Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Extraction of Phenolic Compounds
2.4. Estimation of Phenolics and Antioxidant Potential
2.5. Characterization and Quantification of Phenolics Using LC-ESI-QTOF-MS/MS and HPLC-PDA
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Estimation (TPC, TFC and TTC)
3.2. Antioxidant Potential (DPPH, ABTS, FRAP and TAC)
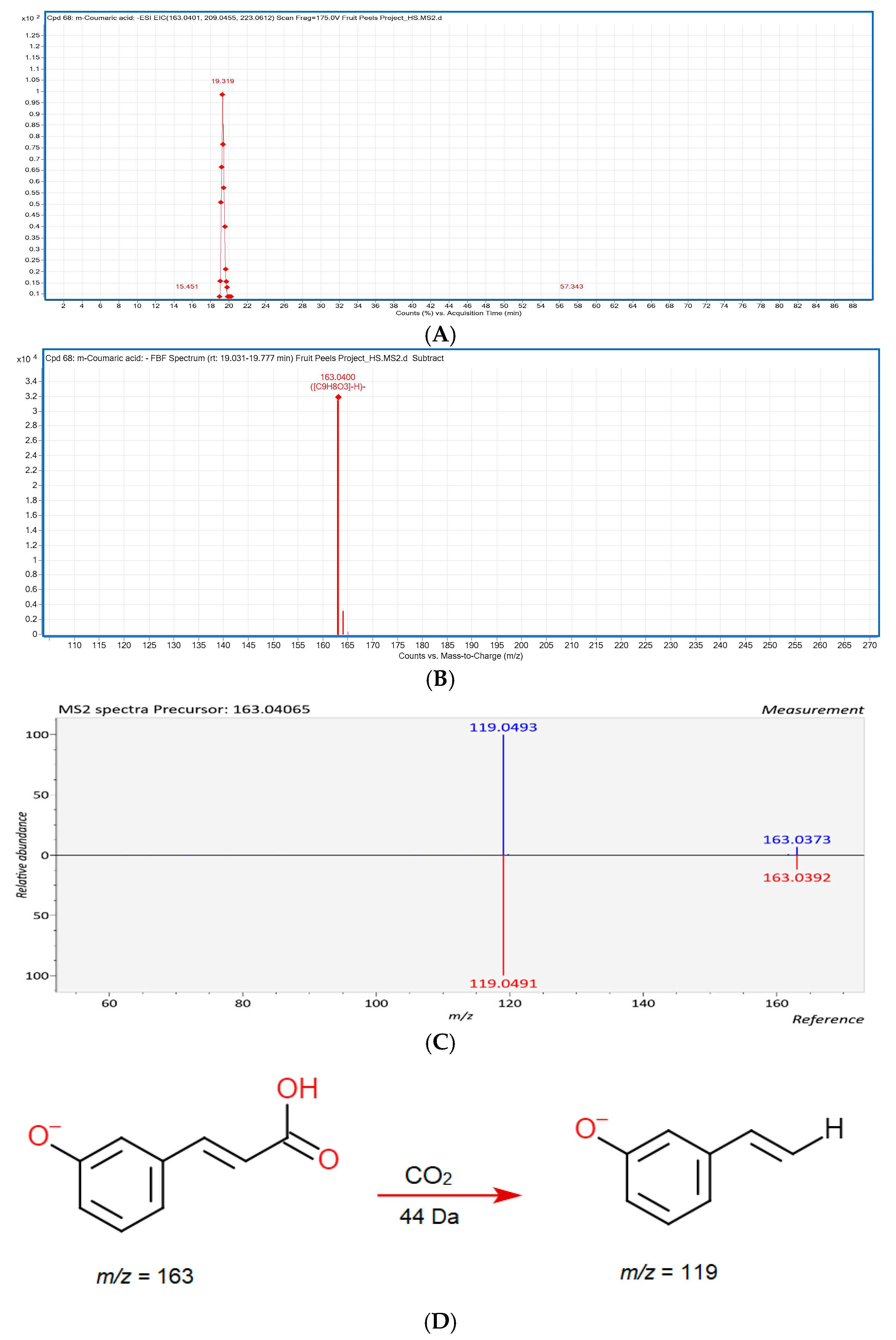
3.3. LC-ESI-QTOF-MS/MS Characterization
3.3.1. Phenolic Acids
3.3.2. Flavonoids
3.3.3. Other Polyphenols
3.3.4. Lignans
3.3.5. Stilbenes
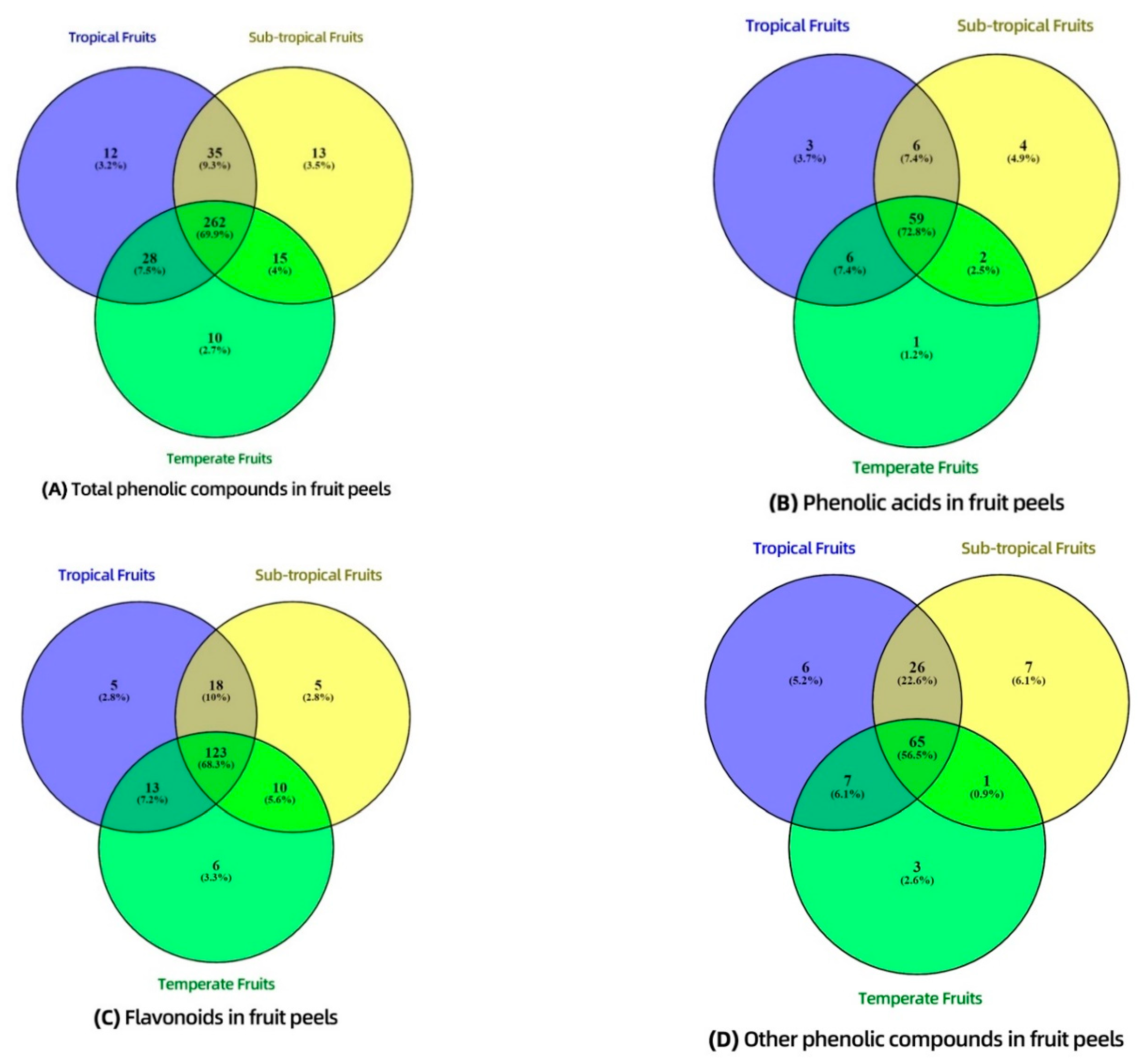
3.4. Distribution of Phenolic Compounds—Venn Diagram
3.5. HPLC-PDA Quantitative Analysis
3.5.1. Phenolic Acids
3.5.2. Flavonoids
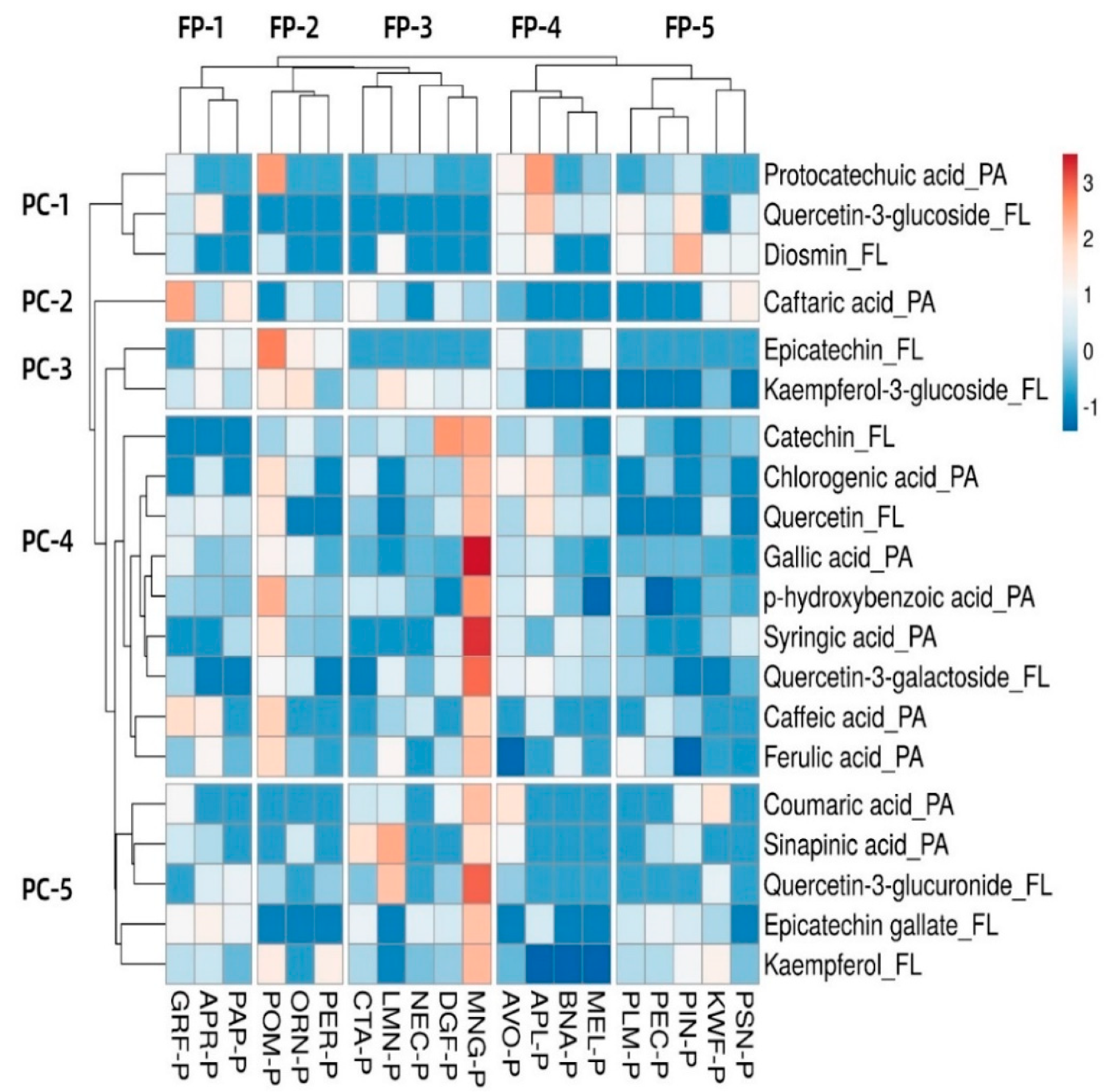
3.6. Heat Map and Hierarchical Clustering Phenolic Compound Analysis
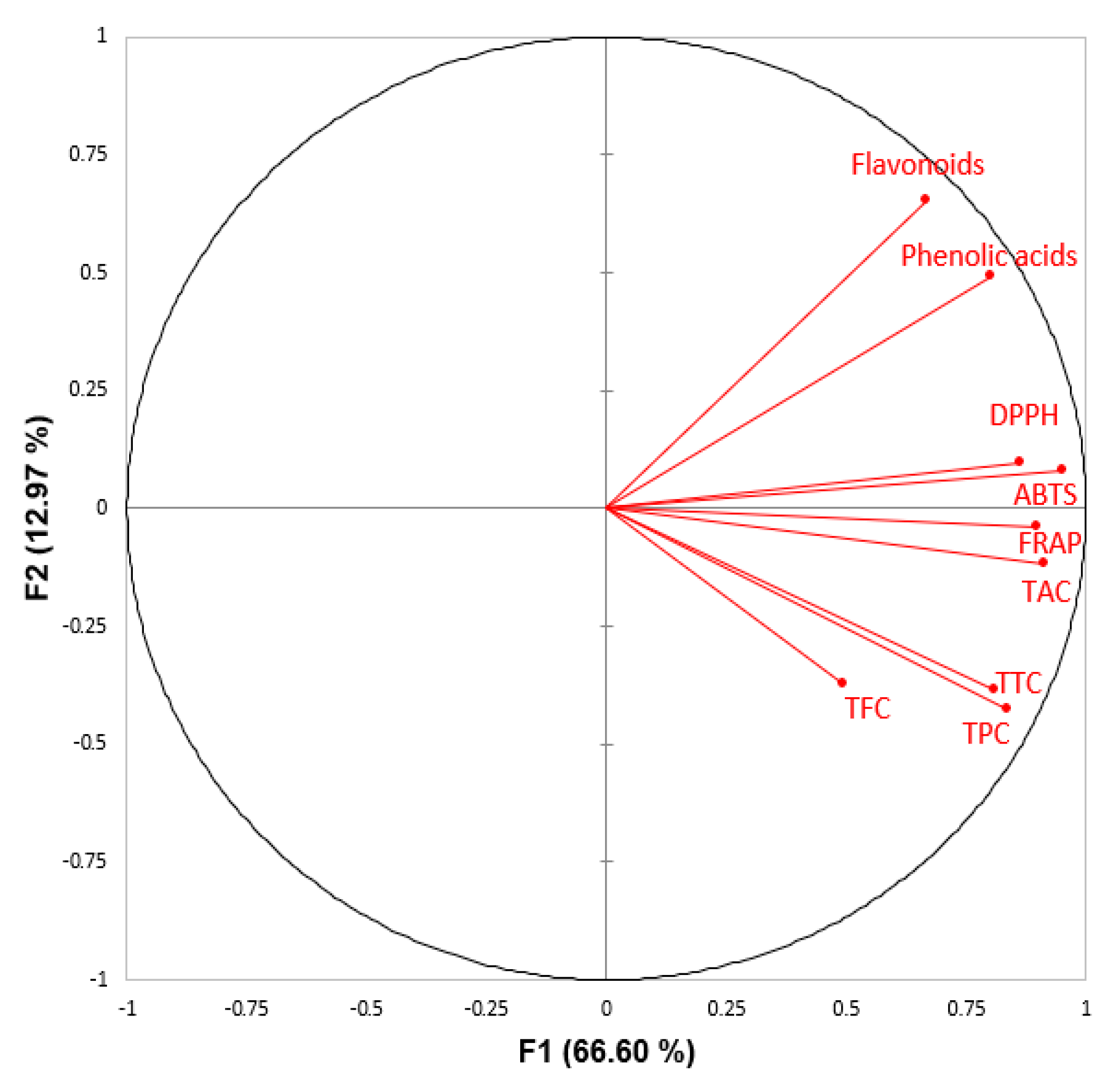
3.7. Correlation between Phenolic Compounds, Targeted Phenolics Quantified through HPLC-PDA and Antioxidant Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ain, H.B.U.; Saeed, F.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Food processing waste: A potential source for bioactive compounds. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 625–649. [Google Scholar]
- Abu Qdais, H.; Wuensch, C.; Dornack, C.; Nassour, A. The role of solid waste composting in mitigating climate change in jordan. Waste Manag. Res. 2019, 37, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Azizan, A.; Xin, L.A.; Abdul Hamid, N.A.; Maulidiani, M.; Mediani, A.; Abdul Ghafar, S.Z.; Zulaikha Zolkeflee, N.K.; Abas, F. Potentially bioactive metabolites from pineapple waste extracts and their antioxidant and α-glucosidase inhibitory activities by (1)h nmr. Foods 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, S.; Li, T.; Ruslan, M.S.; Amran, N.A. Antioxidant recovery from pomegranate peel waste by integrating maceration and freeze concentration technology. Int. J. Food Eng. 2020. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Borges, G.; Mullen, W.; Crozier, A. Comparison of the polyphenolic composition and antioxidant activity of european commercial fruit juices. Food Funct. 2010, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of european and tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate akko peel extracts and β-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Profiling lc-dad-esi-tof ms method for the determination of phenolic metabolites from avocado (persea americana). J. Agric. Food Chem. 2011, 59, 2255–2267. [Google Scholar] [CrossRef]
- Toh, P.Y.; Leong, F.S.; Chang, S.K.; Khoo, H.E.; Yim, H.S. Optimization of extraction parameters on the antioxidant properties of banana waste. Acta Sci. Pol. Technol. Aliment. 2016, 15, 65–78. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach [prunus persica (l.) batsch] cultivars in china. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef]
