due to the presence of blue light receptor proteins [128]. These photoreceptors include the blue light-sensing flavin adenine diphosphate (BLUF) proteins that can undergo conformational changes upon illumination by blue light and subsequently elicit downstream effects on bacterial surface attachments, biofilm formation and motility [129]. For instance, two studies found that a BLUF-associated protein, namely YcgF, downregulated the synthesis of curli fibres, but upregulated biofilm formation in E. coli [123,124]. In contrast, others reported that A. baumannii-harboring blsA gene, which encodes BLUF-containing photoreceptor proteins, did not form biofilms under blue light (462 nm), whereas biofilms were observed in a mutant strain with no functional blsA [125]. The viability of these bacteria was not affected by blue light in both wild and mutated strains, although blue light had a negative effect on bacterial motility and pellicle formation [125]. These findings indicate that there is a variety of blue light-sensing pathways in bacteria, which could be further explored as an alternative method for controlling the growth of bacteria. Another study also observed an alternative molecular mechanism of bactericidal blue light (460 nm) that involved the activation of prophage genes in MRSA, which subsequently led to the killing of the bacteria [126]. Future studies could aim at investigating the presence of similar genes (light-sensing and prophage genes) in food-borne bacteria and subsequently at designing targeted blue light-mediated interventions for controlling the persistence of these bacteria in food or food-processing environments.
In summary, antimicrobial blue light can act upon different parts of the bacterial cell, primarily through the action of ROS. These ROS can induce oxidative damages to a range of macromolecules, such as lipids (cell membrane), proteins (cell wall-associated proteins), nucleic acids (DNA, RNA or plasmids) and polysaccharides (extracellular matrix of biofilms). Additionally, several bacterial species, such as E. coli and A. baumannii, possess blue light receptors that control biofilm formation and motility, and thus can be targeted to reduce their persistence in the environments. Further, several prophage genes may be activated by blue light and induce inactivation of the carrying bacteria (Figure 1).
3.2. Available Technologies
The majority of studies on antimicrobial blue light have used light-emitting diodes (LED) as a light source. LED is commonly comprised of semiconductor materials that are doped with impurities, which create free electrons on the n side and holes (absence of electrons) on the p side—also known as the p–n junction. When electrical voltage is applied, current flows from the positively-charged end (p side; anode) to the negatively-charged end (n side; cathode), with electrons moving in the opposite direction. Subsequently, as an electron interacts with a hole, it falls to a lower energy state through the release of a photon. In this process, the resulting color emitted corresponds to the band gap energy within the p–n junction, which depends on the semiconductor materials and impurities used [130,131]. Currently, a typical blue LED is made of indium/gallium nitride (InGaN) layers grown on sapphire or silicon substrates, which can theoretically cover the entire visible light spectrum—365 nm (GaN) to 1771 nm (InN)—albeit the quality of materials deposited within the LED structure declines continuously beyond 480 nm due to a range of inherent material challenges [132].
Laser diode, which emits light with a higher coherence and narrower emission band than LED, is another source of blue light that has been used in clinical settings. The photomodulative effects of these two light sources on biological systems have been a subject of debates, especially due to their differences in light coherence and wavelength bandwidth. However, accumulating evidence suggests that these parameters have little effect on the biological efficacy of light-based technologies—for example, two studies found similar effects of red LED and laser diode upon tissue repair in rats [133,134]. Others also proposed that biological effects of light were dependent on dosage and wavelength, but not on light sources, with similar healing effects of LEDs and laser diodes on skin wounds reported across different studies [135]. Similarly, the antimicrobial potency of blue light is independent of the light source used, as one in vitro study revealed that LED (405 nm; non-coherent light) and laser diode (405 nm; coherent light) were equally efficient in inactivating MRSA across four light dosages (40, 54, 81 or 121 J/cm2) [136]. Although LED is relatively cheaper than laser diodes, it remains unclear which technology is more efficient based upon their germicidal output per unit electrical power input. In addition, superluminous diode (SLD; 405 or 470 nm), which is an intermediate between LED and laser diode in its light intensity, coherence and emission bandwidth [137,138,139], has also shown bactericidal activities against P. aeruginosa, MRSA and S. aureus in vitro [140,141,142].
A femtosecond laser, which emits ultrasecond pulses at approximately 10−15 s per pulse, is another technology that can be used to deliver antimicrobial blue light. At light dosages of 18.9–75.6 J/cm2 (5–20 min), a blue femtosecond laser (400, 410 and 420 nm) inhibited the growth of S. aureus and P. aeruginosa on agar plates (inhibition zones observed), possibly due to DNA damages induced by ROS [143]. In agreement, a femtosecond laser (425 nm; 800 J/cm2; 1 h) reduced a mutant S. Typhimurium lacking RecA proteins (reponsible for damaged DNA repair) by 5 log colony forming units (CFU), whereas only 0.5-log reduction (CFU) was observed for the wild-type bacteria and thus this finding enhanced the view that DNA damage was a predominant inactivation mechanism of a bactericidal femtosecond laser [144]. However, the two studies used different methods for measuring bactericidal activity (qualitative or quantitative), and also differed in their light dosages (max. 75.6 J/cm2 or 800 J/cm2) and treatment times (max. 20 min or 60 min) [143,144]. Therefore, the potential use of a femtosecond laser as an antimicrobial technology depends on future investigations into its energy efficiency and also its efficacy against different types of bacteria.
3.3. Blue Light Regimes
Antimicrobial blue light may be delivered at high irradiance with short duration times (HI-SD) or low irradiance with long duration times (LI-SD). A study demonstrated that the bactericidal activity of blue light (405 nm) was dependent on light dosage: the highest inactivation of four bacteria, namely S. aureus, Streptococcus pneumoniae, E. coli and P. aeruginosa, was achieved at the highest irradiance (approximately 9 mW/cm2) for a constant treatment time (120 min) or in the longest illumination time (250 min) at a constant irradiance (approximately 9 mW/cm2) [145].
In the same study, HI-SD treatment (approximately 9 mW/cm2 for 250 min) was also less effective than LI-SD (approximately 2.25 mW/cm2 for 1000 min) in inactivating pathogenic bacteria. Isolated colonies were observed on the perimeter of plates exposed to HI-SD, whereas confluent border present on LI-SD plates, indicating post-treatment migration of bacteria to the nutrient-rich and non-treated areas on HI-SD plates. Thus, LI-SD seemed to exhibit higher bactericidal and bacteriostatic effects on a qualitative level [145]. A similar finding was reported for L. monocytogenes, with LI-SD treatment of 10 mW/cm2 for 180 min yielding a 5.18-log reduction (CFU/mL), whereas HI-SD treatments of 20 mW/cm2 for 90 or 30 mW/cm2 for 60 min produced bacterial inactivation of approximately 5 log CFU/mL—the differences were not statistically significant [146]. However, neither study assessed the germicidal efficiency of HI-SD or LI-SD treatments per unit energy [145,146] and thus it remains inconclusive whether either regime is more suitable for practical applications in the food industry.
Alternatively, blue light can be delivered as pulses to increase its bactericidal efficiency. Pulsed blue light technology (450 nm; 33% duty cycle; three times a day for 3 days at 30 min intervals between each treatment) was reported to inactivate planktonic MRSA and Propionibacterium acnes (7 log CFU/mL) at light dosages of 7.6 and 5 J/cm2, respectively [147]. The same technology (7.6 J/cm2) also disrupted the biofilm networks of both bacteria and reduced the number of viable bacteria within the biofilm structures by approximately 1.89 and 1.56 log CFU/mL for MRSA and P. acnes, respectively [147]. In support of this view, pulsed blue LED (450 nm; 33% duty cycle) had a higher bactericidal efficiency against P. acnes than two other regimes (20% or 100% duty cycle), with a 7-log reduction (CFU/mL) achieved at a light dosage of 5 J/cm2 (2 mW/cm2 repeated nine times at 3-h intervals) [148].
For S. aureus, pulsed blue LED (405 nm; 25, 50 or 75% duty cycle) and continuous blue light (405 nm; 100% duty cycle) had similar inactivation efficiency (95–98%), albeit the pulsed blue light had approximately 83% higher optical efficiency (bacterial reduction in CFU/mL per J/cm2) [149]. Based upon these findings [147,148,149], pulsed blue light is preferred than continuous blue light in vitro, but its utilization in food settings is a subject of further investigations into its ability to remain energy efficient during scale up.
3.4. Safety of Blue Light
Safety assessments of blue light have mostly been conducted in clinical settings. Generally, exposure of skin to blue light is safe, albeit high fluences at certain wavelengths could induce cytotoxic effects. In one study, eight volunteers were exposed to blue light (380–480 nm; peak at 420 nm; 100 J/cm2 per day) for five consecutive days and the subsequent results of their skin biopsies were reported as follows: (1) no significant change in the expression of p53, i.e., no DNA damage; (2) no inflammatory cells and sunburn before and after treatment; (3) transient melanogenesis and vacuolization of keratinocytes observed, although these changes did not result in cell apoptosis [150]. Similarly, an in vitro study demonstrated that blue light (415 nm) could be used to inactivate P. aeruginosa on skin burns without inflicting any damage on the mouse skin at an effective antimicrobial dosage of 55.8 J/cm2 [151]. Exposure to the same blue light at a dosage of 109.9 J/cm2 inactivated human keratinocytes and P. aeruginosa by 0.16 log cell/mL and 7.48 log CFU/mL, respectively. However, cytotoxic effects of blue light on human endothelial and keratinocyte cells were observed at wavelengths of 412, 419 and 426 nm (66–100 J/cm2) or 453 nm (>500 J/cm2) [152].
In an in vitro study, damages on human corneal and conjuctival epithelial cells were observed after prolonged (17 h) exposure to blue light (420 and 430 nm at 1.13 and 1.16 W/cm2, respectively), with the authors reporting decreased cellular viabilities, morphological changes of the cells, accumulation of ROS and altered mRNA expression of biomarkers associated with cellular inflammatory response and antioxidant defense system [153]. A review article presented evidence of the adverse effects that blue light (415–455 nm) inflicted on retina (oxidative stress), lens (cataract due to accumulating ROS) and blood-retinal barrier functions [154]. Another group of researchers also reported the suppression of plasma melatonin in eight human subjects exposed to blue light (469 nm; corneal irradiance 0.1–600 W/cm2 for 90 min)—the extent of suppression was significantly higher at higher irradiances (p < 0.0001)—which suggests that blue light has the potential to disrupt circadian rhythm [155].
Widespread implementation of blue light-based technologies requires robust safety standards. According to the American Conference of Governmental Industrial Hygienists, daily exposure of workers to blue light is recommended to follow these rules: (1) for an exposure of 10,000 s (2.8 h) or more, the maximum intensity of the light source is ≤0.01 W/cm2.sr; (2) for light intensity above 0.01 W/cm2.sr, the maximum light dosage is 100 J/cm2.sr, where light dosage (J/cm2.sr) = light intensity (W/cm2.sr) × time of exposure (s); (3) for a light source subtending an angle less than 0.011 radian, the maximum light intensity is 10−4 W/cm2 for viewing durations greater than 100 s [156]. In accordance with these recommendations, a study analyzed blue light-related hazards through optical radiation measurements of several light sources [157]—the methodology in this study can be applied within the food industry for assessing the safety of different antimicrobial blue light technologies.
Based upon the findings presented in this section, blue light is innocuous on the skin, but deleterious to the eyes. Thus, safety glasses can be prescribed for personnel working within the proximity of high-intensity blue light sources. A study reported that several glasses and light filters significantly reduced (p < 0.001) the transmission of blue light from two LEDs (389–500 nm at 1625 mW/cm2 or 410–510 nm at 1680 mW/cm2; 10 s) by at least 97% [158]. Others reported that the use of blue light-blocking amber glasses improved the sleep quality of people with sleep disorders (self-reported or clinically diagnosed) [159,160], with an earlier endogenous dim-light melatonin onset observed when patients wore amber glasses [160]. However, there are no available data on whether anti-blue light glasses or filters can prevent damages to ocular cells. In addition, there is a need for a universal safety standard that governs the use of antimicrobial blue light within the food industry and thus the scientific community should aim at establishing the effective antimicrobial light dosages for different food-borne bacteria.
4. Application of Antimicrobial Blue Light on Surfaces and in Food Matrixes
4.1. Inactivation of Bacteria on Food Packaging and Work Surfaces
Heating effects induced by blue light treatment would be undesirable in industrial settings. Two studies reported that the surface temperatures of stainless steel increased to approximately 50–56 °C when treated with antimicrobial blue light (405 nm; 150–306 mW/cm2; 180–185 J/cm2) [161,162]. In these studies, bacterial reductions of 5 and <1 log CFU were achieved for Campylobacter spp. [162] and other pathogenic bacteria [161], respectively (Table 1). However, others observed a temperature increase of only 2.5 °C when stainless steel was continuously illuminated with blue light for 8 h (405 nm; 26 mW/cm2, 748.8 J/cm2) [163]. The discrepancies between these studies can be attributed to the different light intensities (mW/cm2) used and thus optimization studies are required to determine the suitable combination of light intensity and treatment time, i.e., high intensity-short duration or low intensity-long duration. For example, the reduction of blue light dosage from approximately 183–186 to 89–92 J/cm2 alleviated surface heating effects—final surface temperatures were approximately 44–56 and 31–36 °C at 183–186 and 89–92 J/cm2, respectively—although the bacterial inactivation was also reduced from 5 log CFU (183–186 J/cm2) to 1.1–3.1 log CFU (89–92 J/cm2) [162].

Table 1.
Blue light inactivation of pathogenic bacteria on surfaces.
At 4, 15, and 20 °C, the formation of biofilm was inhibited by blue light (405 nm; 748.8 J/cm2) on stainless steel and acrylic coupons contaminated with L. monocytogenes-laden salmon exudates. However, the bacterial population within blue light-treated pre-formed biofilms was only significantly reduced (p < 0.05) at 25 °C [163]. This finding suggests that the blue light is more effective when used on cells contained in forming biofilms than in established biofilms. The efficacy of blue light in inactivating biofilms on other surfaces is a subject of future studies.
Blue light was able to traverse transparent solid surfaces, such as glass and acrylic slides, which was evident from the same inactivation rates of E. coli biofilms on top (direct exposure) or at the bottom (indirect exposure) of these slides, although four percent of the light irradiance was lost during transmission across both slides [164]. However, another study found that the inactivation of L. monocytogenes on tryptic soy agar was dependent on the ability of blue light (406–470 nm) to penetrate several packaging materials used to cover the agar—for example, no inhibition was observed when polyethylene + nylon was used, whereas maximum inhibition was obtained with polypropylene [165]. Thus, it is pertinent that packaging or surface materials are taken into considerations prior to designing blue light treatments intended to inactivate bacteria located behind these materials.
4.2. Inactivation of Bacteria in Dairy and Liquid Foods: Milk, Cheese and Orange Juice
Current data suggest that antimicrobial blue light is effective against pathogenic and spoilage bacteria in dairy and liquid foods. At least 3-log reduction was achieved in all studies reviewed (Table 2), with the extent of bactericidal efficacy depending on temperature and light wavelength. For milk products, two studies assessed the blue light-mediated inactivation of pathogenic bacteria in skim and whole milk, but no data are available on blue light inactivation in concentrated milk. A study reported that pulsed white light (200–1100 nm) was able to inactivate E. coli in skim and whole milk, albeit not in concentrated milk [170]. Thus, future research is needed to ascertain whether antimicrobial blue light can retain its bactericidal potency in milk products with varying total solid contents.

