RETRACTED: Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Fermentation of Plant Bases
2.1.1. Post Acidification and Titratable Acidity during Storage
2.1.2. Cell Growth during Storage
2.2. Physicochemical Analysis of Fermented Bases
2.2.1. Rheological Measurements
2.2.2. Identification of Carbohydrates and Acids
2.3. Identification of Volatile Organic Compounds (VOCs)
2.3.1. Identification of Targeted VOCs
2.3.2. Identification of Untargeted VOCs
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Effect of LGG® in Acidification Time
3.2. Changes during Storage
3.2.1. Post-Acidification and Titratable Acidity
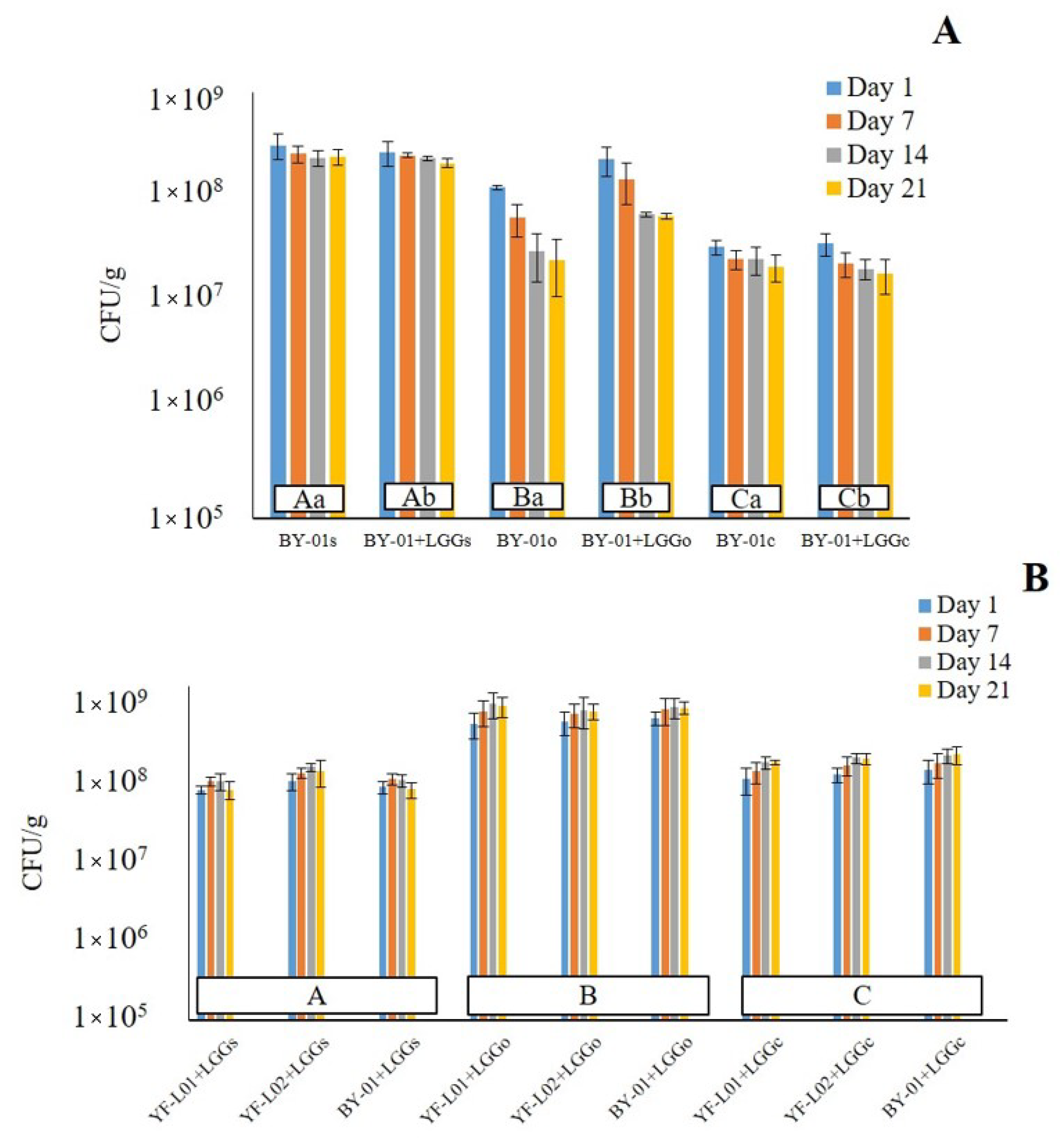
3.2.2. Viability of BB-12® and LGG® during Storage
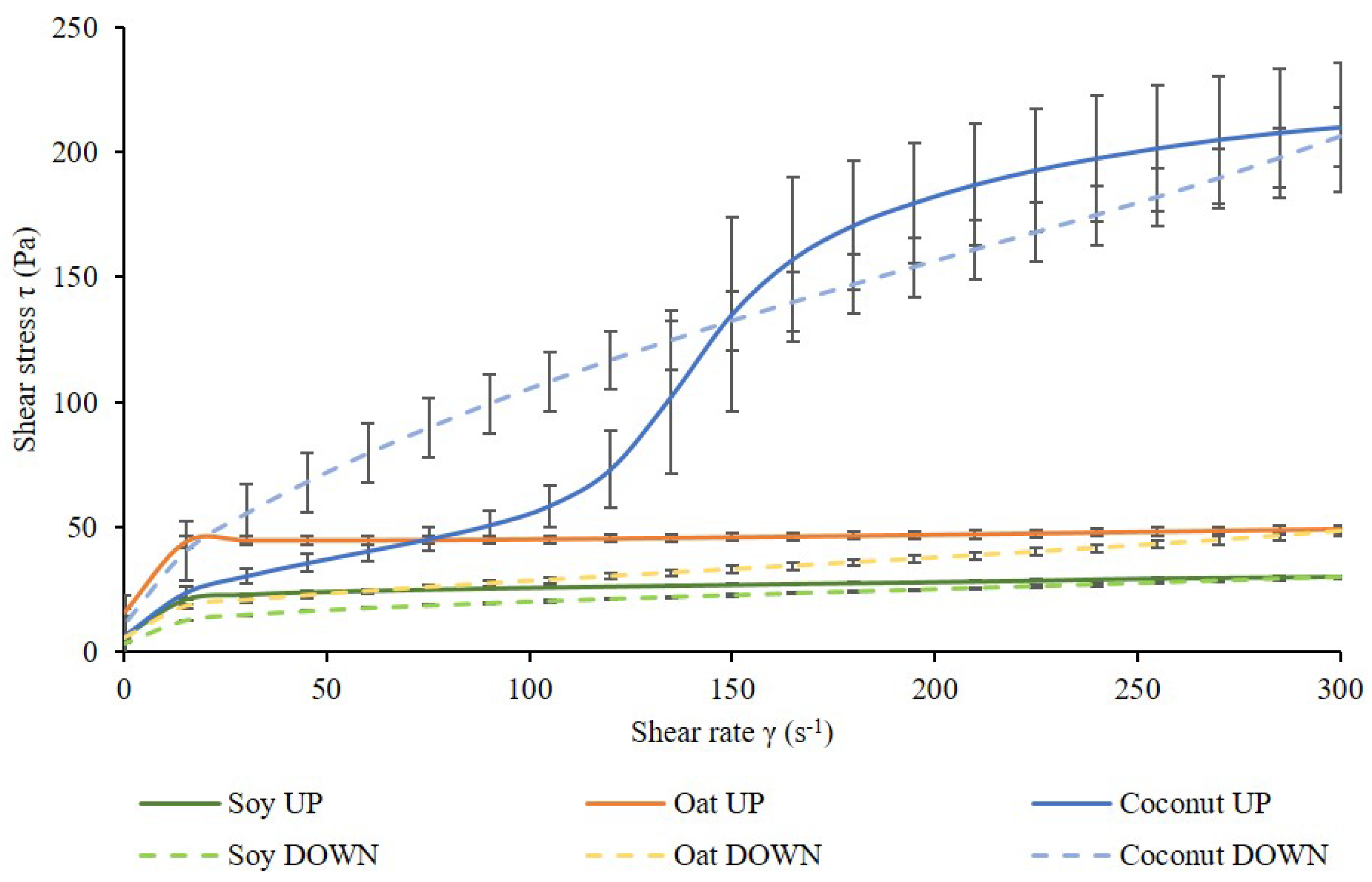
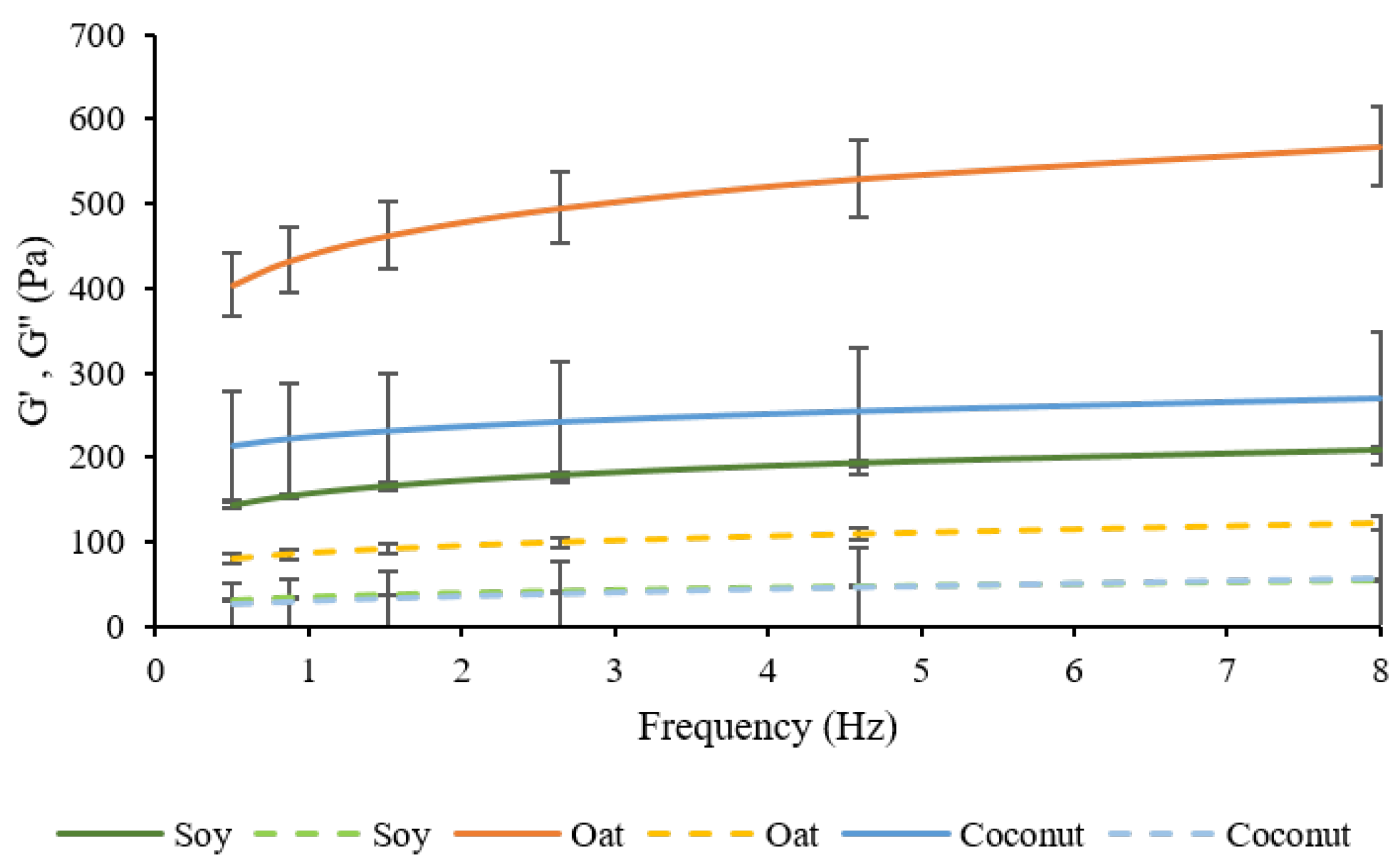
3.3. Rheological Behavior
3.4. Carbohydrates and Acids in Fermented Samples
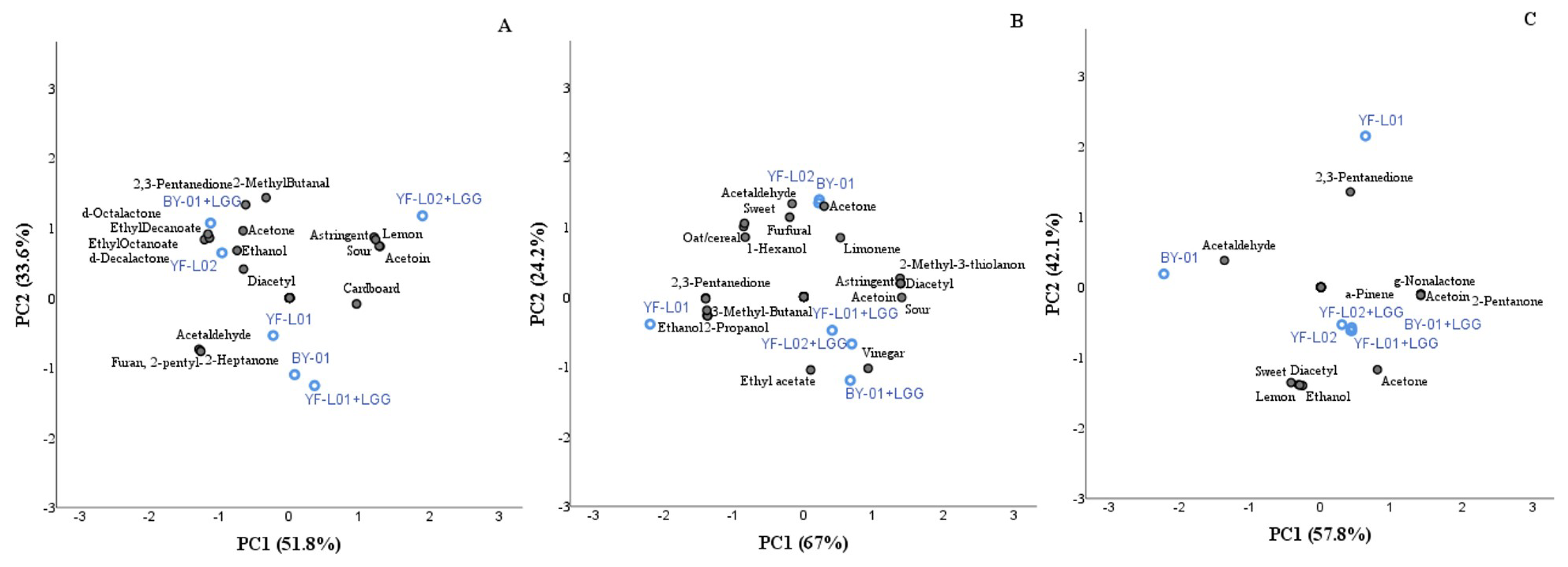
3.5. Volatile Organic Compounds
3.5.1. Major Contributors to Fermented Dairy Flavor
3.5.2. Characteristic VOCs of Soy, Oat, and Coconut
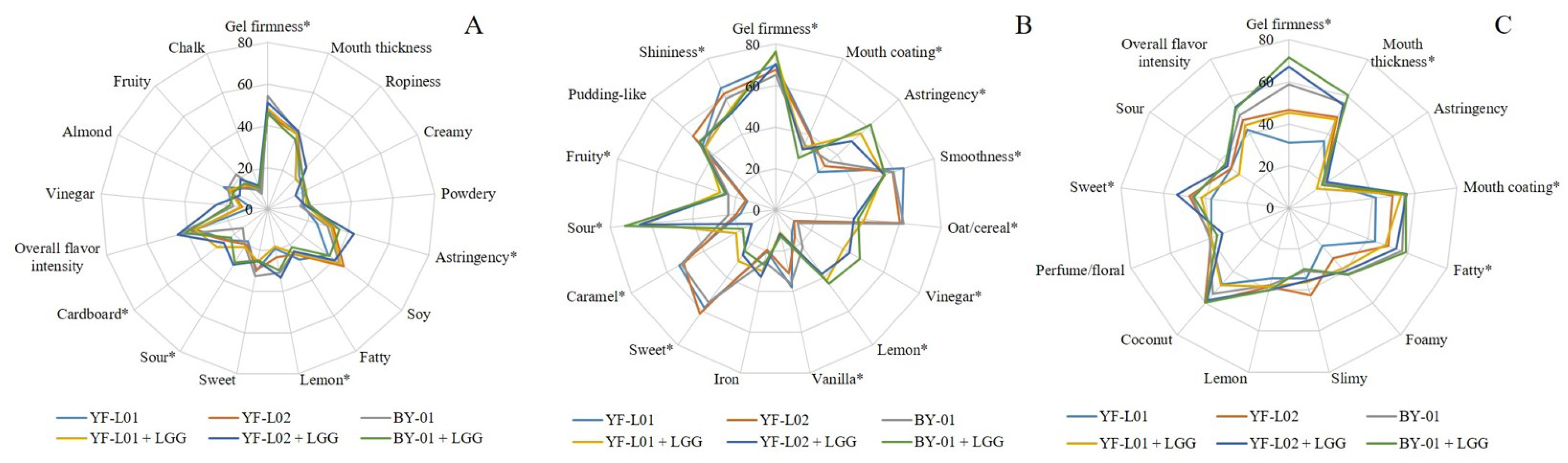
3.6. Sensory Perception of Fermented Soy, Oat, and Coconut Samples
3.7. Correlation of Instrumental Measurements with Flavor Perception
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Heenan, C.N.; Adams, M.C.; Hosken, R.W.; Fleet, G.H. Survival and sensory acceptability of probiotic microorganisms in a nonfermented frozen vegetarian dessert. LWT Food Sci. Technol. 2004, 37, 461–466. [Google Scholar] [CrossRef]
- Saint-Eve, A.; Granada, P.; Legay, G.; Cuvelier, G.; Delarue, J. Consumer acceptance and sensory drivers of liking for high plant protein snacks. J. Sci. Food Agric. 2019, 99, 3983–3991. [Google Scholar] [CrossRef]
- Marko, A.; Rakická, M.; Mikušová, L.; Valík, L.; Šturdík, E. Lactic acid Fermentation of Cereal Substrates in Nutritional Perspective. Int. J. Res. Chem. Environ. 2014, 4, 80–92. [Google Scholar]