- Rodrigo, R.; Gil, D.; Miranda-Merchak, A.; Kalantzidis, G. Antihypertensive role of polyphenols. Adv. Clin. Chem. 2012, 58, 225. [Google Scholar]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process Preserv. 2020. [Google Scholar] [CrossRef]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in lc-ms and lc-hrms analysis and characterization of polyphenols in food. Trac. Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds in palm fruits (jelly and fishtail palm) and their potential antioxidant activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.P.; Le, T.T.; Vissenaekens, H.; Gonzales, G.B.; Van Camp, J.; Smagghe, G.; Raes, K. In vitro antioxidant activity and phenolic profiles of tropical fruit by-products. Int. J. Food Sci. Technol. 2019, 54, 1169–1178. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Shrestha, N.; Shrestha, S.; Bhattarai, A. Determination of Ascorbic Acid in Different Citrus Fruits of Kathmandu Valley. J. Med. Biol. Sci. Res. 2016, 2, 9–14. [Google Scholar]
- Xi, W.; Zhang, G.; Jiang, D.; Zhou, Z. Phenolic compositions and antioxidant activities of grapefruit (citrus paradisi macfadyen) varieties cultivated in china. Int. J. Food Sci. Nutr. 2015, 66, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Irkin, R.; Dogan, S.; Degirmenioglu, N.; Diken, M.E.; Guldas, M. Phenolic content, antioxidant activities and stimulatory roles of citrus fruits on some lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1313–1321. [Google Scholar] [CrossRef]
- Nurliyana, R.d.; Syed Zahir, I.; Mustapha Suleiman, K.; Aisyah, M.R.; Kamarul Rahim, K. Antioxidant study of pulps and peels of dragon fruits: A comparative study. Int. Food Res. J. 2010, 17, 367–375. [Google Scholar]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of caesalpinia bonduc (l.) roxb. Polym. J. 2017, 10, 123–127. [Google Scholar] [CrossRef]
- Marina, Z.; Noriham, A. Quantification of total phenolic compound and in vitro antioxidant potential of fruit peel extracts. Int. Food Res. J. 2014, 21. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant activity, phenolics and uplc–esi(–)–ms of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; YANO, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.C.; Azlan, A.; Chan, S.H.; Khoo, H.E. Extracts of peel and different parts of md2 pineapple as potent nutraceuticals. TJPS 2017, 41, 49. [Google Scholar]
- Wong, Y.S.; Sia, C.M.; Eng, H.; Ang, Y.K.; Chang, S.K.; Yim, H.S. Influence of extraction conditions on antioxidant properties of passion fruit (passiflora edulis) peel. Acta Sci. Pol. Technol. Aliment. 2014, 13, 257–265. [Google Scholar] [CrossRef]
- Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Velázquez-de la Cruz, G.; de León, J.A.; Navarro-Ocaña, A. Evaluation of extraction methods for preparative scale obtention of mangiferin and lupeol from mango peels (mangifera indica l.). Food Chem. 2014, 159, 267–272. [Google Scholar]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability; Phenolic Compounds Biological Activity, Ed.; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez, M.d.M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (citrus paradisi macf.). Oxid. Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef]