Table 2.
Blue light inactivation of pathogenic bacteria on dairy products.
Interestingly, a study found that blue light inactivation of several bacterial strains in milk was more efficient than in a clear liquid matrix (PBS), except for Mycobacterium fortuitum. Two explanations were proposed: (1) blue light was absorbed by riboflavin (photosensitizer) in milk, as apparent from the significant (p < 0.05) reduction in the amount of riboflavin post-treatment, which subsequently generated ROS; (2) milk strongly scattered light and retained the light longer within its matrix, relative to PBS [171]. In contrast, blue light inactivation of Campylobacter spp. was significantly higher (p < 0.05) in transparent Brucella broth than in opaque chicken exudate [162]. These findings suggest that the type of solid particulate in liquid matrixes determines whether bactericidal efficacy of blue light is enhanced or attenuated.
Further, the bactericidal efficacy of blue light in liquid matrix also varies across different bacterial species/strains tested. In PBS, blue- light treatment (405 nm) resulted in a 5-log reduction (CFU/mL) of C. jejuni (18 J/cm2) [172] and L. monocytogenes (185 J/cm2) [173], whereas Salmonella spp. and E. coli O157:H7 was only reduced by less than 1.5 log CFU/mL at light dosages of 180–185 J/cm2 [172,173]. Thus, future studies are required to establish the effects of liquid opacity, particularly for liquid foods, on blue light inactivation of different bacteria.
Blue light inactivation of E. coli in liquid milk was more efficient at lower wavelengths and higher temperatures, with optimal treatment (reduction of 5 log CFU/mL and minimum color change) achieved at 405 nm, 13.8 °C and for 37.83 min [174]. This view was corroborated by others, who found that S. enterica in orange juice was inactivated by blue light (460 min; 4500 J; 92 W/cm2) to a higher degree at 12 or 20 °C than at 4 °C, although the inactivation rate was the same across these temperatures at higher light intensities (147.7 or 254.7 mW/cm2) [175].
Further, major milk components, namely proteins, lipids and lactose, were retained after 2 h of blue light treatment (720 J/cm2), albeit the loss of riboflavin (vitamin B2) had resulted in a bleaching effect that was perceptible to naked eyes [171]. In orange juice, blue light treatment also induced a color change in a temperature- and light intensity-dependent manner, particularly when the treatment was applied at a low intensity with long duration (light dosage was constant) [175].
On cheeses, blue light treatments were effective against L. monocytogenes and Pseudomonas fluorescens (Table 2), with no color changes observed in treated ricotta [176] and packaged slice cheeses [165]. Hyun and Lee (2020) also found that the efficacy of blue light on packaged sliced cheese was higher at 4 °C than at 25 °C [165].
4.3. Inactivation of Bacteria in Horticultural Products
Several studies found that blue light sanitization of fruits and vegetables was dependent on the type of product. Glueck et al. observed that the photosensitizer-mediated inactivation of blue light (435 nm; 33.8 J/cm2) was affected by the geometry of the food, with higher efficacy observed in flat-surfaced vegetables (cucumber, tomatoes and lettuce) than in those with complex structures (fenugreek seeds, mung bean seeds and mung bean germlings) (Table 3) [177]. In support of this view, three other studies showed varying bactericidal efficacies of blue light across different application media. Tortik et al. demonstrated that the combination of blue light (435 nm; 33.8 J/cm2) and curcumin (50 µM) reduced the bacterial load of S. aureus on peppers and cucumber by 2.5–2.6 log CFU [178], whereas an identical treatment in a clear liquid matrix (PBS) resulted in a 7-log reduction (CFU) in the number of S. aureus [179]. Buchovec et al. also found that S. Typhimurium was inactivated by chlorophyllin/chitosan-mediated blue light treatment (405 nm; 38 J/cm2) to a lower degree on strawberries (2.2 log CFU/mL) than in PBS (6.5 log CFU/mL) [180]. Possible explanations include the varying light-reflecting properties of different matrixes, the adsorption of photosensitizers onto the cuticle of vegetables/fruits and the presence of antioxidants in vegetables/fruits that reduced the efficacy of blue light [178,180].

Table 3.
Blue light inactivation of pathogenic bacteria in horticultural products.
In the majority of studies that we reviewed, blue light treatments resulted in no detrimental effects upon the sensorial and nutritional properties of fruits and vegetables. For instance, the antioxidant activities of cherry tomatoes, fresh-cut mangoes and papayas were retained after treatment with blue light (405 nm) [116,181,182]. Most nutrients (vitamin C, β-carotene and lycopene) were also preserved in blue light-treated papayas or fresh-cut mangoes (405 nm), although there was a significant increase (p < 0.05) in the amount of flavonoids during storage (4 °C or 20 °C) in the illuminated papayas [116]—flavonoid content remained stable in fresh-cut mangoes [182]. Others observed no adverse visual quality on blue light-treated strawberries (405 nm), as compared with the untreated controls [180]. In fresh-cut Fuji apples, polyphenol oxidase and peroxidase was inhibited by the combination of curcumin (2 µM) and blue light (420 nm) and thus there was significantly less browning (p < 0.01) in treated apples as compared with untreated controls [183]. On the contrary, blue light (460 nm) induced a bleaching effect in fresh-cut pineapples, as measured by the reduction in its yellowness index [184], although no human observers were used to determine whether this change would be perceived as undesirable.
The non-thermal nature of blue light treatments also allows for their application on low-moisture products, such as almonds, albeit improvements on its bactericidal efficiency would be required (at least 4-log reduction is needed) [185]. Additionally, blue light (405 nm) delayed the regrowth of L. monocytogenes on cherry tomatoes by 14 days, albeit this finding should prompt food producers to be vigilant in determining whether the blue light used is bacteriostatic or bactericidal against pathogenic bacteria [181].
4.4. Inactivation of Bacteria in Meat Products and Seafood (Chicken, Beef and Fish)
Generally, blue light treatment is less effective in meat and seafood products than on surfaces or in dairy and horticultural products (Table 1, Table 2, Table 3 and Table 4), possibly due to the presence of absorptive materials and ROS-neutralizing substances, such as proteins. For instance, the inactivation of S. Enteritidis by blue light was less efficient on cooked chicken meat (0.8–0.9 log CFU/cm2) than in the transparent PBS (1.3–2.4 log CFU/mL) [189]. However, blue light could still induce injuries on bacterial cells that render them more susceptible to subsequent stresses. On cooked chicken meat, S. Enteritidis lost its resistance to four antibiotics, relative to the untreated controls (details in Section 7.1.) [189]. Similarly, blue light treatment rendered L. monocytogenes and Salmonella spp. on fresh salmon significantly more susceptible (p < 0.05) to gastric digestion (pH 2) than untreated cells, especially at lower temperatures [190]. These findings indicate that bactericidal efficacy of blue light-mediated treatments could be improved by combining it with other treatments, such as organic acids, essential oils agents or polyphenols (details in Section 6).

Table 4.
Blue light inactivation of pathogenic bacteria in meat and seafood products.
The efficacy of blue light treatment on chicken products is dependent on the wavelength used, possibly related to wavelength-specific activation of different endogenous photosensitizers. Two studies found that Campylobacter spp. in chicken (fillet or skin) were reduced by 1.7–2.4 log CFU or 0.7–6.7 log CFU/g through treatments with blue light at 405 nm [162] or 395 nm [169], respectively. Other than the type of light, these differences may also be attributable to the different bacterial species, treatment distances and treatment lengths used (Table 4) [162,169]. In agreement, light dosage was found to be inversely proportional to treatment distance, for example, the inactivation of C. jejuni on chicken skin was higher at a treatment distance of 3 cm (6.7 log CFU/cm2) than at 12 cm (1 log CFU/cm2) or 23 cm (0.7 log CFU/cm2) (Table 4) [169].
Sensorial properties of chicken and salmon could be affected by light treatment, especially when using light at the UV–vis region or photosensitizers. A study reported on the heating effects of ultraviolet/blue light (395 nm), especially at shorter treatment distance and longer treatment length, which resulted in significant color changes (p < 0.05) in chicken fillet and skin [169]. Others found that while discoloration was absent in smoked salmon illuminated with blue light alone (460 nm), the whiteness index significantly increased (p < 0.05) in samples treated with riboflavin-mediated blue light, relative to the untreated control samples [191]. Consistently, an extended illumination (8 h) of fresh salmon with blue light alone (405 nm) did not result in color changes [190].
Further, the introduction of exogenous photosensitizers could improve the inactivation of pathogenic bacteria on cooked food. The combination of curcumin (50 or 100 µM) and blue light (435 nm; 33.8 J/cm2) resulted in a 1.7-log reduction (CFU) of S. aureus in cooked chicken meat, whereas the treatment of blue light alone had no effect on the bacterial load. Albeit, the authors suggested that the lipophilicity of curcumin could make it susceptible to attenuation by the fatty regions on the chicken skin and thus modification of this photosensitizer (or alternative photosensitizers) would be required to achieve a higher bactericidal activity [178]. Another study found that blue light (460 nm) reduced the population of L. monocytogenes on smoked salmon fillets by up to 1.12 log from the initial concentration of 3.5 log CFU/cm2, but only when riboflavin was present. The light dosage required to achieve the first log reduction was lower at 4 °C (1600 J/cm2) than at 12 °C (2000 J/cm2), although the difference was not statistically significant [191].
5. Potential Application of Blue Light in Food Supply Chain
5.1. Food Processing and Farms: Airborne and Surface Inactivation
Practical applications of any blue light technology within the food industry are dependent on its ability to inactivate pathogens over distances beyond those typically used in laboratory-scale experiments. In clinical settings, three studies found that a ceiling-mounted high-intensity narrow-spectrum light environmental decontamination system (HINS-light EDS; 405 nm) significantly reduced (p < 0.05) the total viable counts, including MRSA and S. aureus, on surfaces (for example, bed, table, chair, worktop or bins) [193,194,195]. The safety of HINS-light EDS also allowed it to be operated in the presence of humans, such as patients and healthcare workers, which is in contrast to ultraviolet germicidal lamps [193,194,195].
These findings suggest that there is a potential for HINS-light EDS (or similar technologies) to be used for environmental sanitization in food-processing plants. While blue light inactivation of planktonic and biofilm-associated bacteria has been tested on food packaging and also on work surfaces (for example, stainless steel, acrylic or glasses), there are limited studies on the sporicidal effects of blue light on food packaging (Table 1). In suspensions, antimicrobial blue light (405 nm; 1730 J/cm2) also reduced the population of bacterial endospores, namely those of B. cereus, B. subtilis, Bacillus megaterium and Clostridium difficile, by 4 log CFU/mL [196]. Thus, future in vivo validations are required to assess the ability of blue light to inactivate different forms of bacteria on a range of surfaces and at varying distances.
Further, bacteria may be aerosolized during food processing, persist in the air and subsequently spread across indoor premises. Interestingly, a study found that aerosolized S. epidermidis was significantly inactivated by approx. 2 log CFU/mL (p < 0.001) by blue light (405 nm; 39.5 J/cm2), with the susceptibility of the bacteria to blue light being 2–4 times higher in aerosols than in liquids or on surfaces [197]. Future studies should explore the potential of blue light for inactivating food-borne bacteria in aerosols.
Light treatments could also be used to treat veterinary diseases in farm animals, such as mastitis in cows. For example, there were significantly lower (p < 0.05) bacterial loads of Streptococcus dysgalactiae and coagulase-negative staphylococci in milk produced by cows treated with the combination red LED (635 nm; 200 J/cm2) and toluidine blue (2%), relative to the untreated groups—this treatment was not intended for direct decontamination milk, but for alleviating incidences of mastitis in cows, with bacteriological characteristic of milk samples only used as an indicator [198]. In vitro, bacteria isolated from bovine mastitis, namely S. dysgalactiae, S. aureus and Streptococcus agalactiae, were inactivated by red LED (662 nm; 3–12 J/cm2) and methylene blue (50 µM) [199]. Currently, there are no data available on the application of blue light against farm-animal pathogens. However, given the fact that blue light can act on endogenous chromophores, it may have practical advantages over the existing red LED that depends on exogenous photosensitizers to inactivate bacteria on cows.
5.2. Aquaculture
Pathogenic bacteria that attack fish include Vibrio spp., Photobacterium damselae subsp. piscicida, Edwardsiella tarda and Edwardsiella ictaluri [200]. Although most of these bacteria are not known to infect humans, high incidences of disease in farmed fish may inflict adverse economic consequences upon fish farmers. Thus, the availability of methods for inactivating these bacteria in aquaculture systems is paramount to sustain viable fisheries. Several studies have assessed the application of antimicrobial blue light against fish pathogens. In PBS, the blue light inactivation of several pathogenic fish bacteria was 132–543.7 and 247–2178 J/cm2 at 405 and 465 nm, respectively. Generally, these bacteria were more susceptible to blue light at 405 nm than at 465 nm, although there were variations across different bacterial species (Figure 2) [201].
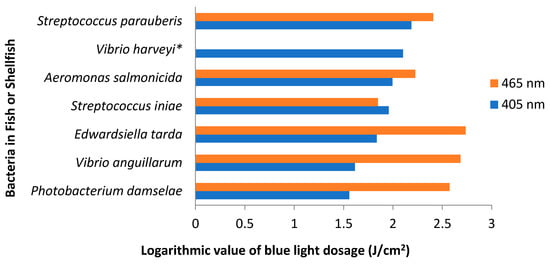
Figure 2.
Blue light dosage required to achieve 1-log reduction of pathogenic bacteria in fish or shellfish. Light dosage was converted to logarithmic values (log) and thus an increase of one unit on the x axis represents a tenfold increase in the light dosage. This graph was created using data taken from Roh et al., which was published under the Creative Commons Attribution 4.0. International License (http://creativecommons.org/licenses/by/4.0/) [201]. * Vibrio harveyi was not inactivated by blue light at 465 nm.
The presence of particulates in aquaculture water reduced the bactericidal efficacy of artificial white light (380–700 nm), in combination with a cationic porphyrin (Tri-Py+-Me-PF), against Vibrio fischeri: (1) in unfiltered water, 50 µM of porphyrin and 43.2–64.8 J/cm2 of light were required to achieve a 7-log reduction (CFU/mL); (2) in filtered water, the combination of porphyrin (at least 10 µM) and light (64.8 J/cm2) led to a bacterial reduction of 7 log CFU/mL. When tested in PBS, a complete inactivation of V. fischeri (7 log CFU/mL) was achieved, regardless of variations in acidity (pH of 6.5–8.5), salinity (20–40 g/L), temperature (10–25 °C) and oxygen concentration (5.3–5.9 mg/L), although the rate of inactivation was highest at the physiological pH (7.4) and ambient temperature (25 °C) [202]. However, when similar treatments (white light at 380–700 nm; Tri-Py+-Me-PF at 5–50 µM) were used against heterotrophic bacteria cultivated from aquaculture water samples, the bactericidal efficacies varied across different water samples, ranging from 1.2 to 2 log CFU/mL. Nevertheless, in PBS, a complete inactivation (8 log CFU/mL) was observed for several pathogenic bacteria isolated from aquaculture water, namely Vibrio spp., P. damselae, Enterococcus faecalis, E. coli and S. aureus, after exposure to a combined treatment of artificial white light (380–700 nm; up to 648 J/cm2) and Tri-Py+-Me-PF (5 µM) [203].