- Peschel, A.O.; Kazemi, S.; Liebichová, M.; Sarraf, S.C.M.; Aschemann-Witzel, J. Consumers’ associative networks of plant-based food product communications. Food Qual. Prefer. 2019, 75, 145–156. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Probiotic fermented almond "milk" as an alternative to cow-milk yoghurt. Int. J. Food Stud. 2015, 4, 201–211. [Google Scholar] [CrossRef]
- Szparaga, A.; Tabor, S.; Kocira, S.; Czerwińska, E.; Kuboń, M.; Płóciennik, B.; Findura, P. Survivability of probiotic bacteria in model systems of non-fermented and fermented coconut and hemp milks. Sustainability 2019, 11, 6093. [Google Scholar] [CrossRef]
- Akin, Z.; Ozcan, T. Functional properties of fermented milk produced with plant proteins. LWT Food Sci. Technol. 2017, 86, 25–30. [Google Scholar] [CrossRef]
- Kocková, M.; Valík, L. Development of new cereal-, pseudocereal-, and cereal-leguminous-based probiotic foods. Czech J. Food Sci. 2014, 32, 391–397. [Google Scholar] [CrossRef]
- Mauro, C.S.I.; Garcia, S. Coconut milk beverage fermented by Lactobacillus reuteri: Optimization process and stability during refrigerated storage. J. Food Sci. Technol. 2019, 56, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Chalupa-krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Belewu, M.A.; Belewu, K.Y. Comparative Physico-Chemical Evaluation of Tiger-nut, Soybean and Coconut Milk Sources. Int. J. Agric. Biol. 2007, 9, 785–787. [Google Scholar]
- Mills, E.N.; Breiteneder, H. Food allergy and its relevance to industrial food proteins. Biotechnol. Adv. 2005, 23, 409–414. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Garro, M.S.; Savoy De Giori, G. Effect of pH on Lactobacillus fermentum growth, raffinose removal, α-galactosidase activity and fermentation products. Appl. Microbiol. Biotechnol. 2004, 65, 119–123. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Ledue-Clier, F.; Bensaada, M.; De Giori, G.S.; Guerekobaya, T.; Sesma, F.; Juillard, V.; Rabot, S.; Piard, J.C. Ability of Lactobacillus fermentum to overcome host α-galactosidase deficiency, as evidenced by reduction of hydrogen excretion in rats consuming soya α-galacto-oligosaccharides. BMC Microbiol. 2008, 8, 1–9. [Google Scholar] [CrossRef]