- Pal, R.S.; Kumar, V.A.; Arora, S.; Sharma, A.K.; Kumar, V.; Agrawal, S. Physicochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Braz. Arch. Biol. Technol. 2015, 58, 262–271. [Google Scholar] [CrossRef]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; Alencar, S.M.d. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Neuroprotective effects of methanolic extract of avocado persea americana (var. Colinred) peel on paraquat-induced locomotor impairment, lipid peroxidation and shortage of life span in transgenic knockdown parkin drosophila melanogaster. Neurochem. Res. 2019, 44, 1986–1998. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A. Characterization of the antioxidant properties of phenolic extracts from some citrus peels. J. Food Sci. Technol. 2012, 49, 729–736. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by frap assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Mehmood, T.; Khan, M.R.; Shabbir, M.A.; Zia, M.A. Phytochemical profiling and hplc quantification of citrus peel from different varieties. Prog. Nutr. 2018, 20, 279–288. [Google Scholar]
- Yang, P.; Xu, F.; Li, H.F.; Wang, Y.; Li, F.C.; Shang, M.Y.; Liu, G.X.; Wang, X.; Cai, S.Q. Detection of 191 taxifolin metabolites and their distribution in rats using hplc-esi-it-tof-msn. Molecules 2016, 21, 1209. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Lozano-Castellon, J.; Mardones, C.; Perez, A.J.; Saez, V.; Riquelme, S.; von Baer, D.; Vallverdu-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by lc-esi-ltq-orbitrap-ms. Molecules 2019, 24, 3763. [Google Scholar]
- Wang, X.Y.; Yan, K.J.; Ma, X.H.; Li, W.; Chu, Y.; Guo, J.H.; Li, S.M.; Zhou, S.P.; Zhu, Y.H.; Liu, C.X. Simultaneous determination and pharmacokinetic study of protocatechuic aldehyde and its major active metabolite protocatechuic acid in rat plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. Sci. 2016, 54, 697–705. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.K.; Moon, J.Y.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. J Food Sci 2011, 76, C38–C45. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, A.; Sun, H.; Zhang, Y. Chapter 23—systematic characterization of the absorbed components of acanthopanax senticosus stem. In Serum Pharmacochemistry of Traditional Chinese Medicine; Wang, X., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 313–336. [Google Scholar]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of chemical constituents of melastoma dodecandrum lour. By uplc-esi-q-exactive focus-ms/ms. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- Sasot, G.; Martinez-Huelamo, M.; Vallverdu-Queralt, A.; Mercader-Marti, M.; Estruch, R.; Lamuela-Raventos, R.M. Identification of phenolic metabolites in human urine after the intake of a functional food made from grape extract by a high resolution ltq-orbitrap-ms approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.B.; Luz, L.R.; Guedes, J.A.C.; Porto, D.D.; Silva, M.F.S.; Silva, G.S.; Riceli, P.R.V.; Canuto, K.M.; Brito, E.S.; Zampieri, D.S.; et al. Metabolomics-based discovery of biomarkers with cytotoxic potential in extracts of myracrodruon urundeuva. J. Braz. Chem. Soc. 2020, 31, 775–787. [Google Scholar] [CrossRef]