In the absence of photosensitizer, blue LED (405 or 465 nm) was able to significantly reduce (p < 0.05 or 0.01) the bacterial loads of Edwardsiella piscida in rearing water, which subsequently also decreased the number of bacterial infections in Fancy carps (Cyprinus caprio) [204]. The light treatment did not induce damages on the fish eyes and skin, with also no increase in the production of heat-shock proteins or unusual feeding behaviour observed in the treated fish, relative to the untreated controls [204]. Albeit, precautions are needed as continuous exposure of some fish, such as sea bass and sole, to blue light (435–500 nm) could result in increased malformations and poor survival of the fish larvae [205]. Thus, additional studies are needed to assess the effects of antimicrobial blue light on live seafood, including fish, oysters and mussels.
5.3. Retail: Prolonging Shelf-Life
As previously discussed, food contamination at retail establishments could lead to outbreaks (Section 2), particularly as inactivation treatments are not usually present at this stage within the food supply chain. In Section 4, we have reviewed studies on the use of antimicrobial blue light against pathogenic bacteria in an array of food products and also on surfaces. However, the use of blue light at the retail level also requires it to inhibit spoilage microorganisms and thus extends the shelf-life of foods. There are also concerns about spoilage bacteria surviving cleaning regimes and persist on surfaces in food-processing plants, including Pseudomonas spp., Serratia spp., Hafnia spp. and LAB [16], and thus additional control measures are required. Further, several review articles have identified major spoilage microorganisms in dairy [206,207], horticultural [208], meat [209] and seafood [210] products, which may be a subject of future studies on antimicrobial blue light.
Although still limited in number, there are several studies that assessed blue light-mediated inactivation of spoilage microorganisms in food products and subsequently the shelf-life of these treated foods. For example, blue light significantly reduced (p < 0.05) the initial loads of mesophilic bacteria, yeasts or other microfungi on strawberries and cherry tomatoes, which delayed the spoilage onset by 2 and 4 days, respectively (Table 3) [181,188]. In Hami melon (cantaloupe), the combination of curcumin (50 µM) and blue LED (470 nm) significantly reduced (p < 0.05) the initial amount of total aerobic microorganisms by 1.38 log CFU/g, with the treated melons also having 1.8-log lower bacterial load (CFU/g) than the untreated controls after 9 days of storage at 4 °C. The soluble solid content, color, water content and firmness of Hami melon were also better preserved in the blue light-treated group than the untreated controls [211].
The combination of curcumin (10 µM) and blue light (470 nm; 5.4 J/cm2) extended the shelf-life of fresh oysters from 8 to 12 days at 4 °C, as determined by total aerobic plate count (shelf-life limit of 107 CFU/g) and total volatile basic nitrogen analysis (shelf-life limit of 30 mg n/100 g oyster) [212]. Sensorial properties of the treated oyster were also improved at the shelf-life terminal point of the untreated group (8th day): (1) human panels rated treated oyster more favorably than untreated control in terms of smell, body color, mucus appearance and texture; (2) electronic nose indicated that the generation of spoilage metabolites was reduced in the treated group, relative to untreated controls. Retention of flavorful free amino acids and reduction in the oxidation of lipids and fatty acids were also observed in the blue light-treated group [212]. Similarly, the treatment of fresh sturgeons with curcumin (30 µM) and blue light (470 nm) significantly reduced (p < 0.05) the prevalence of spoilage Pseudomonas spp. during storage at 4 °C for 6–9 days [213].
The number of spoilage P. fluorescens was lowered by 1.85–3.60 log CFU/g in blue light-treated (460–470 nm) packaged sliced cheese, relative to untreated controls, after 2 and 7 d of storage at 25 and 4 °C, respectively (Table 2) [165].
6. Hurdle Technology
6.1. Photosensitizers
In all studies that combined exogenous photosensitizers with blue light treatments, the addition of photosensitizing agents improved the bactericidal efficacy of blue light, relative to when blue light was used alone (Table 1, Table 3 and Table 4). These photosensitizers facilitate the production of ROS, which subsequently induces bacterial inactivation. However, the bactericidal efficacy may also vary with the type of photosensitizer used. For instance, the combination of blue LED (427–470 nm; 30 J/cm2) and rose bengal (160 µg/mL) inactivated 7.1 log CFU/mL of Porphyromonas gingivalis, whereas when the same blue light was combined with erythrosine or phloxine, the bacterial reductions were only 0.9 or 1 log (CFU/mL), respectively [214].
Similarly, the efficacy of photosensitizers also depends on the delivery method within different application matrixes. For example, curcumin bound to polyvinylpyrrolidone did not improve the blue light inactivation on chicken skin, but was effective on vegetables. The lipophilic curcumin may be readily released by the hydrophilic polyvinylpyrrolidone to the fatty regions of the chicken skin. In contrast, micellar formulation of curcumin (NovaSol®-C) was effective on chicken skin, potentially due to the retention of curcumin in micellar form prior to contact with the bacterial cells [178].
Non-toxic inorganic salts can be used to potentiate photosensitization in photodynamic treatments, particularly through the production of non-oxygen reactive species, such as azide radicals from sodium azide salts or reactive iodine species from potassium iodide [215]. In an in vitro study, the combination of blue light (415 nm; 10 J/cm2), Photofrin (10 µM) and potassium iodide (100 mM) inactivated 6 log of five Gram-negative bacterial species, namely E. coli, P. aeruginosa, Klebsiella pneumoniae, Proteus mirabilis and A. baumannii, whereas in the absence of potassium iodide, photosensitization treatments resulted in no inactivation [216].
Precursors of endogenous photosensitizers, such as 5-aminolevulinic acid (ALA), can also be added to facilitate photo-inactivation of bacteria [110]. A study found that ALA and its derivatives induced the formation of photo-active porphyrins in Gram-negative (E. coli K-12, E. coli Ti05 and P. aeruginosa) and Gram-positive (S. aureus) bacteria, although the amount or type of porphyrin produced, and also the extent of bacterial photo-inactivation (white light; 120 J/cm2), depended on three factors: (1) type of precursor used; (2) bacterial species/strain tested; (3) concentration of precursor added [217]. In support of this view, another study demonstrated that ALA-mediated inactivation of bacteria by blue light (407–420 nm; 50–100 mJ/cm2) was more profound in Gram-positive (5–7 log CFU/mL; except for B. cereus and Streptococcus faecalis) than in Gram-negative bacteria (1–2 log CFU/mL)—this difference was possibly due to the higher amount of coproporphyrin present in Gram-positive bacteria tested (B. cereus produced 37–45% lower coproporphyrin than the other Gram-positive bacteria and was reduced by only 1–2 log CFU/mL; porphyrin production was not observed in S. faecalis and no reduction was reported) [218].
6.2. Acidity and Temperature
In clear liquid suspensions, the susceptibility of E. coli and L. monocytogenes to blue light (405 nm) was enhanced in the presence of environmental stresses: (1) the light dosages required to inactivate both bacteria (5 log CFU/mL) at stressful temperatures (4 °C or 45 °C) were significantly lower (p < 0.05) than at a non-stressful temperature (22 °C); (2) the light dosages required to inactivate E. coli and L. monocytogenes at pH 3 were reduced by 77% and 50%, respectively, relative to that required at pH 7; (3) the bacterial inactivation was significantly higher (p < 0.05) under osmotically-stressful conditions (salt concentrations of 10% and 15% for E. coli or 10% for L. monocytogenes) than under non-stressful conditions (salt concentrations of 0 or 0.8%) [219]. On nitrocellulose surface, both bacteria were also inactivated by blue light (405 nm; 36 J/cm2) to a higher extent at pH 3 (reduction of 95–99% reduction) than at pH 7 (reduction of 13–26%) [219]. In addition, the type of acid present determined the extent of bactericidal inactivation of blue LED (461 nm; 596.7 J/cm2) against E. coli O157:H7, S. Typhimurium, L. monocytogenes and S. aureus, with the highest inactivation rates at pH 4.5 achieved using lactic acid, followed by citric and malic acids [220].
However, there has been no consensus on how temperature affects the efficacy of antimicrobial blue light, with current data suggesting that it depends on the bacterial species and light wavelength used. The growth of S. Enteritidis on cooked chicken meat was only delayed when treated with blue light (405 nm) at 10 and 20 °C, but it was inactivated at 4 °C [189]. Similarly, five S. enterica serovars on fresh-cut pineapple, namely Typhimurium, Newport, Gaminara, Montevideo and Saintpaul, were inactivated by blue light (460 nm) at 7 and 16 °C (bactericidal), but only inhibited at 25 °C (bacteriostatic) [184]. On the contrary, S. aureus, Lactobacillus plantarum and V. parahaemolyticus in PBS were inactivated by blue light (405 or 460 nm) at all experimental temperatures (4, 10 or 25 °C), albeit the extent of bactericidal effect varied across bacterial species and also light wavelengths [221]. Another study also demonstrated that E. coli O157:H7, L. monocytogenes and Salmonella spp. on fresh-cut mangoes were inactivated by blue light (405 nm) at 4 and 10 °C, but only inhibited at 20 °C, with both bactericidal and bacteriostatic effects varying with bacterial species [182]. Interestingly, the population of L. monocytogenes in pre-biofilms on stainless steel and acrylic coupons was significantly reduced (p < 0.05) at 25 °C, but not at 4 °C [163].
6.3. Nanoparticle
Silver nanoparticles (AgNPs) are able to interact with negatively-charged molecules in bacterial cells, such as proteins or nucleic acids and subsequently induce damages to the cells, for example, by increasing cell membrane permeability, causing DNA damage or inhibiting protein synthesis [222]. A review by Carbone et al. summarized available data on the potential use of AgNPs as an antimicrobial agent in food packaging [223]. In photodynamic treatments, the combination of blue light (460 nm) with AgNPs was significantly more effective (p < 0.001) against MRSA and P. aeruginosa than each treatment alone [224,225]. The formation of P. aeruginosa biofilm on gelatin-based discs was also significantly inhibited (p < 0.001) by the combined treatment, relative to treatments with blue light or AgNPs alone [225].
Similarly, metal oxides can be used in photocatalytic processes to generate superoxides or hydroxyl radicals for inactivating microorganisms or oxidizing organic substances. In an in vitro study, zinc oxide nanoparticles (ZnO-NPs; 0.5 mg/mL) were combined with blue light (462 nm; 5.4 J/cm2) to inactivate 5 log CFU/mL of A. baumannii, with ZnO-NPs and blue light alone resulted in zero and less than 1-log reduction (CFU/mL), respectively. The combination of ZnO-NPs (0.1 mg/mL) and blue light (462 nm; 10.8 J/cm2) also significantly reduced the number of antibiotic-resistant (colistin or imipenem) A. baumannii (p < 0.005), K. pneumoniae (p < 0.005) or Candida albicans (fungus; p < 0.05) in culture medium. Further, transmission electron microscopical image revealed that the cell membrane was damaged in A. baumannii, but an analysis using gel electrophoresis showed no fragmentations of plasmid DNA post-treatment and thus these findings indicate that ZnO-NPs/blue light photocatalysis only attacks cytoplasmic membrane and not the bacterial genome [226].
6.4. Plant Extracts: Polyphenols and Essential Oils
Polyphenols are known to exhibit antimicrobial potency through their ability to bind to the cell membrane, cell wall and their associated proteins and thus compromising the structural integrity of the bacterial cell [227,228]. Inhibition of bacterial adhesins and quorum sensing by polyphenols also resulted in failures to form biofilms [228]. Interestingly, several studies have reported on the synergistic antimicrobial effects of blue light and plant-derived polyphenols, particularly at the blue light wavelength range of 385–400 nm. For example, gallic acid (4 mM) was combined with blue light (400 nm; 72 J/cm2) to inactivate 7.5 log CFU/mL of S. aureus, with lipid peroxidation observed through the detection of malondialdehyde. This lipid peroxidation was likely to be caused by the formation of hydroxyl radicals, which were detected by electron spin resonance [229]. In a follow-up study, the combination of blue light (400 nm; 75–150 J/cm2) and several polyphenols (1 mg/mL; caffeic acid, gallic acid, chlorogenic acid, epigallocatechin, epigallocatechin gallate and proanthocyanidin) induced significant inactivation (p < 0.05 or p < 0.01) of E. faecalis, S. aureus, Streptococcus mutans, Aggregatibacter actinomycetemcomitans, E. coli and P. aeruginosa. Damage to the DNA was also reported, which suggested that the polyphenols were incorporated into the bacterial cells, probably facilitated by the high affinity of polyphenols to the cell membrane [230].
Similarly, inactivation of S. mutans within biofilms was reported after exposure to the combination of caffeic acid (0–2 mg/mL) and light (365, 385 and 400 nm; 120–480 J/cm2), with the highest inactivation (5 log CFU/mL) achieved at caffeic concentration of 2 mg/mL and blue light dosage of 480 J/cm2 (385 nm) [231]. Other authors also reported the synergistic antimicrobial activities of blue light (400 nm) and wine grape-derived polyphenols (for example, catechin and its isotopic ingredients) against S. aureus or P. aeruginosa (5 log CFU/mL) [232,233].
In addition, essential oils possess antimicrobial activity by inducing membrane breakage and permeability, albeit Gram-negative bacteria are known to be more resistant than Gram-positive bacteria due to the presence of hydrophilic outer membrane [234,235]. Membrane leakages can lead to loss of cellular constituents, such as ions, genetic materials or adenosine triphosphate (ATP), and subsequently cell death [236,237]. One study found that essential oils derived from eucalyptus (5%), clove (0.5%) and thyme (0.5%) improved the blue light (469–470 nm) inactivation of S. epidermidis and P. aeruginosa by 2–7 and 3–8 log CFU/mL, respectively, as compared with inactivation achieved by light alone. Relative to treatments with essential oils alone, samples treated with the combination of essential oils and blue light had 3–6 and 3–5 log CFU/mL lower counts of S. epidermidis and P. aeruginosa, respectively [238].
7. Blue Light versus Antimicrobial Resistance and Consequences of Sub-Lethal Light Exposures
Antimicrobial resistance presents a challenge to the food industry, with multiroute transmissions of resistant bacteria occurring through the contamination of food-processing environments, transfer of genes originating from microorganisms intentionally added to foods (starter cultures, bio-preservative bacteria or bacteriophage) and cross-contamination of foods [239,240,241,242]. Throughout the years, antimicrobial-resistant pathogenic bacteria have emerged across different food industries: horticultural (vegetables and fruits) [240], seafood [241] and meat (food and livestock) [242]. In previous sections, we have reviewed several studies that successfully used blue light against drug-resistant bacteria, such as MRSA. However, in this section, we focus on the ability of blue light to sensitize multidrug-resistant bacteria to antibiotics and subsequently on the inactivation of biofilms (monomicrobial or polymicrobial) and also on the potential development of bacterial tolerance/resistance to blue light.