- Sumarna. Changes of raffinose and stachyose in soy milk fermentation by lactic acid bacteria from local fermented foods of Indonesian. Malays. J. Microbiol. 2008, 4, 26–34. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Cruz Cansino, N. Efecto de la ultra alta presión de homogeneización en licuado de soja y su comportamiento en el desarrollo de un producto fermentado. Rev. Colomb. Biotecnol. 2009, 3, 179. [Google Scholar]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Rheological properties and sensory characteristics of set-type soy yogurt. J. Agric. Food Chem. 2007, 55, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.R.; Lopes-da Silva, J.A. Effect of the molecular weight of a neutral polysaccharide on soy protein gelation. Food Res. Int. 2017, 102, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Nishinari, K. Rheological Studies on the Gelation Process of Soybean 7S and 11S Proteins in the Presence of Glucono-δ-lactone. J. Agric. Food Chem. 1993, 41, 8–14. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.L.; Sun, Y.; Li, A.L.; Liu, F.; Meng, X.C. Microencapsulation of Lactobacillus rhamnosus GG by Transglutaminase Cross-Linked Soy Protein Isolate to Improve Survival in Simulated Gastrointestinal Conditions and Yoghurt. J. Food Sci. 2016, 81, M1726–M1734. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, H.N. Effect of Synbiotic Interaction of Fructooligosaccharide and Probiotics on the Acidification Profile, Textural and Rheological Characteristics of Fermented Soy Milk. Food Bioprocess Technol. 2013, 6, 3166–3176. [Google Scholar] [CrossRef]
- Hefnawy, H.T.M.; Ramadan, M.F. Physicochemical characteristics of soy protein isolate and fenugreek gum dispersed systems. J. Food Sci. Technol. 2011, 48, 371–377. [Google Scholar] [CrossRef]
- Yang, M.; Li, L. Physicochemical, textural and sensory characteristics of probiotic soy yogurt prepared from germinated soybean. Food Technol. Biotechnol. 2010, 48, 490–496. [Google Scholar]
- O’Toole, D.K. Soybean: Soy-Based Fermented Foods, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3–4, pp. 124–133. [Google Scholar] [CrossRef]