- Wang, Y.F.; Vorsa, N.; Harrington, P.D.; Chen, P. Nontargeted metabolomic study on variation of phenolics in different cranberry cultivars using uplc-im—Hrms. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Zeng, Y.; Lu, Y.; Chen, Z.; Tan, J.; Bai, J.; Li, P.; Wang, Z.; Du, S. Rapid characterization of components in bolbostemma paniculatum by uplc/ltq-orbitrap msn analysis and multivariate statistical analysis for herb discrimination. Molecules 2018, 23, 1155. [Google Scholar] [CrossRef]
- Rychlik, M. Quantification of free coumarin and its liberation from glucosylated precursors by stable isotope dilution assays based on liquid chromatography-tandem mass spectrometric detection. J. Agric. Food Chem. 2008, 56, 796–801. [Google Scholar] [CrossRef]
- Geng, C.A.; Chen, H.; Chen, X.L.; Zhang, X.M.; Lei, L.G.; Chen, J.J. Rapid characterization of chemical constituents in saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014, 361, 9–22. [Google Scholar] [CrossRef]
- Yang, S.; Shan, L.; Luo, H.; Sheng, X.; Du, J.; Li, Y. Rapid classification and identification of chemical components of schisandra chinensis by uplc-q-tof/ms combined with data post-processing. Molecules 2017, 22, 1778. [Google Scholar] [CrossRef]
- Stella, L.; De Rosso, M.; Panighel, A.; Vedova, A.D.; Flamini, R.; Traldi, P. Collisionally induced fragmentation of m-h (-) species of resveratrol and piceatannol investigated by deuterium labelling and accurate mass measurements. Rapid Commun. Mass Spectrom. 2008, 22, 3867–3872. [Google Scholar] [CrossRef]
- Reed, K.A. Identification of Phenolic Compounds from Peanut Skin Using hplc-msn; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2009. [Google Scholar]
- Pereira-Netto, A.B. Tropical fruits as natural, exceptionally rich, sources of bioactive compounds. Int. J. Fruit Sci. 2018, 18, 231–242. [Google Scholar] [CrossRef]
- He, S.; Wu, B.; Pan, Y.; Jiang, L. Stilbene oligomers from parthenocissus laetevirens: Isolation, biomimetic synthesis, absolute configuration, and implication of antioxidative defense system in the plant. J. Org. Chem. 2008, 73, 5233–5241. [Google Scholar] [CrossRef]
- Cammell, M.E.; Knight, J.D. Effects of climatic change on the population dynamics of crop pests. In Advances in Ecological Research; Begon, M., Fitter, A.H., Macfadyen, A., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 22, pp. 117–162. [Google Scholar]
- Gang, D.R.; Dinkova-Kostova, A.T.; Davin, L.B.; Lewis, N.G. Phylogenetic links in plant defense systems: Lignans, isoflavonoids, and their reductases. In Phytochemicals for Pest Control; Hedin, P.A., Hollingworth, R.M., Eds.; American Chemical Society: Washington, DC, USA, 1997; Volume 658, pp. 58–89. [Google Scholar]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (mangifera indica, cv. Ataulfo) fruit by hplc–dad–ms/ms-esi and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (mangifera indica l.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 1055, 1327–1334. [Google Scholar] [CrossRef]