7.1. Resistant Bacteria: Improved Sensitivity to Antibiotics
Generally, bacteria achieve antimicrobial resistance through three mechanisms: (1) preventing the drug from reaching the target (limiting uptake or active efflux); (2) modifying the target sites, such as alterations of penicillin-binding proteins in Gram-positive bacteria; (3) inactivating the drug through degradation or chemical modulation [243]. Theoretically, blue light could induce damages that disrupt the ability of bacteria to limit drug uptakes or to perform active efflux (mechanism 1). As mentioned previously (Section 3.1), blue light could induce breakage of cell walls, increase the permeability of the cell membrane (lipid peroxidation) and inactivate lipopolysaccharides (constituents of outer membrane in Gram-negative bacteria)—all of these structures play roles in conferring barriers against antibiotics [243]. In addition, one study showed that blue light-treated MRSA suffered from potassium ion leakages, which suggested that several transmembrane proteins (for example, Na+/K+ ion pumps) may have been denatured [113] and thus potentially limiting the role of these transmembrane proteins in pumping drugs out of the bacterial cells (active efflux) [243].
Several studies also found that blue light rendered bacteria more susceptible to antibiotics. In an in vitro study, blue light-treated (411 nm; 150 J/cm2 per cycle; 15 cycles) S. aureus (methicillin-sensitive and resistant) had a higher susceptibility to gentamycin and doxycycline, but not vancomycin, ciprofloxacin, chloramphenicol and rifampicin, than untreated controls [244]. Another report showed that the minimum inhibitory concentration (MIC) of gentamycin, ceftazidime and meropenem against drug-resistant P. aeruginosa was reduced by up to 8-fold in the blue light-treated groups (405 nm; 10 or 12 J/cm2), relative to the untreated controls [245]. On cooked chicken meat, blue light-treated (405 nm; 1700 J/cm2) S. Enteritidis became more susceptible to ampicillin, chloramphenicol, nalidixic acid and rifampicin, relative to freshly-cultured or non-illuminated controls [189]. On the contrary, one study reported no change in the susceptibility of S. aureus (methicillin sensitive and resistant) to antibiotics (panel of 10, including gentamycin) after fifteen cycles of sub-lethal exposures to blue light (405 nm; 108 J/cm2 per cycle) [246].
Further, He et al. found that blue light could be combined with tetracycline-class antibiotics to inactivate drug-resistant E. coli and MRSA: (1) demeclocycline (DMCT; 10–50 µM) could be activated as a photosensitizer by blue light (415 nm; 10 J/cm2) and reduced the bacterial load of resistant E. coli and MRSA by 6 log CFU/mL; (2) sub-lethally injured E. coli underwent inactivation during the sub-culturing of these bacteria post-treatment. This indicated that the antibiotic was still active, even in the absence of light, and continued to inactivate the sensitized bacteria by inhibiting their ribosomes; (3) minimum inhibitory concentrations of DMCT, doxycycline, minocycline and tetracycline against drug-resistant E. coli and MRSA were reduced by up to 8-fold, when applied in the presence of blue light (415 nm), relative to the dark controls [247].
7.2. Inactivation of Biofilms
Biofilms facilitate horizontal gene transfers between individual bacteria within the matrix, especially in those that contain more than one bacterial species. These exchanges of mobile genetic elements may lead to an increase in antimicrobial resistance and environmental persistence [248]. To mitigate this issue, a group of researchers deployed antimicrobial blue light (405 nm; 500 J/cm2) against polymicrobial biofilms and achieved log reductions of 2.37 and 3.40 CFU/mL for MRSA and P. aeruginosa, respectively, within a dual-species biofilm. The same blue light treatment on another dual-species biofilm inactivated P. aeruginosa and C. albicans by 6.34 and 3.11 log CFU/mL, respectively. As expected, monomicrobial biofilms were more susceptible to blue light, with damages to the exopolysaccharide matrix also observed across different types of biofilm [249].
The efficacy of blue light against biofilms also varies across bacterial species. For example, blue light (405 nm; 108–206 J/cm2) significantly inactivated monomicrobial biofilms of drug-resistant A. baumannii, P. aeruginosa and Neisseria gonorrhoeae (4–8 log CFU/mL; p < 0.01 or p < 0.0001), whereas the same blue light treatment did not significantly affect the biofilms of E. coli, E. faecalis and Proteus mirabilis. Further, blue light (405 nm; 216 J/cm2) significantly reduced (p < 0.01) the number of MRSA in biofilms grown for 24 h, but not in biofilms grown for 48 h [250].
7.3. Sub-Lethal Exposures Induce Cellular Processes Potentially Leading to Tolerance
A study found that fifteen cycles of sub-lethal exposures of S. aureus to blue light (411 nm; 150 J/cm2 per cycle) resulted in the development of tolerance due to genetic alterations, which was stable after five successive sub-culturing. There was an increase in the expression of recA and umuC genes in the blue light-tolerant S. aureus, whereas mutant strains with non-functional recA and umuC genes did not develop tolerance. This finding confirmed that SOS-dependent mechanism played a role in the development of blue light-tolerant phenotype, although direct mutations from DNA damage were also possible [244]. In contrast, another study showed that S. aureus did not develop tolerance to blue light (405 nm; 108 J/cm2 per cycle) after fifteen cycles of sub-lethal treatment [246]. Exposures of P. aeruginosa, A. baumannii and E. coli to twenty cycles of blue light (405 nm; dosages enough to induce bacterial reduction of 4 log CFU) also did not result in the development of tolerance [251]. Possible explanations for the discrepancies between these studies include different wavelengths and blue light dosages used, albeit further investigations are needed to ascertain the effects of sub-lethal exposures of bacteria to blue light.
Two reports found that exposure of S. aureus to blue light had reduced its susceptibility to H2O2 [244,246]. In response to oxidative stress induced by the blue light, bacteria may up-regulate the expression of katA that encodes the production of H2O2-scavenging catalase protein and thus exhibit higher tolerance to H2O2 [118]. Adair and Drum identified thirty-two other genes in S. aureus regulated (up- or downregulated) by blue light (465 nm; 250 J/cm2), which included those responsible for the production of cell envelope components and heat-shock proteins [252]. Similarly, light-mediated gene regulations occurred in other major food-borne pathogens, namely V. cholerae and C. sazakii. In response to ROS generated by blue light (fluorescent black light; UV-filtered), V. cholerae showed differential expression of 222 genes (6.3%), relative to untreated cells, especially those encoding enzymes that protect or repair lipids and nucleic acids (genome)—these transcriptional responses to blue light were regulated by ChrR and MerR-like proteins [253]. Blue light (415 nm; up to 20.04 J/cm2) was also found to upregulate the expression of genes in C. sakazakii that encode oxidative stress-resistance chaperone, an adhesin and a capsule biosynthesis protein (CapC) [115].
These findings indicate that sub-lethal exposures of bacteria to blue light induce cell responses that may lead to the development of tolerance over time. However, complete resistance has not been reported, as even when tolerance was observed at a particular blue light dosage, increasing the light dosage was sufficient to eliminate the tolerant bacteria [244]. Nevertheless, other strategies are required to antagonize any development of bacterial tolerance to blue light. For example, the combination of blue light at 460 and 405 nm was reported to be effective against blue light-tolerant S. aureus. Leanse et al. [254] revealed that blue light at 460 nm (90–360 J/cm2) inactivated staphyloxanthin (a ROS scavenger; antioxidant) through photolysis and thus disrupted the ability of S. aureus to resist blue light treatment. Subsequent treatment with blue light at 405 nm (90–180 J/cm2) inactivated the bacteria (planktonic or in biofilm) at a higher rate than when single wavelengths were used, albeit inactivation was dependent on the dosage of both 405- and 460-nm blue light. In addition, as blue light attacks multiple targets in the bacterial cells, the development of resistance to blue light is likely to be slower than resistance to antibiotics.
8. Research Gap and Future Outlook
While it is a well-established fact that bacterial cells contain photo-active endogenous photosensitizers, the amount and type of these intracellular chromophores—and thus the susceptibility to blue light—may vary across bacterial species. For example, spectroscopic measurements revealed the presence of flavins and porphyrins in the cell lysates of A. actinomycetecomitans, although these compounds were not detected in E. coli. When illuminated by blue light (460 nm; 150 J/cm2), A. actinomycetecomitans (serotype b; ATCC 43718) were reduced by 5 log CFU, whereas E. coli (ATCC 25922) remained unaffected [255]. Another group of researchers also used spectroscopic technique to identify protoporphyrin IX and coproporphyrin as the main intracellular photosensitizers in Helicobacter pylori, which could exist in monomeric, dimeric or aggregated forms [256]. Others utilized high-performance liquid chromatography to characterize the endogenous photosensitizers in P. aeruginosa and A. baumannii, including coproporphyrin (I or III) and protporphyrin IX [257,258]. These studies provide technical foundation that future researches could build upon, particularly for characterizing endogenous photosensitizers in food-borne pathogenic bacteria.
There are limited data on the bactericidal activity of blue light against spoilage microorganisms, especially bacteria that are capable of growing in anaerobic conditions, such as Clostridium estertheticum in vacuum-packed meats. A study showed that while E. coli, S. aureus and E. faecalis in liquid media were unaffected by blue light (405 nm; 5.73 J/cm2) under anaerobic environments, the bacterial loads of Prevotella intermedia and Prevotella nigrescenes were significantly reduced by 1 and 2 log CFU/mL (p < 0.05), respectively [259]. Others observed 1-log reduction of P. gingivalis after illumination with blue light (405 nm; 3.42 J/cm2) [260]. The authors of both studies attributed blue light-mediated anaerobic inactivation of bacteria to the generation of organic radicals directly from the triplet state of endogenous photosensitizers [259,260]. These findings indicate that there is a potential for anaerobic application of blue light, although there is a need for improvements in the bactericidal efficacy of blue light under oxygen-scarce conditions. Azide salts can be used to facilitate anaerobic photodynamic treatments [216]. In addition, future studies are required to assess the effects of blue light on spore germination or the production of bacterial toxins.
Sensorial properties of blue light-treated foods need to be assessed beyond the quantitative measurements conducted within laboratory settings. For example, the quality of blue light-treated oysters were evaluated using both chemical/microbiological analyses and human panels (sensory evaluation), which provided the researchers with a comprehensive information to determine the shelf-life of seafood [212]. Future research can be designed to assess the sensorial properties of other types of blue light-treated food, including fruits, vegetables, meat and dairy products.
Lastly, spectral readings from Fourier-transform infrared (FTIR) indicated that blue light (470 nm) and UV (254 nm) primarily attacked the DNA during inactivation of MRSA, although the two lights targeted different DNA conformations: blue light induced damage on A-DNA, whereas UV predominantly inactivated B-DNA—these are two of the three conformations of double helical DNA, with the other one being Z-DNA. These findings suggest that blue and UV lights may be used as complementary treatments against microbes [261]. For safety, far ultraviolet-C (UV-C; 207 or 222 nm) can be used as an alternative to the conventional UV-C (254 nm). Accumulating evidence indicates that unlike the conventional UV-C, far UV-C exhibits bactericidal activity, but only has minimal effects on mammalian cells, such as the eye and skin of mice or human skin cells [262,263,264,265,266,267].
Author Contributions
Conceptualization, G.B.; investigation, J.H. and S.W.; writing—original draft preparation, J.H.; writing—review and editing, J.H., S.W. and G.B.; visualization, J.H.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded through the AgResearch Strategic Science Investment Fund (SSIF) Food Integrity (contract A25768).
Acknowledgments
The authors wish to thank Aswathi Soni and Delphine Rapp for their technical inputs. Elisabeth Belianty and Erinna Hadi are also acknowledged for their assistance with the illustrations.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the work.
References
- World Health Organization. WHO|WHO Estimates of the Global Burden of Foodborne Diseases. Available online: http://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/ (accessed on 10 October 2020).
- Dickson-Spillmann, M.; Siegrist, M.; Keller, C. Attitudes toward Chemicals Are Associated with Preference for Natural Food. Food Qual. Prefer. 2011, 22, 149–156. [Google Scholar] [CrossRef]
- Ha, T.M.; Shakur, S.; Pham Do, K.H. Consumer Concern about Food Safety in Hanoi, Vietnam. Food Control 2019, 98, 238–244. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus Spores in the Food Industry: A Review on Resistance and Response to Novel Inactivation Technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef]
- Wang, M.S.; Wang, L.H.; Bekhit, A.E.D.A.; Yang, J.; Hou, Z.P.; Wang, Y.Z.; Dai, Q.Z.; Zeng, X.A. A Review of Sublethal Effects of Pulsed Electric Field on Cells in Food Processing. J. Food Eng. 2018, 223, 32–41. [Google Scholar] [CrossRef]
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current Status and Future Trends of High-Pressure Processing in Food Industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D.J. Cost Analysis and Environmental Impact of Pulsed Electric Fields and High Pressure Processing in Comparison with Thermal Pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- D’Souza, C.; Yuk, H.G.; Khoo, G.H.; Zhou, W. Application of Light-Emitting Diodes in Food Production, Postharvest Preservation, and Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and Potential Applications of Ultraviolet Light in the Food Industry—A Critical Review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Koutchma, T. UV Light Microbial Inactivation in Foods. In Ultraviolet Light in Food Technology: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–344. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Kütting, B.; Drexler, H. UV-Induced Skin Cancer at Workplace and Evidence-Based Prevention. Int. Arch. Occup. Environ. Health 2010, 83, 843–854. [Google Scholar] [CrossRef]
- Zaffina, S.; Camisa, V.; Lembo, M.; Vinci, M.R.; Tucci, M.G.; Borra, M.; Napolitano, A.; Cannatã, V. Accidental Exposure to UV Radiation Produced by Germicidal Lamp: Case Report and Risk Assessment. Photochem. Photobiol. 2012, 88, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; De Melo, W.C.M.A.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light Based Anti-Infectives: Ultraviolet C Irradiation, Photodynamic Therapy, Blue Light, and Beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 17 December 2020).