- Marazza, J.A.; Garro, M.S.; Savoy de Giori, G. Aglycone production by Lactobacillus rhamnosus CRL981 during soymilk fermentation. Food Microbiol. 2009, 26, 333–339. [Google Scholar] [CrossRef]
- Biel, W.; Bobko, K.; Maciorowski, R. Chemical composition and nutritive value of husked and naked oats grain. J. Cereal Sci. 2009, 49, 413–418. [Google Scholar] [CrossRef]
- Dong, J.L.; Yu, X.; Dong, L.E.; Shen, R.L. In vitro fermentation of oat β-glucan and hydrolysates by fecal microbiota and selected probiotic strains. J. Sci. Food Agric. 2017, 97, 4198–4203. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.A.; González Martinez, C.; Rodriguez-García, J.; Chiralt, A. Optimisation of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Sci. Technol. Int. 2013, 21, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Anttila, H.; Sontag-Strohm, T.; Salovaara, H. Viscosity of beta-glucan in oat products. Agric. Food Sci. 2004, 13, 80–87. [Google Scholar] [CrossRef]
- Zeidan, A.A.; Poulsen, V.K.; Janzen, T.; Buldo, P.; Derkx, P.M.; Øregaard, G.; Neves, A.R. Polysaccharide production by lactic acid bacteria: From genes to industrial applications. FEMS Microbiol. Rev. 2017, 41, S168–S200. [Google Scholar] [CrossRef]
- Peterson, D.M. Storage Proteins, 2nd ed.; AACC International, Inc.: Eagan, Minnesota, 2011; pp. 123–142. [Google Scholar] [CrossRef]
- Pedó, I.; Sgarbieri, V.C.; Gutkoski, L.C. Protein evaluation of four oat (Avena sativa L.) cultivars adapted for cultivation in the south of Brazil. Plant Foods Hum. Nutr. 1999, 53, 297–304. [Google Scholar] [CrossRef]
- Ma, C.Y.; Harwalkar, V.R. Thermal Coagulation of Oat Globulin. Cereal Chem. 1987, 64, 212–218. [Google Scholar]
- Sayar, S.; White, P.J. Oat Starch: Physicochemical Properties and Function, 2nd ed.; AACC International, Inc.: Eagan, Minnesota, 2011; pp. 109–122. [Google Scholar] [CrossRef]
- Lehtinen, P.; Kaukovirta-Norja, A. Oat Lipids, Enzymes, and Quality, 2nd ed.; AACC International, Inc.: Eagan, Minnesota, 2011; pp. 143–155. [Google Scholar] [CrossRef]