- Hu, K.; Dars, A.G.; Liu, Q.; Xie, B.; Sun, Z. Phytochemical profiling of the ripening of chinese mango (mangifera indica l.) cultivars by real-time monitoring using uplc-esi-qtof-ms and its potential benefits as prebiotic ingredients. Food Chem. 2018, 256, 171–180. [Google Scholar] [CrossRef]
- Gorinstein, S.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Vearasilp, S.; Haruenkit, R.; Ruamsuke, P.; Katrich, E.; Tashma, Z. Antioxidant properties and bioactive constituents of some rare exotic thai fruits and comparison with conventional fruits: In vitro and in vivo studies. Food Res. Int. 2011, 44, 2222–2232. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of banana (musa aaa) peel as a source of antioxidant phenolic compounds. Food Res. Int. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Pal, J.; Prakash, A.; Pandey, G.; Raju, C. Comparatives study of antioxidant activity and total phenolic contents of pomegranate and orange peels extracts. J. Pharmacogn. Phytochem. 2017, 6, 1359–1362. [Google Scholar]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin c and antioxidant activity in wasted parts of sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Labat, A.; Scobbie, L.; Duncan, G.J.; Duthie, G.G. Phenolic acid content of fruits commonly consumed and locally produced in scotland. Food Chem. 2009, 115, 100–104. [Google Scholar] [CrossRef]
- Mihailović, N.; Mihailovic, V.; Kreft, S.; Ciric, A.; Joksović, L.; Djurdjevic, P. Analysis of phenolics in the peel and pulp of wild apples ( malus sylvestris (l.) mill.). J. Food Compos. Anal. 2017, 67. [Google Scholar]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (malus domestica borkh) cultivars of organic and integrated production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Gómez-Caravaca, A.M. Use of hplc-and gc-qtof to determine hydrophilic and lipophilic phenols in mango fruit (mangifera indica l.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar]
- Zain, N.M.; Nazeri, M.A.; Azman, N.A. Assessment on bioactive compounds and the effect of microwave on pitaya peel. J. Teknol. 2019, 81, 11–19. [Google Scholar]
- Silva, L.M.R.d.; Figueiredo, E.A.T.d.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Figueiredo, R.W.d.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef]
- Singh, S.; Immanuel, G. Extraction of antioxidants from fruit peels and its utilization in paneer. J. Food Process. Technol. 2014, 5, 1. [Google Scholar]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of abts/dpph assays to measure antioxidant capacity in popular antioxidant-rich us foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]

| Sample | TPC (mg GAE/g) | TFC (mg QE/g) | TTC (mg CE/g) | DPPH (mg AAE/g) | ABTS (mg AAE/g) | FRAP (mg AAE/g) | TAC (mg AAE/g) |
|---|---|---|---|---|---|---|---|
| Apple peel | 10.82 ± 0.51 e | 1.22 ± 0.10 b–e | 2.25 ± 0.12 e | 5.20 ± 0.25 b | 4.96 ± 0.17 d | 3.20 ± 0.04 d | 2.97 ± 0.16 d |
| Apricot peel | 5.60 ± 0.27 f,g | 1.22 ± 0.09 b–e | 1.07 ± 0.05 f | 3.73 ± 0.55 c | 3.21 ± 0.04 e | 2.27 ± 0.11 e | 2.28 ± 0.04 e,f |