- Møretrø, T.; Langsrud, S. Residential Bacteria on Surfaces in the Food Industry and Their Implications for Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef]
- Fox, E.M.; Jiang, Y.; Gobius, K.S. Key Pathogenic Bacteria Associated with Dairy Foods: On-Farm Ecology and Products Associated with Foodborne Pathogen Transmission. Int. Dairy J. 2018, 84, 28–35. [Google Scholar] [CrossRef]
- Cancino-Padilla, N.; Fellenberg, M.A.; Franco, W.; Ibáñez, R.A.; Vargas-Bello-Pérez, E. Foodborne Bacteria in Dairy Products: Detection by Molecular Techniques. Cien. Inv. Agr. 2017, 44, 215–229. [Google Scholar] [CrossRef]
- Castro, H.; Jaakkonen, A.; Hakkinen, M.; Korkeala, H.; Lindström, M. Occurrence, Persistence, and Contamination Routes of Listeria Monocytogenes Genotypes on Three Finnish Dairy Cattle Farms: A Longitudinal Study. Appl. Environ. Microbiol. 2018, 84, 2000–2017. [Google Scholar] [CrossRef]
- Haley, B.J.; Sonnier, J.; Schukken, Y.H.; Karns, J.S.; Van Kessel, J.A.S. Diversity of Listeria Monocytogenes Within a U.S. Dairy Herd, 2004–2010. Foodborne Pathog. Dis. 2015, 12, 844–850. [Google Scholar] [CrossRef]
- Browne, A.S.; Midwinter, A.C.; Withers, H.; Cookson, A.L.; Biggs, P.J.; Marshall, J.C.; Benschop, J.; Hathaway, S.; Haack, N.A.; Akhter, R.N.; et al. Molecular Epidemiology of Shiga Toxinproducing Escherichia Coli (STEC) on New Zealand Dairy Farms: Application of a Culture-Independent Assay and Whole-Genome Sequencing. Appl. Environ. Microbiol. 2018, 84, 481–499. [Google Scholar] [CrossRef]
- Venegas-Vargas, C.; Henderson, S.; Khare, A.; Mosci, R.E.; Lehnert, J.D.; Singh, P.; Ouellette, L.M.; Norby, B.; Funk, J.A.; Rust, S.; et al. Factors Associated with Shiga Toxin-Producing Escherichia Coli Shedding by Dairy and Beef Cattle. Appl. Environ. Microbiol. 2016, 82, 5049–5056. [Google Scholar] [CrossRef]
- Ross, C.M.; Rapp, D.; Cave, V.M.; Brightwell, G. Prevalence of Shiga Toxin-Producing Escherichia Coli in Pasture-Based Dairy Herds. Lett. Appl. Microbiol. 2019, 68, 112–119. [Google Scholar] [CrossRef]
- McAuley, C.M.; McMillan, K.E.; Moore, S.C.; Fegan, N.; Fox, E.M. Characterization of Escherichia Coli and Salmonella from Victoria, Australia, Dairy Farm Environments. J. Food Prot. 2017, 80, 2078–2082. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, L.D.; Wright, E.M.; Siler, J.D.; Elton, M.; Cummings, K.J.; Warnick, L.D.; Wiedmann, M. Subtype Analysis of Salmonella Isolated from Subclinically Infected Dairy Cattle and Dairy Farm Environments Reveals the Presence of Both Human- and Bovine-Associated Subtypes. Vet. Microbiol. 2014, 170, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, A.; Sartori, C.; Boss, R.; Resch, G.; Oechslin, F.; Steiner, A.; Moreillon, P.; Graber, H.U. Genotypes of Staphylococcus Aureus: On-Farm Epidemiology and the Consequences for Prevention of Intramammary Infections. J. Dairy Sci. 2019, 102, 3295–3309. [Google Scholar] [CrossRef] [PubMed]
- McAuley, C.M.; McMillan, K.; Moore, S.C.; Fegan, N.; Fox, E.M. Prevalence and Characterization of Foodborne Pathogens from Australian Dairy Farm Environments. J. Dairy Sci. 2014, 97, 7402–7412. [Google Scholar] [CrossRef]
- Hunter, C.J.; Bean, J.F. Cronobacter: An Emerging Opportunistic Pathogen Associated with Neonatal Meningitis, Sepsis and Necrotizing Enterocolitis. J. Perinatol. 2013, 33, 581–585. [Google Scholar] [CrossRef]
- Norberg, S.; Stanton, C.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Cotter, P.D. Cronobacter spp. in Powdered Infant Formula. J. Food Prot. 2012, 75, 607–620. [Google Scholar] [CrossRef]
- Vojkovska, H.; Karpiskova, R.; Orieskova, M.; Drahovska, H. Characterization of Cronobacter Spp. Isolated from Food of Plant Origin and Environmental Samples Collected from Farms and from Supermarkets in the Czech Republic. Int. J. Food Microbiol. 2016, 217, 130–136. [Google Scholar] [CrossRef]
- Jaradat, Z.W.; Ababneh, Q.O.; Saadoun, I.M.; Samara, N.A.; Rashdan, A.M. Isolation of Cronobacter spp. (Formerly Enterobacter Sakazakii) from Infant Food, Herbs and Environmental Samples and the Subsequent Identification and Confirmation of the Isolates Using Biochemical, Chromogenic Assays, PCR and 16S RRNA Sequencing. BMC Microbiol. 2009, 9, 225. [Google Scholar] [CrossRef]
- Schmid, M.; Iversen, C.; Gontia, I.; Stephan, R.; Hofmann, A.; Hartmann, A.; Jha, B.; Eberl, L.; Riedel, K.; Lehner, A. Evidence for a Plant-Associated Natural Habitat for Cronobacter spp. Res. Microbiol. 2009, 160, 608–614. [Google Scholar] [CrossRef]
- Molloy, C.; Cagney, C.; O’Brien, S.; Iversen, C.; Fanning, S.; Duffy, G. Surveillance and Characterisation by Pulsed-Field Gel Electrophoresis of Cronobacter Spp. in Farming and Domestic Environments, Food Production Animals and Retail Foods. Int. J. Food Microbiol. 2009, 136, 198–203. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The Complex Microbiota of Raw Milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E. Milk and Raw Milk Consumption as a Vector for Human Disease. In Zoonotic Pathogens in the Food Chain; Krause, D., Hendrick, S., Eds.; CABI: Oxfordshire, UK, 2010; pp. 99–118. [Google Scholar]
- Gaulin, C.; Ramsay, D.; Bekal, S. Widespread Listeriosis Outbreak Attributable to Pasteurized Cheese, Which Led to Extensive Cross-Contamination Affecting Cheese Retailers, Quebec, Canada, 2008. J. Food Prot. 2012, 75, 71–78. [Google Scholar] [CrossRef]
- Germinario, C.; Caprioli, A.; Giordano, M.; Chironna, M.; Gallone, M.S.; Tafuri, S.; Minelli, F.; Maugliani, A.; Michelacci, V.; Santangelo, L.; et al. Community-Wide Outbreak of Haemolytic Uraemic Syndrome Associated with Shiga Toxin 2-Producing Escherichia Coli O26: H11 in Southern Italy, Summer 2013. Eurosurveillance 2016, 21, 30343. [Google Scholar] [CrossRef]
- Oxaran, V.; Lee, S.H.I.; Chaul, L.T.; Corassin, C.H.; Barancelli, G.V.; Alves, V.F.; de Oliveira, C.A.F.; Gram, L.; De Martinis, E.C.P. Listeria Monocytogenes Incidence Changes and Diversity in Some Brazilian Dairy Industries and Retail Products. Food Microbiol. 2017, 68, 16–23. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Isolation and Molecular Characterization of Salmonella Enterica, Escherichia Coli O157: H7 and Shigella Spp. from Meat and Dairy Products in Egypt. Int. J. Food Microbiol. 2014, 168–169, 57–62. [Google Scholar] [CrossRef]
- Derra, F.A.; Karlsmose, S.; Monga, D.P.; Mache, A.; Svendsen, C.A.; Félix, B.; Granier, S.A.; Geyid, A.; Taye, G.; Hendriksen, R.S. Occurrence of Listeria Spp. in Retail Meat and Dairy Products in the Area of Addis Ababa, Ethiopia. Foodborne Pathog. Dis. 2013, 10, 577–579. [Google Scholar] [CrossRef]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A Systematic Review of Bacterial Foodborne Outbreaks Related to Red Meat and Meat Products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- Tesson, V.; Federighi, M.; Cummins, E.; de Oliveira Mota, J.; Guillou, S.; Boué, G. A Systematic Review of Beef Meat Quantitative Microbial Risk Assessment Models. Int. J. Environ. Res. Public Health 2020, 17, 688. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Am. Soc. Microbiol. 2019. [Google Scholar] [CrossRef]
- Mor-Mur, M.; Yuste, J. Emerging Bacterial Pathogens in Meat and Poultry: An Overview. Food Bioprocess Technol. 2010, 3, 24–35. [Google Scholar]
- Gieraltowski, L.; Higa, J.; Peralta, V.; Green, A.; Schwensohn, C.; Rosen, H.; Libby, T.; Kissler, B.; Marsden-Haug, N.; Booth, H.; et al. National Outbreak of Multidrug Resistant Salmonella Heidelberg Infections Linked to a Single Poultry Company. PLoS ONE 2016, 11, e0162369. [Google Scholar] [CrossRef] [PubMed]
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of Foodborne Disease Outbreaks Caused by Clostridium Perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Wimalarathna, H.; Mills, J.; Saldana, L.; Pang, W.; Richardson, J.F.; Maiden, M.C.J.; McCarthy, N.D. Duck Liver–Associated Outbreak of Campylobacteriosis among Humans, United Kingdom, 2011. Emerg. Infect. Dis. 2013, 19, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.C.; Harding, O.; Fisher, L.; Cowden, J. A Continuous Common-Source Outbreak of Campylobacteriosis Associated with Changes to the Preparation of Chicken Liver Pâté. Epidemiol. Infect. 2009, 137, 383–388. [Google Scholar] [CrossRef]
- Chai, S.J.; Cole, D.; Nisler, A.; Mahon, B.E. Poultry: The Most Common Food in Outbreaks with Known Pathogens, United States, 1998–2012. Epidemiol. Infect. 2017, 145, 316–325. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Outbreak of Salmonella Heidelberg Infections Linked to a Single Poultry Producer—13 States, 2012-2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 553–556. [Google Scholar]
- Little, C.L.; Gormley, F.J.; Rawal, N.; Richardson, J.F. A Recipe for Disaster: Outbreaks of Campylobacteriosis Associated with Poultry Liver Pâté in England and Wales. Epidemiol. Infect. 2010, 138, 1691–1694. [Google Scholar] [CrossRef]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef]
- Yang, B.; Qu, D.; Zhang, X.; Shen, J.; Cui, S.; Shi, Y.; Xi, M.; Sheng, M.; Zhi, S.; Meng, J. Prevalence and Characterization of Salmonella Serovars in Retail Meats of Marketplace in Shaanxi, China. Int. J. Food Microbiol. 2010, 141, 63–72. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, J.; Zhang, F.; Yang, X.; Wu, H.; Zeng, H.; Chen, M.; Ding, Y.; et al. Staphylococcus Aureusisolated from Retail Meat and Meat Products in China: Incidence, Antibiotic Resistance and Genetic Diversity. Front. Microbiol. 2018, 9, 2767. [Google Scholar] [CrossRef]
- Ali, N.H.; Farooqui, A.; Khan, A.; Khan, A.Y.; Kazmi, S.U. Microbial Contamination of Raw Meat and Its Environment in Retail Shops in Karachi, Pakistan. J. Infect. Dev. Ctries. 2010, 4, 382–388. [Google Scholar]
- Ge, B.; Mukherjee, S.; Hsu, C.H.; Davis, J.A.; Tran, T.T.T.; Yang, Q.; Abbott, J.W.; Ayers, S.L.; Young, S.R.; Crarey, E.T.; et al. MRSA and Multidrug-Resistant Staphylococcus Aureus in U.S. Retail Meats, 2010–2011. Food Microbiol. 2017, 62, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of Seafood-Associated Infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valciņa, O.; Bartkevičs, V.; Bērziņš, A. Major Foodborne Pathogens in Fish and Fish Products: A Review. Ann. Microbiol. 2016, 66, 1–15. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, L.; Yang, Q.; Han, F.; Chen, S.; Pu, S.; Vance, A.; Ge, B. Prevalence and Antimicrobial Susceptibility of Major Foodborne Pathogens in Imported Seafood. J. Food Prot. 2011, 74, 1451–1461. [Google Scholar] [CrossRef]
- Dumen, E.; Ekici, G.; Ergin, S.; Bayrakal, G.M. Presence of Foodborne Pathogens in Seafood and Risk Ranking for Pathogens. Foodborne Pathog. Dis. 2020, 17, 541–546. [Google Scholar] [CrossRef]
- Novotny, L.; Dvorska, L.; Lorencova, A.; Beran, V.; Pavlik, I. Fish: A Potential Source of Bacterial Pathogens for Human Beings. Vet. Med. 2004, 49, 343–358. [Google Scholar] [CrossRef]
- Ali, A.; Parisi, A.; Conversano, M.C.; Iannacci, A.; D’Emilio, F.; Mercurio, V.; Normanno, G. Food-Borne Bacteria Associated with Seafoods: A Brief Review. J. Food Qual. Hazards Control. 2020, 7, 4–10. [Google Scholar] [CrossRef]
- Herwig, R. Pathogens Transmitted by Seafood. In Foodborne Disease Handbook: Volume IV: Seafood and Environmental Toxins; Hui, Y.H., Kitts, D., Stanfield, P.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; p. 109. [Google Scholar]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria Monocytogenes in Aquatic Food Products—A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Jung, S.W. A Foodborne Outbreak of Gastroenteritis Caused by Vibrio Parahaemolyticus Associated with Cross-Contamination from Squid in Korea. Epidemiol. Health 2018, 40, e2018056. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Hong, S.; Lee, S.; Na, H.Y.; Jeong, Y.-I.; Choi, E.J.; Kim, J.; Kawk, H.S.; Cho, E. Cholera Outbreak Due to Raw Seafood Consumption in South Korea, 2016. Am. J. Trop. Med. Hyg. 2018, 99, 168–170. [Google Scholar] [CrossRef]
- Hassan, R.; Tecle, S.; Adcock, B.; Kellis, M.; Weiss, J.; Saupe, A.; Sorenson, A.; Klos, R.; Blankenship, J.; Blessington, T.; et al. Multistate Outbreak of Salmonella Paratyphi B Variant L(+) Tartrate(+) and Salmonella Weltevreden Infections Linked to Imported Frozen Raw Tuna: USA, March–July 2015. Epidemiol. Infect. 2018, 146, 1461–1467. [Google Scholar] [CrossRef]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E.; Tatini, S.R. Incidence of Salmonella in Fish and Seafood. J. Food Prot. 2000, 63, 579–592. [Google Scholar] [CrossRef]
- Taylor, M.; Cheng, J.; Sharma, D.; Bitzikos, O.; Gustafson, R.; Fyfe, M.; Greve, R.; Murti, M.; Stone, J.; Honish, L.; et al. Outbreak of Vibrio Parahaemolyticus Associated with Consumption of Raw Oysters in Canada, 2015. Foodborne Pathog. Dis. 2018, 15, 554–559. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors Influencing the Microbial Safety of Fresh Produce: A Review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Beuchat, L.R. Ecological Factors Influencing Survival and Growth of Human Pathogens on Raw Fruits and Vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Araújo, S.; Silva, I.A.T.; Tacão, M.; Patinha, C.; Alves, A.; Henriques, I. Characterization of Antibiotic Resistant and Pathogenic Escherichia Coli in Irrigation Water and Vegetables in Household Farms. Int. J. Food Microbiol. 2017, 257, 192–200. [Google Scholar] [CrossRef]
- Akinde, S.B.; Sunday, A.A.; Adeyemi, F.M.; Fakayode, I.B.; Oluwajide, O.O.; Adebunmi, A.A.; Oloke, J.K.; Adebooye, C.O. Microbes in Irrigation Water and Fresh Vegetables: Potential Pathogenic Bacteria Assessment and Implications for Food Safety. Appl. Biosaf. 2016, 21, 89–97. [Google Scholar] [CrossRef]
- Steele, M.; Odumeru, J. Irrigation Water as Source of Foodborne Pathogens on Fruit and Vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar] [CrossRef]
- Okafo, C.N.; Umoh, V.J.; Galadima, M. Occurrence of Pathogens on Vegetables Harvested from Soils Irrigated with Contaminated Streams. Sci. Total Environ. 2003, 311, 49–56. [Google Scholar] [CrossRef]
- Hounmanou, Y.M.G.; Mdegela, R.H.; Dougnon, T.V.; Mhongole, O.J.; Mayila, E.S.; Malakalinga, J.; Makingi, G.; Dalsgaard, A. Toxigenic Vibrio Cholerae O1 in Vegetables and Fish Raised in Wastewater Irrigated Fields and Stabilization Ponds during a Non-Cholera Outbreak Period in Morogoro, Tanzania: An Environmental Health Study. BMC Res. Notes 2016, 9, 466. [Google Scholar] [CrossRef]
- Willis, C.; McLauchlin, J.; Aird, H.; Amar, C.; Barker, C.; Dallman, T.; Elviss, N.; Lai, S.; Sadler-Reeves, L. Occurrence of Listeria and Escherichia Coli in Frozen Fruit and Vegetables Collected from Retail and Catering Premises in England 2018–2019. Int. J. Food Microbiol. 2020, 334, 108849. [Google Scholar] [CrossRef]
- Sagoo, S.K.; Little, C.L.; Ward, L.; Gillespie, I.A.; Mitchell, R.T. Microbiological Study of Ready-to-Eat Salad Vegetables from Retail Establishments Uncovers a National Outbreak of Salmonellosis. J. Food Prot. 2003, 66, 403–409. [Google Scholar] [CrossRef]
- Ponniah, J.; Robin, T.; Paie, M.S.; Radu, S.; Ghazali, F.M.; Kqueen, C.Y.; Nishibuchi, M.; Nakaguchi, Y.; Malakar, P.K. Listeria Monocytogenes in Raw Salad Vegetables Sold at Retail Level in Malaysia. Food Control 2010, 21, 774–778. [Google Scholar] [CrossRef]
- Ajayeoba, T.A.; Atanda, O.O.; Obadina, A.O.; Bankole, M.O.; Adelowo, O.O. The Incidence and Distribution of Listeria Monocytogenes in Ready-to-Eat Vegetables in South-Western Nigeria. Food Sci. Nutr. 2016, 4, 59–66. [Google Scholar] [CrossRef]