- McGorrin, R.J. Key Aroma Compounds in Oats and Oat Cereals. J. Agric. Food Chem. 2019, 67, 13778–13789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Robards, K.; Glennie-Holmes, M.; Helliwell, S. Oat Lipids. J. Am. Oil Chem. Soc. 1999, 76, 159–169. [Google Scholar] [CrossRef]
- Heiniö, R.L. Influence of Processing on the Flavour Formation of Oat and Rye; VTT Publications: Helsinki, Finland, 2003; pp. 5–72. [Google Scholar]
- Parrish, C.R. Moo-ove Over, Cow’s Milk: The Rise of Plant-Based Dairy Alternatives The Rise of Plant-Based Milks. Pract. Gastroenterol. 2018, XLII, 20–27. [Google Scholar]
- Saranov, I.A.; Kuznetsov, I.A.; Kuznetsova, I.V.; Magomedov, G.O. Investigation of melting and crystallization processes of fat components of praline masses. Proc. Voronezh State Univ. Eng. Technol. 2018, 80, 323–327. [Google Scholar] [CrossRef]
- Devi, A.; Khatkar, B. Thermo-Physical Properties of Fats and Oils. Int. J. Eng. Technocal Res. 2017, 7, 45. [Google Scholar] [CrossRef]
- Lakshmi, T.; Mary Pramela, A. Coconut milk kefir: Nutrient composition and assessment of microbial quality. Int. J. Food Sci. Nutr. 2018, 3, 1–4. [Google Scholar]
- Johansen, E. Use of Natural Selection and Evolution to Develop New Starter Cultures for Fermented Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 411–428. [Google Scholar] [CrossRef]
- Wright, V.A.; Axelsson, L. Lactic Acid Bacteria: An Introduction. In Lactic Acid Bacteria: Microbiological and Functional Aspects, 4th ed.; Taylor & Francis: London, UK, 2012; pp. 1–16. [Google Scholar]
- Coolbear, T.; Weimer, B.; Wilkinson, M.G. Lactic Acid Bacteria: Lactic Acid Bacteria in Flavor Development. In Encyclopedia of Dairy Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 160–165. [Google Scholar] [CrossRef]