| Avocado peel | 18.79 ± 1.46 c | 1.24 ± 0.11 b–d | 9.01 ± 0.20 a | 8.67 ± 0.44 a | 7.19 ± 0.72 c | 3.65 ± 0.07 c | 4.50 ± 0.16 c |
| Banana peel | 6.13 ± 0.25 f,g | 1.32 ± 0.12 b,c | 1.22 ± 0.08 f | 1.20 ± 0.12 e | 1.31 ± 0.03 g,h | 0.81 ± 0.03 i,j | 2.36 ± 0.22 e,f |
| Custard apple peel | 15.72 ± 0.74 d | 1.21 ± 0.08 b–e | 8.32 ± 0.56 a–c | 2.52 ± 0.52 d | 4.00 ± 0.44 e | 1.51 ± 0.02 f | 2.58 ± 0.04 d,e |
| Dragon fruit peel | 0.45 ± 0.12 k | 0.03 ± 0.01 h | 0.03 ± 0.01 h | 1.03 ± 0.16 e | 0.56 ± 0.08 h | 0.06 ± 0.01 l | 0.19 ± 0.02 i |
| Grapefruit peel | 27.22 ± 1.00 a | 0.82 ± 0.14 f | 7.60 ± 0.35 c | 9.17 ± 0.19 a | 10.79 ± 0.56 a | 9.22 ± 0.25 a | 8.77 ± 0.34 a |
| kiwi fruit peel | 5.30 ± 0.40 g,h | 0.45 ± 0.06 g | 3.51 ± 0.33 d | 5.03 ± 0.39 b | 8.95 ± 0.18 b | 1.13 ± 0.10 g–i | 0.79 ± 0.05 h |
| Lime peel | 23.32 ± 2.07 b | 1.14 ± 0.17 c–e | 8.42 ± 0.63 a,b | 2.73 ± 0.34 d | 1.46 ± 0.14 g | 0.92 ± 0.07 h–j | 2.27 ± 0.08 e,f |
| Mango peel | 27.51 ± 0.63 a | 1.75 ± 0.08 a | 8.99 ± 0.13 a | 8.67 ± 0.49 a | 9.32 ± 0.24 b | 6.19 ± 0.26 b | 6.19 ± 0.23 b |
| Melon peel | 2.39 ± 0.02 i–k | 0.03 ± 0.01 h | 0.02 ± 0.01 h | 0.48 ± 0.28 e | 1.16 ± 0.20 g,h | 0.08 ± 0.01 l | 0.93 ± 0.23 g–h |
| Nectarine peel | 1.53 ± 0.04 j,k | 0.09 ± 0.01 h | 0.23 ± 0.18 h | 1.29 ± 0.09 e | 1.25 ± 0.13 g,h | 0.91 ± 0.07 h–j | 0.97 ± 0.05 g,h |
| Orange peel | 21.31 ± 1.37 b | 1.08 ± 0.06 c–f | 8.12 ± 0.26 b,c | 4.79 ± 0.31 b | 3.36 ± 0.16 e | 2.44 ± 0.12 e | 2.55 ± 0.08 d,e |
| Papaya peel | 3.13 ± 0.15 h–j | 1.06 ± 0.07 c–f | 1.09 ± 0.04 f | 1.13 ± 0.11 e | 3.30 ± 0.17 e | 0.91 ± 0.07 i,j | 1.12 ± 0.13 g,h |
| Passion fruit peel | 1.55 ± 0.21 j,k | 0.04 ± 0.01 h | 0.19 ± 0.02 h | 0.72 ± 0.13 e | 1.04 ± 0.07 g,h | 0.42 ± 0.04 k | 1.32 ± 0.05 g |
| Peach peel | 5.84 ± 0.33 f,g | 1.02 ± 0.08 d–f | 0.16 ± 0.05 h | 1.33 ± 0.11 e | 1.03 ± 0.06 g,h | 0.89 ± 0.07 i,j | 1.13 ± 0.07 g,h |
| Pear peel | 4.30 ± 0.29 g–i | 1.07 ± 0.12 c–f | 0.10 ± 0.03 h | 0.84 ± 0.12 e | 1.21 ± 0.06 g,h | 0.65 ± 0.08 j,k | 1.18 ± 0.03 g,h |
| Pineapple peel | 7.83 ± 0.35 f | 1.47 ± 0.07 b | 1.23 ± 0.05 f | 1.30 ± 0.07 e | 2.36 ± 0.06 f | 1.30 ± 0.16 f,g | 2.00 ± 0.14 f |
| Plum peel | 4.81 ± 0.30 g,h | 0.96 ± 0.08 e,f | 0.29 ± 0.05 g,h | 1.01 ± 0.10 e | 1.19 ± 0.08 g,h | 0.71 ± 0.04 j,k | 0.87 ± 0.04 h |
| Pomegranate peel | 3.89 ± 0.21 g–i | 0.97 ± 0.10 e,f | 0.99 ± 0.02 f–g | 4.60 ± 0.08 b,c | 3.34 ± 0.09 e | 1.25 ± 0.13 f–h | 2.40 ± 0.18 e,f |
| Fruit Peels | Gallic Acid | Protocatechuic Acid | Caftaric Acid | Chlorogenic Acid | p-hydroxybenzoic Acid | Caffeic Acid | Syringic Acid | Coumaric Acid | Ferulic Acid | Sinapinic Acid | Sum of Phenolic Acids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APL-P | 4.2 ± 0.9 d | 7.4 ± 0.4 a | - | 11.2 ± 0.1 b | 6.5 ± 0.8 c | 2.1 ± 0.9 c | 1.1 ± 0.7 g | - | 1.2 ± 0.3 h | - | 33.7 ± 1.5 C |
| APR-P | 2.1 ± 0.6 g | - | 2.4 ± 0.4 e | 5.9 ± 0.2 e | 3.1 ± 0.3 f | 3.5 ± 0.1 b | - | - | 4.5 ± 0.1 c | 1.3 ± 0.1 e | 22.8 ± 1.7 D |
| AVO-P | 3.2 ± 0.5 e | 4.2 ± 0.2 b | 1.2 ± 0.1 g | 9.5 ± 0.1 c | 4.5 ± 0.6 d | - | 3.5 ± 0.3 d | 4.1 ± 0.1 b | - | 2.7 ± 0.6 c | 32.9 ± 2.5 C |