- Gomez-Govea, M.; Solis, L.; Heredia, N.; García, S. Analysis of Microbial Contamination Levels of Fruits and Vegetables at Retail in Monterrey, Mexico. J. Food Agric. Environ. 2005, 10, 152–156. [Google Scholar]
- Denis, N.; Zhang, H.; Leroux, A.; Trudel, R.; Bietlot, H. Prevalence and Trends of Bacterial Contamination in Fresh Fruits and Vegetables Sold at Retail in Canada. Food Control 2016, 67, 225–234. [Google Scholar] [CrossRef]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Detection of Listeria Species in Fresh Produce Samples from Different Retail Shops in Canterbury, New Zealand. Adv. Food Technol. Nutr. Sci.Open J. 2016, 2, 96–102. [Google Scholar] [CrossRef]
- Nunes Silva, B.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Meta-Analysis of the Incidence of Foodborne Pathogens in Vegetables and Fruits from Retail Establishments in Europe. Curr. Opin. Food Sci. 2017, 18, 21–28. [Google Scholar] [CrossRef]
- Møretrø, T.; Hermansen, L.; Holck, A.L.; Sidhu, M.S.; Rudi, K.; Langsrud, S. Biofilm Formation and the Presence of the Intercellular Adhesion Locus Ica among Staphylococi from Food and Food Processing Environments. Appl. Environ. Microbiol. 2003, 69, 5648–5655. [Google Scholar] [PubMed]
- Fagerlund, A.; Langsrud, S.; Heir, E.; Mikkelsen, M.I.; Møretrø, T. Biofilm Matrix Composition Affects the Susceptibility of Food Associated Staphylococci to Cleaning and Disinfection Agents. Front. Microbiol. 2016, 7, 856. [Google Scholar] [PubMed]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide Intercellular Adhesin in Biofilm: Structural and Regulatory Aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [PubMed]
- Fratamico, P.M.; Annous, B.A.; Guenther, N.W. Biofilms in the Food and Beverage Industries; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Furkanur, M.; Mizan, R.; Jahid, I.K.; Ha, S.-D. Microbial Biofilms in Seafood: A Food-Hygiene Challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31, 572–585. [Google Scholar]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar]
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus Cereus Biofilms-Same, Only Different. Front. Microbiol. 2016, 7, 1054. [Google Scholar]
- Liu, N.T.; Nou, X.; Lefcourt, A.M.; Shelton, D.R.; Lo, Y.M. Dual-Species Biofilm Formation by Escherichia Coli O157: H7 and Environmental Bacteria Isolated from Fresh-Cut Processing Facilities. Int. J. Food Microbiol. 2014, 171, 15–20. [Google Scholar]
- Alonso, A.N.; Perry, K.J.; Regeimbal, J.M.; Regan, P.M.; Higgins, D.E. Identification of Listeria Monocytogenes Determinants Required for Biofilm Formation. PLoS ONE 2014, 9, e113696. [Google Scholar]
- Zhang, T.; Bae, D.; Wang, C. Listeria Monocytogenes DNA Glycosylase AdlP Affects Flagellar Motility, Biofilm Formation, Virulence, and Stress Responses. Appl. Environ. Microbiol. 2016, 82, 5144–5152. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.W.; Reeve, K.E.; Gunn, J.S. Flagellated but Not Hyperfimbriated Salmonella Enterica Serovar Typhimurium Attaches to and Forms Biofilms on Cholesterol-Coated Surfaces. J. Bacteriol. 2010, 192, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- Jonas, K.; Tomenius, H.; Kader, A.; Normark, S.; Römling, U.; Belova, L.M.; Melefors, Ö. Roles of Curli, Cellulose and BapA in Salmonella Biofilm Morphology Studied by Atomic Force Microscopy. BMC Microbiol. 2007, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.Q.; Louie, J.W.; Feng, D.; Zhong, W.; Brandl, M.T. Curli Fimbriae Are Conditionally Required in Escherichia Coli O157: H7 for Initial Attachment and Biofilm Formation. Food Microbiol. 2016, 57, 81–89. [Google Scholar] [CrossRef]
- Giacomucci, S.; Cros, C.D.N.; Perron, X.; Mathieu-Denoncourt, A.; Duperthuy, M. Flagella-Dependent Inhibition of Biofilm Formation by Sub-Inhibitory Concentration of Polymyxin B in Vibrio Cholerae. PLoS ONE 2019, 14, e0221431. [Google Scholar] [CrossRef]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef]
- Aurum, F.S.; Nguyen, L.T. Efficacy of Photoactivated Curcumin to Decontaminate Food Surfaces under Blue Light Emitting Diode. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Josewin, S.W.; Kim, M.J.; Yuk, H.G. Inactivation of Listeria Monocytogenes and Salmonella spp. on Cantaloupe Rinds by Blue Light Emitting Diodes (LEDs). Food Microbiol. 2018, 76, 219–225. [Google Scholar] [CrossRef]
- Bumah, V.V.; Whelan, H.T.; Masson-Meyers, D.S.; Quirk, B.; Buchmann, E.; Enwemeka, C.S. The Bactericidal Effect of 470-Nm Light and Hyperbaric Oxygen on Methicillin-Resistant Staphylococcus Aureus (MRSA). Lasers Med. Sci. 2015, 30, 1153–1159. [Google Scholar] [CrossRef][Green Version]
- De Lucca, A.J.; Carter-Wientjes, C.; Williams, K.A.; Bhatnagar, D. Blue Light (470 nm) Effectively Inhibits Bacterial and Fungal Growth. Lett. Appl. Microbiol. 2012, 55, 460–466. [Google Scholar] [CrossRef]
- Dietel, W.; Pottier, R.; Pfister, W.; Schleier, P.; Zinner, K. 5-Aminolaevulinic Acid (ALA) Induced Formation of Different Fluorescent Porphyrins: A Study of the Biosynthesis of Porphyrins by Bacteria of the Human Digestive Tract. J. Photochem. Photobiol. B Biol. 2007, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Skaar, E.P. Heme Synthesis and Acquisition in Bacterial Pathogens. J. Mol. Biol. 2016, 428, 3408–3428. [Google Scholar] [CrossRef] [PubMed]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic Heme Biosynthesis: Multiple Pathways to a Common Essential Product. Microbiol. Mol. Biol. Rev. 2017, 81. [Google Scholar] [CrossRef] [PubMed]
- Lukšienè, Ž. New Approach to Inactivation of Harmful and Pathogenic Microorganisms by Photosensitization. Food Technol. Biotechnol. 2005, 43, 411–418. [Google Scholar]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Kim, M.J.; Mikš-Krajnik, M.; Kumar, A.; Yuk, H.G. Inactivation by 405 ± 5 nm Light Emitting Diode on Escherichia Coli O157: H7, Salmonella Typhimurium, and Shigella Sonnei under Refrigerated Condition Might Be Due to the Loss of Membrane Integrity. Food Control 2016, 59, 99–107. [Google Scholar] [CrossRef]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of Intracellular Porphyrin, Reactive Oxygen Species, and Fatty Acids Profiles During Inactivation of Methicillin-Resistant Staphylococcus Aureus by Antimicrobial Blue Light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Umesh Hebbar, H. Efficacy of Blue LED in Microbial Inactivation: Effect of Photosensitization and Process Parameters. Int. J. Food Microbiol. 2019, 290, 296–304. [Google Scholar] [CrossRef]
- Chu, Z.; Hu, X.; Wang, X.; Wu, J.; Dai, T.; Wang, X. Inactivation of Cronobacter Sakazakii by Blue Light Illumination and the Resulting Oxidative Damage to Fatty Acids. Can. J. Microbiol. 2019, 65, 922–929. [Google Scholar] [CrossRef]
- Kim, M.J.; Bang, W.S.; Yuk, H.G. 405 ± 5 nm Light Emitting Diode Illumination Causes Photodynamic Inactivation of Salmonella Spp. on Fresh-Cut Papaya without Deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial Blue Light Inactivation of Pathogenic Microbes: State of the Art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Millioni, R.; Puricelli, L.; Tessari, P.; Arrigoni, G.; Franchin, C.; Segalla, A.; Teardo, E.; Reddi, E. Molecular Targets of Antimicrobial Photodynamic Therapy Identified by a Proteomic Approach. J. Proteomics 2012, 77, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Kielian, T.; Hamblin, M.R. Blue Light Eliminates Community-Acquired Methicillin-Resistant Staphylococcus Aureus in Infected Mouse Skin Abrasions. Photomed. Laser Surg. 2013, 31, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Therapy for Multidrug-Resistant Acinetobacter Baumannii Infection in a Mouse Burn Model: Implications for Prophylaxis and Treatment of Combat-Related Wound Infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of Photodynamic Laser and Violet-Blue Led Irradiation on Staphylococcus Aureus Biofilm and Escherichia Coli Lipopolysaccharide Attached to Moderately Rough Titanium Surface: In Vitro Study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Tschowri, N.; Lindenberg, S.; Hengge, R. Molecular Function and Potential Evolution of the Biofilm-Modulating Blue Light-Signalling Pathway of Escherichia Coli. Mol. Microbiol. 2012, 85, 893–906. [Google Scholar] [CrossRef]
- Tschowri, N.; Busse, S.; Hengge, R. The BLUF-EAL Protein YcgF Acts as a Direct Anti-Repressor in a Blue-Light Response of Escherichia Coli. Genes Dev. 2009, 23, 522–534. [Google Scholar] [CrossRef]
- Mussi, M.A.; Gaddy, J.A.; Cabruja, M.; Arivett, B.A.; Viale, A.M.; Rasia, R.; Actis, L.A. The Opportunistic Human Pathogen Acinetobacter Baumannii Senses and Responds to Light. J. Bacteriol. 2010, 192, 6336–6345. [Google Scholar] [CrossRef]
- Yang, P.; Wang, N.; Wang, C.; Yao, Y.; Fu, X.; Yu, W.; Cai, R.; Yao, M. 460 Nm Visible Light Irradiation Eradicates MRSA via Inducing Prophage Activation. J. Photochem. Photobiol. B Biol. 2017, 166, 311–322. [Google Scholar] [CrossRef]
- Jayaraman, P.; Devarajan, K.; Chua, T.K.; Zhang, H.; Gunawan, E.; Poh, C.L. Blue Light-Mediated Transcriptional Activation and Repression of Gene Expression in Bacteria. Nucleic Acids Res. 2016, 44, 6994–7005. [Google Scholar] [CrossRef] [PubMed]
- Braatsch, S.; Klug, G. Blue Light Perception in Bacteria. Photosynth. Res. 2004, 79, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, M.; Hoff, W.D. Light Helps Bacteria Make Important Lifestyle Decisions. Trends Microbiol. 2011, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yeh, N.; Chung, J.P. High-Brightness LEDs-Energy Efficient Lighting Sources and Their Potential in Indoor Plant Cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of Light-Emitting Diodes (LEDs) in Food Processing and Water Treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Feezell, D.; Nakamura, S. Invention, Development, and Status of the Blue Light-Emitting Diode, the Enabler of Solid-State Lighting. Comptes Rendus Phys. 2018, 19, 113–133. [Google Scholar] [CrossRef]
- Dall Agnol, M.A.; Nicolau, R.A.; De Lima, C.J.; Munin, E. Comparative Analysis of Coherent Light Action (Laser) versus Non-Coherent Light (Light-Emitting Diode) for Tissue Repair in Diabetic Rats. Lasers Med. Sci. 2009, 24, 909–916. [Google Scholar] [CrossRef]
- Corazza, A.V.; Jorge, J.; Kurachi, C.; Bagnato, V.S. Photobiomodulation on the Angiogenesis of Skin Wounds in Rats Using Different Light Sources. Photomed. Laser Surg. 2007, 25, 102–106. [Google Scholar] [CrossRef]
- de Abreu Chaves, M.E.; Piancastelli, A.C.C.; de Araújo, A.R.; Pinotti, M. Effects of Low-Power Light Therapy on Wound Healing: LASER x LED. An. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Bumah, V.V.; Biener, G.; Raicu, V.; Enwemeka, C.S. The Relative Antimicrobial Effect of Blue 405 Nm LED and Blue 405 Nm Laser on Methicillin-Resistant Staphylococcus Aureus in Vitro. Lasers Med. Sci. 2015, 30, 2265–2271. [Google Scholar] [CrossRef]
- Feltin, E.; Castiglia, A.; Cosendey, G.; Sulmoni, L.; Carlin, J.F.; Grandjean, N.; Rossetti, M.; Dorsaz, J.; Laino, V.; Duelk, M.; et al. Broadband Blue Superluminescent Light-Emitting Diodes Based on GaN. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Rossetti, M.; Napierala, J.; Matuschek, N.; Achatz, U.; Duelk, M.; Vélez, C.; Castiglia, A.; Grandjean, N.; Dorsaz, J.; Feltin, E. Superluminescent Light Emitting Diodes: The Best out of Two Worlds. MOEMS Miniaturized Syst. XI 2012, 8252, 825208. [Google Scholar]
- Zhang, Z.; Hogg, R.; Lv, X.; Wang, Z. Self-Assembled Quantum-Dot Superluminescent Light-Emitting Diodes. Adv. Opt. Photonics 2010, 2, 201–228. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Williams, D.; Hollosi, S.; Yens, D.; Enwemeka, S.K. Visible 405 Nm SLD Light Photo-Destroys Methicillin-Resistant Staphylococcus Aureus (MRSA) in Vitro. Lasers Surg. Med. 2008, 40, 734–737. [Google Scholar] [CrossRef]
- Guffey, J.S.; Wilborn, J. Effects of Combined 405-Nm and 880-Nm Light on Staphylococcus Aureus and Pseudomonas Aeruginosa in Vitro. Photomed. Laser Surg. 2006, 24, 680–683. [Google Scholar]
- Guffey, J.S.; Wilborn, J. In Vitro Bactericidal Effects of 405-Nm and 470-Nm Blue Light. Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Gendy, A.O.; Moniem Radi, N.A.; Mohamed, T. The Bactericidal Efficacy of Femtosecond Laser-Based Therapy on the Most Common Infectious Bacterial Pathogens in Chronic Wounds: An in Vitro Study. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.-W.D.; Fu, Q.; Lindsay, S.M.; Li, Z.; Cope, S.; Vaiana, S.; Kiang, J.G. Studies of Inactivation of Encephalomyocarditis Virus, M13 Bacteriophage, and Salmonella Typhimurium by Using a Visible Femtosecond Laser: Insight into the Possible Inactivation Mechanisms. J. Biomed. Opt. 2011, 16, 078003. [Google Scholar]
- Barneck, M.D.; Rhodes, N.L.R.; de la Presa, M.; Allen, J.P.; Poursaid, A.E.; Nourian, M.M.; Firpo, M.A.; Langell, J.T. Violet 405-Nm Light: A Novel Therapeutic Agent against Common Pathogenic Bacteria. J. Surg. Res. 2016, 206, 316–324. [Google Scholar]
- Murdoch, L.E.; MacLean, M.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Bactericidal Effects of 405nm Light Exposure Demonstrated by Inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium Species in Liquid Suspensions and on Exposed Surfaces. Sci. World J. 2012, 2012, 137805. [Google Scholar]
- Bumah, V.V.; Masson-Meyers, D.S.; Enwemeka, C.S. Pulsed 450 nm Blue Light Suppresses MRSA and Propionibacterium Acnes in Planktonic Cultures and Bacterial Biofilms. J. Photochem. Photobiol. B Biol. 2020, 202, 111702. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Bumah, V.V.; Castel, C.; Castel, D.; Enwemeka, C.S. Pulsed 450 Nm Blue Light Significantly Inactivates Propionibacterium Acnes More than Continuous Wave Blue Light. J. Photochem. Photobiol. B Biol. 2020, 202, 111719. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.B.; Maclean, M.; Given, M.J.; Wilson, M.P.; Judd, M.D.; Timoshkin, I.V.; Macgregor, S.J. Efficacy of Pulsed 405-Nm Light-Emitting Diodes for Antimicrobial Photodynamic Inactivation: Effects of Intensity, Frequency, and Duty Cycle. Photomed. Laser Surg. 2017, 35, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and Histological Effects of Blue Light on Normal Skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue Light Rescues Mice from Potentially Fatal Pseudomonas Aeruginosa Burn Infection: Efficacy, Safety, and Mechanism of Action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-Light Irradiation Regulates Proliferation and Differentiation in Human Skin Cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Marek, V.; Mélik-Parsadaniantz, S.; Villette, T.; Montoya, F.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A. Blue Light Phototoxicity toward Human Corneal and Conjunctival Epithelial Cells in Basal and Hyperosmolar Conditions. Free Radic. Biol. Med. 2018, 126, 27–40. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Zhou, Y.; Tan, G.; Li, J. Research Progress about the Effect and Prevention of Blue Light on Eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar]
- West, K.E.; Jablonski, M.R.; Warfield, B.; Cecil, K.S.; James, M.; Ayers, M.A.; Maida, J.; Bowen, C.; Sliney, D.H.; Rollag, M.D.; et al. Blue Light from Light-Emitting Diodes Elicits a Dose-Dependent Suppression of Melatonin in Humans. J. Appl. Physiol. 2011, 110, 619–626. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists. ACGIH—Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs). Available online: https://www.nsc.org/Portals/0/Documents/facultyportal/Documents/fih-6e-appendix-b.pdf (accessed on 21 October 2020).