- Ikujenlola, A.V.; Adurotoye, E.A.; Adeniran, H.A. Chemical and Sensory Properties of Probioticated Drinks from Blends of African Yam Bean, Soybean and Coconut Milk Analogues. Acta Univ. Cibiniensis. Ser. E Food Technol. 2020, 23, 147–156. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, D.K.; An, H.M.; Kim, J.R.; Kim, M.J.; Cha, M.K.; Lee, S.W.; Kim, S.O.; Choi, K.S.; Lee, K.O.; et al. Producing functional soy-based yogurt incubated with bifidobacterium longum spm1205 isolated from healthy adult koreans. Biotechnol. Biotechnol. Equip. 2012, 26, 2759–2764. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Hristozova, T.; Gargova, S. Application of pure and mixed probiotic lactic acid bacteria and yeast cultures for oat fermentation. J. Sci. Food Agric. 2005, 2141, 2134–2141. [Google Scholar] [CrossRef]
- Petruláková, M.; Valík, L. Evaluation of legumes as a substrate for probiotic strain Lactobacillus rhamnosus GG. Acta Aliment. 2015, 44, 268–275. [Google Scholar] [CrossRef]
- Kocková, M.; Valík, L. Suitability of cereal porridges as substrate for probiotic strain Lactobacillus rhamnosus GG. Potravinarstvo 2013, 7, 22–27. [Google Scholar] [CrossRef]
- Laneuville, S.I.; Turgeon, S.L. Microstructure and stability of skim milk acid gels containing an anionic bacterial exopolysaccharide and commercial polysaccharides. Int. Dairy J. 2014, 37, 5–15. [Google Scholar] [CrossRef]
- De Vuyst, L. Technology Aspects Related to the Application of Functional Starter Cultures. Food Technol. Biotechnol. 2000, 38, 105–112. [Google Scholar]
- Mårtensson, O.; Öste, R.; Holst, O. Texture promoting capacity and EPS formation by lactic acid bacteria in three different oat-based non-dairy media. Eur. Food Res. Technol. 2002, 214, 232–236. [Google Scholar] [CrossRef]
- Mårtensson, O.; Öste, R.; Holst, O. Lactic Acid Bacteria in an Oat-based Non-dairy Milk Substitute: Fermentation Characteristics and Exopolysaccharide Formation. LWT Food Sci. Technol. 2000, 33, 525–530. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Wasko, A.; Kubik-Komar, A. Optimization of culture conditions for exopolysaccharide production by a probiotic strain of Lactobacillus rhamnosus E/N. Pol. J. Microbiol. 2014, 63, 253–257. [Google Scholar] [CrossRef]