| BNA-P | 1.2 ± 0.2 i | - | - | 4.5 ± 0.5 f | 2.8 ± 0.1 g | - | 4.1 ± 0.5 c | - | 3.7 ± 0.2 d | - | 16.3 ± 1.9 G |
| CTA-P | 1.4 ± 0.2 h | - | 4.7 ± 0.9 c | 7.5 ± 0.3 d | 4.5 ± 0.9 d | - | - | 1.8 ± 0.4 f | 1.8 ± 0.3 g | 3.9 ± 0.8 b | 25.6 ± 1.6 D |
| DGF-P | 1.1 ± 0.5 i | - | 3.5 ± 0.5 d | 4.1 ± 0.9 g | 1.2 ± 0.7 i | - | 3.1 ± 0.9 d | 2.8 ± 0.1 d | 2.7 ± 0.8 e | - | 18.5 ± 2.1 F |
| GRF-P | 5.4 ± 0.9 c | 3.4 ± 0.4 c | 7.8 ± 0.5 a | - | 3.5 ± 0.9 e | 4.2 ± 0.5 a | - | 3.1 ± 0.8 c | 2.1 ± 0.4 f | 1.7 ± 0.7 d | 31.2 ± 1.9 C |
| KWF-P | 1.1 ± 0.9 i | - | 4.2 ± 0.4 c | 3.2 ± 0.5 i | 2.8 ± 0.1 g | - | 2.1 ± 0.1 e | 4.1 ± 0.8 b | 1.2 ± 0.7 h | - | 18.7 ± 1.7 F |
| LMN-P | - | 1.2 ± 0.8 e | 2.4 ± 0.5 e | - | 4.2 ± 0.4 d | 1.2 ± 0.6 e | - | 2.1 ± 0.4 e | 4.5 ± 0.9 c | 4.9 ± 0.7 a | 20.5 ± 2.1 E |
| MNG-P | 14.5 ± 0.4 a | - | 2.1 ± 0.1 f | 13.8 ± 0.9 a | 10.5 ± 0.4 a | 4.5 ± 0.4 a | 11.5 ± 0.7 a | 5.1 ± 0.2 a | 6.3 ± 0.4 a | 3.9 ± 0.9 b | 72.2 ± 4.5 A |
| MEL-P | - | 1.1 ± 0.7 e | - | 1.6 ± 0.3 j | - | - | 2.3 ± 0.1 e | - | 1.2 ± 0.2 h | - | 6.2 ± 1.2 M |
| NEC-P | 1.5 ± 0.7 h | 1.2 ± 0.2 e | - | 4.5 ± 0.4 f | 2.8 ± 0.1 g | 1.7 ± 0.9 d | - | - | 1.1 ± 0.1 h | - | 12.8 ± 1.9 I |
| ORN-P | 5.4 ± 0.9 c | - | 3.1 ± 0.4 d | 5.6 ± 0.3 e | 3.6 ± 0.1 e | - | 1.8 ± 0.2 f | - | 2.1 ± 0.7 f | 1.8 ± 0.2 d | 23.4 ± 2.3 D |
| PAP-P | 2.4 ± 0.7 f | - | 5.6 ± 0.1 b | - | 2.9 ± 0.2 g | - | 2.4 ± 0.9 e | - | 1.8 ± 0.4 g | - | 15.1 ± 1.1 H |
| PSN-P | - | - | 5.2 ± 0.8 b | - | 2.1 ± 0.4 h | - | 3.5 ± 0.3 d | - | 1.1 ± 0.1 h | - | 11.9 ± 1.9 J |
| PEC-P | 1.5 ± 0.4 h | 1.2 ± 0.1 e | - | 3.7 ± 0.9 h | - | 1.8 ± 0.2 d | - | - | 2.7 ± 0.1 e | 1.4 ± 0.9 e | 12.3 ± 1.3 I |
| PER-P | 1.1 ± 0.7 i | - | 2.1 ± 0.8 f | - | 3.2 ± 0.3 f | - | 1.5 ± 0.4 f | - | 1.2 ± 0.1 h | - | 9.1 ± 1.7 L |
| PIN-P | 1.5 ± 0.9 h | 2.1 ± 0.2 d | - | - | 1.2 ± 0.1 i | 1.1 ± 0.5 e | - | 2.8 ± 0.3 d | - | 1.9 ± 0.7 d | 10.6 ± 1.9 K |
| PLM-P | 1.4 ± 0.3 h | - | - | - | 3.8 ± 0.1 e | - | 1.7 ± 0.7 f | - | 4.2 ± 0.4 c | - | 11.1 ± 2.1 J |
| POM-P | 6.7 ± 0.1 b | 7.4 ± 0.6 a | - | 11.8 ± 0.7 b | 9.8 ± 0.1 b | 4.5 ± 0.7 a | 6.7 ± 0.9 b | - | 5.8 ± 0.2 b | - | 52.7 ± 3.9 B |
| Fruit Peels | Catechin | Epicatechin | Epicatechin Gallate | Quercetin-3-Galactoside | Quercetin-3-Glucuronide | Quercetin-3-Glucoside | Kaempferol-3-Glucoside | Diosmin | Quercetin | Kaempferol | Sum of Flavonoids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APL-P | 3.2 ± 0.8 b | - | 1.5 ± 0.1 d | 5.7 ± 0.6 b | - | 4.5 ± 0.9 a | - | 2.1 ± 0.4 b | 9.6 ± 0.9 b | - | 26.6 ± 1.9 C |
| APR-P | - | 2.1 ± 0.8 b | 2.3 ± 0.1 b | - | 3.8 ± 0.7 d | 3.5 ± 0.2 b | 3.2 ± 0.4 b | - | 6.9 ± 0.7 c | 4.5 ± 0.1 d | 26.3 ± 2.1 C |
| AVO-P | 2.1 ± 0.9 d | 1.8 ± 0.4 c | - | 4.9 ± 0.7 c | 1.7 ± 0.8 f | 2.8 ± 0.1 c | 1.9 ± 0.5 e | 1.7 ± 0.1 c | 3.9 ± 0.9 g | 2.9 ± 0.4 f | 23.7 ± 2.7 D |
| BNA-P | 1.5 ± 0.7 e | - | - | 3.8 ± 0.9 d | - | 1.7 ± 0.1 e | - | - | 4.8 ± 0.8 e | - | 11.8 ± 2.1 H |
| CTA-P | 2.1 ± 0.4 d | - | 1.9 ± 0.7 c | - | 1.4 ± 0.1 f | - | 1.7 ± 0.5 e | - | 3.2 ± 0.9 h | 3.9 ± 0.4 d | 14.2 ± 1.9 G |