- Leccese, F.; Vandelanotte, V.; Salvadori, G.; Rocca, M. Blue Light Hazard and Risk Group Classification of 8 W LED Tubes, Replacing Fluorescent Tubes, through Optical Radiation Measurements. Sustainability 2015, 7, 13454–13468. [Google Scholar] [CrossRef]
- Soares, C.J.; de Paula Rodrigues, M.; Vilela, A.B.F.; Rizo, E.R.C.; Ferreira, L.B.; Giannini, M.; Price, R.B. Evaluation of Eye Protection Filters Used with Broad-Spectrum and Conventional LED Curing Lights. Braz. Dent. J. 2017, 28, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, B.; James, R.P. Amber Lenses to Block Blue Light and Improve Sleep: A Randomized Trial. Chronobiol. Int. 2009, 26, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Kitajima, T.; Ito, Y.; Koike, S.; Nakao, Y.; Tsuchiya, A.; Hirose, M.; Iwata, N. Wearing Blue Light-Blocking Glasses in the Evening Advances Circadian Rhythms in the Patients with Delayed Sleep Phase Disorder: An Open-Label Trial. Chronobiol. Int. 2016, 33, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.; Gunther, N.W.; Sheen, S. Inactivation of Salmonella Spp., Pathogenic Escherichia Coli, Staphylococcus Spp., or Listeria Monocytogenes in Chicken Purge or Skin Using a 405-nm LED Array. Food Microbiol. 2017, 64, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Gunther, N.W.; Phillips, J.G.; Sommers, C. The Effects of 405-Nm Visible Light on the Survival of Campylobacter on Chicken Skin and Stainless Steel. Foodborne Pathog. Dis. 2016, 13, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.J.; Bang, W.S.; Yuk, H.G. Anti-Biofilm Effect of 405-Nm LEDs against Listeria Monocytogenes in Simulated Ready-to-Eat Fresh Salmon Storage Conditions. Food Control 2018, 84, 513–521. [Google Scholar] [CrossRef]
- McKenzie, K.; Maclean, M.; Timoshkin, I.V.; Endarko, E.; Macgregor, S.J.; Anderson, J.G. Photoinactivation of Bacteria Attached to Glass and Acrylic Surfaces by 405 Nm Light: Potential Application for Biofilm Decontamination. Photochem. Photobiol. 2013, 89, 927–935. [Google Scholar] [CrossRef]
- Hyun, J.E.; Lee, S.Y. Antibacterial Effect and Mechanisms of Action of 460–470 nm Light-Emitting Diode against Listeria Monocytogenes and Pseudomonas Fluorescens on the Surface of Packaged Sliced Cheese. Food Microbiol. 2020, 86, 103314. [Google Scholar] [CrossRef]
- Luksiene, Z.; Buchovec, I.; Paskeviciute, E. Inactivation of Food Pathogen Bacillus Cereus by Photosensitization in Vitro and on the Surface of Packaging Material. J. Appl. Microbiol. 2009, 107, 2037–2046. [Google Scholar] [CrossRef]
- Luksiene, Z.; Paskeviciute, E. Reprint of: Novel Approach to Decontaminate Food-Packaging from Pathogens in Non-Thermal and Not Chemical Way: Chlorophyllin-Based Photosensitization Q. J. Food Eng. 2012, 110, 317–323. [Google Scholar] [CrossRef]
- Buchovec, I.; Paskeviciute, E.; Luksiene, Z. Photosensitization-Based Inactivation of Food Pathogen Listeria Monocytogenes in Vitro and on the Surface of Packaging Material. J. Photochem. Photobiol. B Biol. 2010, 99, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Haughton, P.N.; Grau, E.G.; Lyng, J.; Cronin, D.; Fanning, S.; Whyte, P. Susceptibility of Campylobacter to High Intensity near Ultraviolet/Visible 395 ± 5 nm Light and Its Effectiveness for the Decontamination of Raw Chicken and Contact Surfaces. Int. J. Food Microbiol. 2012, 159, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.M.; Sauer, A.; Moraru, C.I. Inactivation of Escherichia Coli in Milk and Concentrated Milk Using Pulsed-Light Treatment. J. Dairy Sci. 2012, 95, 5597–5603. [Google Scholar] [CrossRef] [PubMed]
- dos Anjos, C.; Sellera, F.P.; de Freitas, L.M.; Gargano, R.G.; Telles, E.O.; Freitas, R.O.; Baptista, M.S.; Ribeiro, M.S.; Lincopan, N.; Pogliani, F.C.; et al. Inactivation of Milk-Borne Pathogens by Blue Light Exposure. J. Dairy Sci. 2020, 103, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, L.E.; MacLean, M.; MacGregor, S.J.; Anderson, J.G. Inactivation of Campylobacter Jejuni by Exposure to High-Intensity 405-Nm Visible Light. Foodborne Pathog. Dis. 2010, 7, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Endarko, E.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. High-Intensity 405 Nm Light Inactivation of Listeria Monocytogenes. Photochem. Photobiol. 2012, 88, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Srimagal, A.; Ramesh, T.; Sahu, J.K. Effect of Light Emitting Diode Treatment on Inactivation of Escherichia Coli in Milk. LWT-Food Sci. Technol. 2016, 71, 378–385. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Zhou, W.; Yuk, H.G. Irradiance and Temperature Influence the Bactericidal Effect of 460-Nanometer Light-Emitting Diodes on Salmonella in Orange Juice. J. Food Prot. 2016, 79, 553–560. [Google Scholar] [CrossRef]
- Ricciardi, E.F.; Pedros-Garrido, S.; Papoutsis, K.; Lyng, J.G.; Conte, A.; Del Nobile, M.A. Novel Technologies for Preserving Ricotta Cheese: Effects of Ultraviolet and near-Ultraviolet–Visible Light. Foods 2020, 9, 580. [Google Scholar] [CrossRef]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New Horizons in Microbiological Food Safety: Photodynamic Decontamination Based on a Curcumin Derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef]
- Tortik, N.; Spaeth, A.; Plaetzer, K. Photodynamic Decontamination of Foodstuff from Staphylococcus Aureus Based on Novel Formulations of Curcumin. Photochem. Photobiol. Sci. 2014, 13, 1402–1409. [Google Scholar] [CrossRef]
- Winter, S.; Tortik, N.; Kubin, A.; Krammer, B.; Plaetzer, K. Back to the Roots: Photodynamic Inactivation of Bacteria Based on Water-Soluble Curcumin Bound to Polyvinylpyrrolidone as a Photosensitizer. Photochem. Photobiol. Sci. 2013, 12, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Lukseviciute, V.; Marsalka, A.; Reklaitis, I.; Luksiene, Z. Effective Photosensitization-Based Inactivation of Gram (−) Food Pathogens and Molds Using the Chlorophyllin-Chitosan Complex: Towards Photoactive Edible Coatings to Preserve Strawberries. Photochem. Photobiol. Sci. 2016, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Towards Better Microbial Safety of Fresh Produce: Chlorophyllin-Based Photosensitization for Microbial Control of Foodborne Pathogens on Cherry Tomatoes. J. Photochem. Photobiol. B Biol. 2018, 182, 130–136. [Google Scholar] [CrossRef] [PubMed]
- MinJeong, K.; CheeHwa, T.; WooSuk, B.; HyunGyun, Y. Antibacterial Effect of 405±5 Nm Light Emitting Diode Illumination against Escherichia Coli O157:H7, Listeria Monocytogenes, and Salmonella on the Surface of Fresh-Cut Mango and Its Influence on Fruit Quality. Int. J. Food Microbiol. 2017, 244, 82–89. [Google Scholar]
- Tao, R.; Zhang, F.; Tang, Q.-J.; Xu, C.-S.; Ni, Z.J.; Meng, X.-H. Effects of Curcumin-Based Photodynamic Treatment on the Storage Quality of Fresh-Cut Apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Kim, M.J.; Bang, W.S.; Zhou, W.; Yuk, H.G. Effect of 460 Nm Light Emitting Diode Illumination on Survival of Salmonella Spp. on Fresh-Cut Pineapples at Different Irradiances and Temperatures. J. Food Eng. 2017, 196, 130–138. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Sites, J.; Boyd, G.; Gurtler, J.B.; Tyrell, B.; Fleck, M. Reduction of Bacterial Pathogens and Potential Surrogates on the Surface of Almonds Using High-Intensity 405-Nanometer Light. J. Food Prot. 2016, 79, 1840–1845. [Google Scholar] [CrossRef]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Innovative Nonthermal Technologies: Chlorophyllin and Visible Light Significantly Reduce Microbial Load on Basil. Food Technol. Biotechnol. 2019, 57, 126–132. [Google Scholar] [CrossRef]
- Guffey, J.S.; Payne, W.C.; Motts, S.D.; Towery, P.; Hobson, T.; Harrell, G.; Meurer, L.; Lancaster, K. Inactivation of Salmonella on Tainted Foods: Using Blue Light to Disinfect Cucumbers and Processed Meat Products. Food Sci. Nutr. 2016, 4, 878–887. [Google Scholar] [CrossRef]
- Luksiene, Z.; Paskeviciute, E. Novel Approach to the Microbial Decontamination of Strawberries: Chlorophyllin-Based Photosensitization. J. Appl. Microbiol. 2011, 110, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Adeline Ng, B.X.; Zwe, Y.H.; Yuk, H.G. Photodynamic Inactivation of Salmonella Enterica Enteritidis by 405 ± 5-Nm Light-Emitting Diode and Its Application to Control Salmonellosis on Cooked Chicken. Food Control 2017, 82, 305–315. [Google Scholar] [CrossRef]
- Li, X.; Kim, M.J.; Yuk, H.G. Influence of 405 nm Light-Emitting Diode Illumination on the Inactivation of Listeria Monocytogenes and Salmonella Spp. on Ready-to-Eat Fresh Salmon Surface at Chilling Storage for 8 h and Their Susceptibility to Simulated Gastric Fluid. Food Control 2018, 88, 61–68. [Google Scholar] [CrossRef]
- Josewin, S.W.; Ghate, V.; Kim, M.J.; Yuk, H.G. Antibacterial Effect of 460 Nm Light-Emitting Diode in Combination with Riboflavin against Listeria Monocytogenes on Smoked Salmon. Food Control 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Stephen Guffey, J.; Stephen, J.; Professor, A. The Use of 405nm and 464nm Blue Light to Inhibit Listeria Monocytogenes in Ready-to-Eat (RTE) Meat. Eur. J. Acad. Essays ISSN 2016, 3, 76–80. [Google Scholar]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Coia, J.E.; Hamilton, K.; Taggart, I.; Watson, S.B.; Thakker, B.; Gettinby, G. Environmental Decontamination of a Hospital Isolation Room Using High-Intensity Narrow-Spectrum Light. J. Hosp. Infect. 2010, 76, 247–251. [Google Scholar] [CrossRef]
- Bache, S.E.; MacLean, M.; MacGregor, S.J.; Anderson, J.G.; Gettinby, G.; Coia, J.E.; Taggart, I. Clinical Studies of the High-Intensity Narrow-Spectrum Light Environmental Decontamination System (HINS-Light EDS), for Continuous Disinfection in the Burn Unit Inpatient and Outpatient Settings. Burns 2012, 38, 69–76. [Google Scholar] [CrossRef]
- Maclean, M.; Booth, M.; Anderson, J.; MacGregor, S.; Woolsey, G.; Coia, J.; Hamilton, K.; Gettinby, G. Continuous Decontamination of an Intensive Care Isolation Room during Patient Occupancy Using 405 Nm Light Technology. J. Infect. Prev. 2013, 14, 176–181. [Google Scholar] [CrossRef]
- MacLean, M.; Murdoch, L.E.; MacGregor, S.J.; Anderson, J.G. Sporicidal Effects of High-Intensity 405 nm Visible Light on Endospore-Forming Bacteria. Photochem. Photobiol. 2013, 89, 120–126. [Google Scholar] [CrossRef]
- Dougall, L.; Anderson, J.G.; Timoshkin, I.V.; MacGregor, S.J.; Maclean, M. Efficacy of Antimicrobial 405 nm Blue-Light for Inactivation of Airborne Bacteria; Dai, T., Ed.; International Society for Optics and Photonics: San Fransisco, CA, USA, 2018; Volume 10479, p. 10479G. [Google Scholar]
- Moreira, L.H.; de Souza, J.C.P.; de Lima, C.J.; Salgado, M.A.C.; Fernandes, A.B.; Andreani, D.I.K.; Villaverde, A.B.; Zângaro, R.A. Use of Photodynamic Therapy in the Treatment of Bovine Subclinical Mastitis. Photodiagn. Photodyn. Ther. 2018, 21, 246–251. [Google Scholar] [CrossRef]
- Sellera, F.P.; Sabino, C.P.; Ribeiro, M.S.; Gargano, R.G.; Benites, N.R.; Melville, P.A.; Pogliani, F.C. In Vitro Photoinactivation of Bovine Mastitis Related Pathogens. Photodiagn. Photodyn. Ther. 2016, 13, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, P.S.; Al-Ghabshi, A.; Al-Mazrooei, N.; Al-Habsi, S. Comparative Pathogenomics of Bacteria Causing Infectious Diseases in Fish. Int. J. Evol. Biol. 2012, 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.J.; Kim, A.; Kang, G.S.; Kim, D.H. Photoinactivation of Major Bacterial Pathogens in Aquaculture. Fish. Aquat. Sci. 2016, 19, 1–7. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Photodynamic Antimicrobial Chemotherapy in Aquaculture: Photoinactivation Studies of Vibrio Fischeri. PLoS ONE 2011, 6, e20970. [Google Scholar] [CrossRef]
- Arrojado, C.; Pereira, C.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; et al. Applicability of Photodynamic Antimicrobial Chemotherapy as an Alternative to Inactivate Fish Pathogenic Bacteria in Aquaculture Systems. Photochem. Photobiol. Sci. 2011, 10, 1691–1700. [Google Scholar] [CrossRef]