- Tsangalis, D.; Shah, N.P. Metabolism of oligosaccharides and aldehydes and production of organic acids in soymilk by probiotic bifidobacteria. Int. J. Food Sci. Technol. 2004, 39, 541–554. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Baba, A.S.; Misran, M. Effects of fermentation by Bifidobacterium bifidum on the rheology and physical properties of ice cream mixes made with cow and vegetable milks. Int. J. Food Sci. Technol. 2015, 50, 942–949. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.d.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013. [Google Scholar] [CrossRef]
- Ross, A.I.; Tyler, P.; Borgognone, M.G.; Eriksen, B.M. Relationships between shear rheology and sensory attributes of hydrocolloid-thickened fluids designed to compensate for impairments in oral manipulation and swallowing. J. Food Eng. 2019, 263, 123–131. [Google Scholar] [CrossRef]
- Bernat Pérez, N. Desarrollo, Caracterización y Optimización de Productos Fermentados a Base de Licuados Vegetales Como Alternativa a los Yogures Convencionales. Ph.D. Thesis, Universidad Politécnica de Valencia, Valencia, Spain, 2013; p. 331. [Google Scholar]
- Helland, M.H.; Wicklund, T.; Narvhus, J.A. Growth and metabolism of selected strains of probiotic bacteria, in maize porridge with added malted barley. Int. J. Food Microbiol. 2004, 91, 305–313. [Google Scholar] [CrossRef]
- Mishra, B.K.; Hati, S.; Das, S.; Prajapati, J.B. Biofunctional attributes and storage study of soy milk fermented by Lactobacillus rhamnosus and Lactobacillus helveticus. Food Technol. Biotechnol. 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Kpodo, M.K.F.; Afoakwa, E.; Saalia, K.; Amoa, B. Changes in physico-chemical characteristics and volatile flavour components of different yoghurt products made from soy, peanuts and cow milk. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 11278–11294. [Google Scholar] [CrossRef]
- Pavunc, A.L.; Penava, L.; Ranilovic, J.; Novak, J.; Banic, M.; Butorac, K.; Petrovic, E.; Mihaljevic-Herman, V.; Bendelja, K.; Mlakar, A.S.; et al. Influence of dehydrated wheat/rice cereal matrices on probiotic activity of Bifidobacterium animalis ssp. lactis BB-12®. Food Technol. Biotechnol. 2019, 57, 147–158. [Google Scholar] [CrossRef]