| DGF-P | 7.5 ± 0.9 a | - | 1.5 ± 0.1 d | 4.5 ± 0.7 c | 1.7 ± 0.4 f | - | 2.4 ± 0.7 d | - | 4.9 ± 0.3 e | 3.5 ± 0.7 e | 26.0 ± 1.1 C |
| GRF-P | - | - | 2.1 ± 0.7 b | 2.9 ± 0.1 e | - | 1.7 ± 0.7 e | 1.9 ± 0.3 e | 1.1 ± 0.7 d | 5.9 ± 0.1 d | 4.2 ± 0.9 d | 19.8 ± 1.9 E |
| KWF-P | 1.5 ± 0.2 e | - | 1.1 ± 0.9 e | - | 4.5 ± 0.3 c | - | 1.2 ± 0.8 f | 1.7 ± 0.1 c | 5.4 ± 0.2 d | 7.1 ± 0.7 b | 22.5 ± 2.7 D |
| LMN-P | 2.7 ± 0.1 c | - | - | 4.8 ± 0.7 c | 8.7 ± 0.2 b | - | 3.7 ± 0.4 a | 1.9 ± 0.6 b | - | 1.2 ± 0.1 h | 23.0 ± 1.3 D |
| MNG-P | 7.1 ± 0.3 a | - | 3.2 ± 0.9 a | 10.9 ± 0.1 a | 11.5 ± 0.7 a | - | 2.7 ± 0.4 c | - | 11.9 ± 0.4 a | 9.8 ± 0.7 a | 57.1 ± 2.4 A |
| MEL-P | - | 1.9 ± 0.3 c | - | 2.8 ± 0.9 e | - | 1.7 ± 0.1 e | - | - | 4.5 ± 0.3 f | - | 10.9 ± 1.2 I |
| NEC-P | 2.1 ± 0.8 d | - | 1.7 ± 0.1 d | 1.8 ± 0.7 g | - | - | 2.9 ± 0.7 c | - | 2.9 ± 0.9 i | 3.1 ± 0.1 e | 14.5 ± 2.1 G |
| ORN-P | 3.5 ± 0.1 b | 2.4 ± 0.4 b | - | 3.8 ± 0.2 d | - | - | 3.9 ± 0.1 a | - | - | 1.9 ± 0.8 g | 15.5 ± 1.7 F |
| PAP-P | - | 1.7 ± 0.3 c | 1.9 ± 0.9 c | - | 4.7 ± 0.1 c | - | 1.7 ± 0.9 e | - | 4.9 ± 0.4 e | 2.9 ± 0.2 f | 17.8 ± 2.1 F |
| PSN-P | 1.8 ± 0.7 e | - | - | 1.7 ± 0.1 f | - | 2.1 ± 0.9 d | - | 1.7 ± 0.1 c | - | 3.1 ± 0.8 e | 10.4 ± 1.4 I |
| PEC-P | 1.2 ± 0.5 f | - | 1.9 ± 0.3 c | 2.1 ± 0.4 f | - | 1.8 ± 0.2 e | - | 1.1 ± 0.1 d | - | 4.1 ± 0.7 d | 12.2 ± 1.7 H |
| PER-P | 1.8 ± 0.7 e | 1.9 ± 0.9 c | - | - | 1.7 ± 0.4 f | - | 1.1 ± 0.1 f | - | - | 7.4 ± 0.7 b | 13.9 ± 2.1 G |
| PIN-P | - | - | 1.5 ± 0.1 d | - | - | 3.7 ± 0.1 b | - | 3.1 ± 0.6 a | - | 6.5 ± 0.7 c | 14.8 ± 1.9 G |
| PLM-P | 3.1 ± 0.1 b | - | 1.4 ± 0.9 d | 2.7 ± 0.3 e | - | 3.1 ± 0.5 c | - | 1.9 ± 0.7 b | - | 4.1 ± 0.2 d | 16.3 ± 2.1 F |
| POM-P | 2.1 ± 0.1 d | 4.1 ± 0.3 a | - | 5.7 ± 0.1 b | 2.1 ± 0.7 e | - | 3.6 ± 0.2 a | 1.1 ± 0.3 d | 9.4 ± 0.9 b | 7.6 ± 0.7 b | 35.7 ± 4.7 B |
| Variables | TPC | TFC | TTC | DPPH | ABTS | FRAP | TAC | Phenolic Acids |
|---|---|---|---|---|---|---|---|---|
| TFC | 0.488 * | |||||||
| TTC | 0.932 ** | 0.457 * | ||||||
| DPPH | 0.718 ** | 0.396 | 0.720 ** | |||||
| ABTS | 0.591 ** | 0.270 | 0.622 ** | 0.904 ** | ||||
| FRAP | 0.722 ** | 0.314 | 0.603 ** | 0.868 ** | 0.835 ** | |||
| TAC | 0.780 ** | 0.397 | 0.668 ** | 0.850 ** | 0.779 ** | 0.967 ** | ||
| Phenolic acids | 0.496 * | 0.343 | 0.515 * | 0.761 ** | 0.628 * | 0.614 ** | 0.640 ** | |
| Flavonoids | 0.349 | 0.232 | 0.355 | 0.633 * | 0.535 * | 0.473 * | 0.452 * | 0.911 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. https://doi.org/10.3390/foods9091206
Suleria HAR, Barrow CJ, Dunshea FR. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods. 2020; 9(9):1206. https://doi.org/10.3390/foods9091206
Chicago/Turabian StyleSuleria, Hafiz A. R., Colin J. Barrow, and Frank R. Dunshea. 2020. "Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels" Foods 9, no. 9: 1206. https://doi.org/10.3390/foods9091206
APA StyleSuleria, H. A. R., Barrow, C. J., & Dunshea, F. R. (2020). Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods, 9(9), 1206. https://doi.org/10.3390/foods9091206