- Roh, H.J.; Kang, G.S.; Kim, A.; Kim, N.E.; Nguyen, T.L.; Kim, D.H. Blue Light-Emitting Diode Photoinactivation Inhibits Edwardsiellosis in Fancy Carp (Cyprinus Carpio). Aquaculture 2018, 483, 1–7. [Google Scholar] [CrossRef]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Sánchez-Vázquez, F.J. Effects of Light during Early Larval Development of Some Aquacultured Teleosts: A Review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Machado, S.G.; Baglinière, F.; Marchand, S.; Van Coillie, E.; Vanetti, M.C.D.; De Block, J.; Heyndrickx, M. The Biodiversity of the Microbiota Producing Heat-Resistant Enzymes Responsible for Spoilage in Processed Bovine Milk and Dairy Products. Front. Microbiol. 2017, 8, 302. [Google Scholar] [CrossRef]
- Yuan, L.; Sadiq, F.A.; Burmølle, M.; Wang, N.; He, G. Insights into Psychrotrophic Bacteria in Raw Milk: A Review. J. Food Prot. 2019, 82, 1148–1159. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Avery, S.V.; Singleton, I. Microbes Associated with Fresh Produce: Sources, Types and Methods to Reduce Spoilage and Contamination. Adv. Appl. Microbiol. 2019, 107, 29–82. [Google Scholar]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage Bacteria and Meat Quality. In Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: London, UK, 2019; pp. 307–334. [Google Scholar]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood Spoilage Microbiota and Associated Volatile Organic Compounds at Different Storage Temperatures and Packaging Conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, J.; Li, S.; Hamzah, S.S.; Jiang, H.; Zhou, A.; Zeng, S.; Lin, S. Curcumin-Based Photodynamic Sterilization for Preservation of Fresh-Cut Hami Melon. Molecules 2019, 24, 2374. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; Cao, B.; Wu, J.; Wang, Y.; Xue, Y.; Xu, J.; Xue, C.; Tang, Q.J. The Effect of a Novel Photodynamic Activation Method Mediated by Curcumin on Oyster Shelf Life and Quality. Food Res. Int. 2016, 87, 204–210. [Google Scholar] [CrossRef]
- Gong, C.; Li, Y.; Gao, R.; Xiao, F.; Zhou, X.; Wang, H.; Xu, H.; Wang, R.; Huang, P.; Zhao, Y. Inactivation of Specific Spoilage Organism (Pseudomonas) of Sturgeon by Curcumin-Mediated Photodynamic Inactivation. Photodiagn. Photodyn. Ther. 2020, 31, 101827. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.; Aoki, A.; Takeuchi, Y.; Sasaki, Y.; Hiratsuka, K.; Abiko, Y.; Izumi, Y. Antimicrobial Effect of Photodynamic Therapy Using High-Power Blue Light-Emitting Diode and Red-Dye Agent on Porphyromonas Gingivalis. J. Periodontal Res. 2013, 48, 696–705. [Google Scholar] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef]
- Fotinos, N.; Convert, M.; Piffaretti, J.C.; Gurny, R.; Lange, N. Effects on Gram-Negative and Gram-Positive Bacteria Mediated by 5-Aminolevulinic Acid and 5-Aminolevulinic Acid Derivatives. Antimicrob. Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef]
- Nitzan, Y.; Salmon-Divon, M.; Shporen, E.; Malik, Z. ALA Induced Photodynamic Effects on Gram Positive and Negative Bacteria. Photochem. Photobiol. Sci. 2004, 3, 430–435. [Google Scholar] [CrossRef]
- McKenzie, K.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. Enhanced Inactivation of Escherichia Coli and Listeria Monocytogenes by Exposure to 405nm Light under Sub-Lethal Temperature, Salt and Acid Stress Conditions. Int. J. Food Microbiol. 2014, 170, 91–98. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Zhou, W.; Yuk, H.G. Effect of Organic Acids on the Photodynamic Inactivation of Selected Foodborne Pathogens Using 461 nm LEDs. Food Control 2015, 57, 333–340. [Google Scholar] [CrossRef]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Antibacterial Efficacy of 405, 460 and 520 Nm Light Emitting Diodes on Lactobacillus Plantarum, Staphylococcus Aureus and Vibrio Parahaemolyticus. J. Appl. Microbiol. 2016, 120, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Akram, F.E.; El-Tayeb, T.; Abou-Aisha, K.; El-Azizi, M. A Combination of Silver Nanoparticles and Visible Blue Light Enhances the Antibacterial Efficacy of Ineffective Antibiotics against Methicillin-Resistant Staphylococcus Aureus (MRSA). Ann. Clin. Microbiol. Antimicrob. 2016, 15, 48. [Google Scholar] [CrossRef]
- El Din, S.N.; El-Tayeb, T.A.; Abou-Aisha, K.; El-Azizi, M. In Vitro and in Vivo Antimicrobial Activity of Combined Therapy of Silver Nanoparticles and Visible Blue Light against Pseudomonas Aeruginosa. Int. J. Nanomed. 2016, 11, 1749–1758. [Google Scholar]
- Yang, M.Y.; Chang, K.C.; Chen, L.Y.; Wang, P.C.; Chou, C.C.; Wu, Z.-B.; Hu, A. Blue Light Irradiation Triggers the Antimicrobial Potential of ZnO Nanoparticles on Drug-Resistant Acinetobacter Baumannii. J. Photochem. Photobiol. B Biol. 2018, 180, 235–242. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamada, Y.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal Action of Photoirradiated Gallic Acid via Reactive Oxygen Species Formation. J. Agric. Food Chem. 2012, 60, 10048–10054. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Kanno, T.; Lingström, P.; Örtengren, U.; Niwano, Y. Photo-Irradiated Caffeic Acid Exhibits Antimicrobial Activity against Streptococcus Mutans Biofilms via Hydroxyl Radical Formation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Sheng, H.; Tada, M.; Mokudai, T.; Oizumi, S.; Kamachi, T.; Niwano, Y. Bactericidal Action of Photo-Irradiated Aqueous Extracts from the Residue of Crushed Grapes from Winemaking. Biocontrol Sci. 2016, 21, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Sheng, H.; Kamachi, T.; Niwano, Y. Microbicidal Action of Photoirradiated Aqueous Extracts from Wine Lees. J. Food Sci. Technol. 2016, 53, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Cubero-Márquez, M.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Antimicrobial Activity of Nanoemulsions Containing Essential Oils and High Methoxyl Pectin during Long-Term Storage. Food Control 2017, 77, 131–138. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia Coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef]
- Marqués-Calvo, M.S.; Codony, F.; Agustí, G.; Lahera, C. Visible Light Enhances the Antimicrobial Effect of Some Essential Oils. Photodiagn. Photodyn. Ther. 2017, 17, 180–184. [Google Scholar] [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.-A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef]
- Zekar, F.M.; Granier, S.A.; Marault, M.; Yaici, L.; Gassilloud, B.; Manceau, C.; Touati, A.; Millemann, Y. From Farms to Markets: Gram-Negative Bacteria Resistant to Third-Generation Cephalosporins in Fruits and Vegetables in a Region of North Africa. Front. Microbiol. 2017, 8, 1569. [Google Scholar] [CrossRef] [PubMed]
- Boss, R.; Overesch, G.; Baumgartner, A. Antimicrobial Resistance of Escherichia Coli, Enterococci, Pseudomonas Aeruginosa, and Staphylococcus Aureus from Raw Fish and Seafood Imported into Switzerland. J. Food Prot. 2016, 79, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, R.; Stijntjes, M.; Rodríguez-Baño, J.; Tacconelli, E.; Babu Rajendran, N.; Voss, A. Review of Antimicrobial Resistance Surveillance Programmes in Livestock and Meat in EU with Focus on Humans. Clin. Microbiol. Infect. 2018, 24, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus Aureus Tolerance to Antimicrobial Photodynamic Inactivation and Antimicrobial Blue Light upon Sub-Lethal Treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef] [PubMed]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue Light Treatment of Pseudomonas Aeruginosa: Strong Bactericidal Activity, Synergism with Antibiotics and Inactivation of Virulence Factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Assessment of the Potential for Resistance to Antimicrobial Violet-Blue Light in Staphylococcus Aureus. Antimicrob. Resist. Infect. Control 2017, 6, 100. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.Y.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Tetracyclines Function as Dual-Action Light-Activated Antibiotics. PLoS ONE 2018, 13, e0196485. [Google Scholar] [CrossRef]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The Interconnection between Biofilm Formation and Horizontal Gene Transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef]
- Ferrer-Espada, R.; Wang, Y.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Microbial Isolates in Biofilms. Lasers Surg. Med. 2020, 52, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Harrington, O.D.; Fang, Y.; Ahmed, I.; Goh, X.S.; Dai, T. Evaluating the Potential for Resistance Development to Antimicrobial Blue Light (at 405 nm) in Gram-Negative Bacteria: In Vitro and in Vivo Studies. Front. Microbiol. 2018, 9, 2403. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.L.; Drum, B.E. RNA-Seq Reveals Changes in the Staphylococcus Aureus Transcriptome Following Blue Light Illumination. Genom. Data 2016, 9, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Tardu, M.; Bulut, S.; Kavakli, I.H. MerR and ChrR Mediate Blue Light Induced Photo-Oxidative Stress Response at the Transcriptional Level in Vibrio Cholerae. Sci. Rep. 2017, 7, 40817. [Google Scholar] [CrossRef]
- Leanse, L.; Goh, X.; Cheng, J.; Hooper, D.; Dai, T. Dual-Wavelength Photo-Killing of Methicillin-Resistant Staphylococcus Aureus. JCl Insight 2020, 5, e134343. [Google Scholar] [CrossRef]
- Cieplik, F.; Späth, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.A.; Maisch, T.; Schmalz, G. Blue Light Kills Aggregatibacter Actinomycetemcomitans Due to Its Endogenous Photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769. [Google Scholar] [CrossRef]
- Battisti, A.; Morici, P.; Ghetti, F.; Sgarbossa, A. Spectroscopic Characterization and Fluorescence Imaging of Helicobacter Pylori Endogenous Porphyrins. Biophys. Chem. 2017, 229, 19–24. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Inactivation of Pseudomonas Aeruginosa by Photo-Excitation of Endogenous Porphyrins: In Vitro and in Vivo Studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and in Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef]
- Hope, C.K.; Strother, M.; Creber, H.K.; Higham, S.M. Lethal Photosensitisation of Prevotellaceae under Anaerobic Conditions by Their Endogenous Porphyrins. Photodiagn. Photodyn. Ther. 2016, 13, 344–346. [Google Scholar] [CrossRef]
- Hope, C.K.; Hindley, J.A.; Khan, Z.; de Josselin de Jong, E.; Higham, S.M. Lethal Photosensitization of Porphyromonas Gingivalis by Their Endogenous Porphyrins under Anaerobic Conditions: An in Vitro Study. Photodiagn. Photodyn. Ther. 2013, 10, 677–682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bumah, V.V.; Aboualizadeh, E.; Masson-Meyers, D.S.; Eells, J.T.; Enwemeka, C.S.; Hirschmugl, C.J. Spectrally Resolved Infrared Microscopy and Chemometric Tools to Reveal the Interaction between Blue Light (470 Nm) and Methicillin-Resistant Staphylococcus Aureus. J. Photochem. Photobiol. B Biol. 2017, 167, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kaidzu, S.; Sugihara, K.; Sasaki, M.; Nishiaki, A.; Igarashi, T.; Tanito, M. Evaluation of Acute Corneal Damage Induced by 222-nm and 254-nm Ultraviolet Light in Sprague–Dawley Rats. Free Radic. Res. 2019, 53, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Asano, K.; Morimoto, Y.; Igarashi, T.; Nakane, A. Chronic Irradiation with 222-nm UVC Light Induces Neither DNA Damage nor Epidermal Lesions in Mouse Skin, Even at High Doses. PLoS ONE 2018, 13, e0201259. [Google Scholar] [CrossRef]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brenner, D.J. Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light. Radiat. Res. 2017, 187, 493–501. [Google Scholar] [CrossRef]
- Yamano, N.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaki-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term Effects of 222-nm Ultraviolet Radiation C Sterilizing Lamps on Mice Susceptible to Ultraviolet Radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef]
- Buonanno, M.; Randers-Pehrson, G.; Bigelow, A.W.; Trivedi, S.; Lowy, F.D.; Spotnitz, H.M.; Hammer, S.M.; Brenner, D.J. 207-nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. I: In Vitro Studies. PLoS ONE 2013, 8, e76968. [Google Scholar] [CrossRef]
- Buonanno, M.; Stanislauskas, M.; Ponnaiya, B.; Bigelow, A.W.; Randers-Pehrson, G.; Xu, Y.; Shuryak, I.; Smilenov, L.; Owens, D.M.; Brenner, D.J. 207-Nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. II: In-Vivo Safety Studies. PLoS ONE 2016, 11, e0138418. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).