- Watson, D.; O’Connell Motherway, M.; Schoterman, M.H.; van Neerven, R.J.; Nauta, A.; Van Sinderen, D. Selective carbohydrate utilization by lactobacilli and bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef]
- Gopal, P.K.; Sullivan, P.A.; Smart, J.B. Utilisation of galacto-oligosaccharides as selective substrates for growth by lactic acid bacteria including Bifidobacterium lactis DR10 and Lactobacillus rhamnosus DR20. Int. Dairy J. 2001, 11, 19–25. [Google Scholar] [CrossRef]
- Içier, F.; Gündüz, G.T.; Yilmaz, B.; Memeli, Z. Changes on some quality characteristics of fermented soy milk beverage with added apple juice. LWT Food Sci. Technol. 2015, 63, 57–64. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Pang, Z.; Luo, Y.; Li, B.; Zhang, M.; Liu, X. Effect of different hydrocolloids on tribological and rheological behaviors of soymilk gels. Food Hydrocoll. 2020, 101, 105558. [Google Scholar] [CrossRef]
- Chetachukwu, A.S.; Thongraung, C.; Yupanqui, C.T. Development of reduced-fat coconut yoghurt: Physicochemical, rheological, microstructural and sensory properties. Int. J. Dairy Technol. 2019, 72, 524–535. [Google Scholar] [CrossRef]
- Rossa, P.N.; Burin, V.M.; Bordignon-Luiz, M.T. Effect of microbial transglutaminase on functional and rheological properties of ice cream with different fat contents. LWT Food Sci. Technol. 2012, 48, 224–230. [Google Scholar] [CrossRef]
- Hui, Y.H. Handbook of Food Science, Technology, and Engineering; CRC Press: Boca Raton, FL, USA, 2006; Volume 3. [Google Scholar]
- Girard, M.; Schaffer-Lequart, C. Gelation of skim milk containing anionic exopolysaccharides and recovery of texture after shearing. Food Hydrocoll. 2007, 21, 1031–1040. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Vegetable milks and their fermented derivative products. Int. J. Food Stud. 2014, 3, 93–124. [Google Scholar] [CrossRef]
- Sutherland, I.W. Bacterial Exopolysaccharides. Compr. Glycosci. Chem. Syst. Biol. 2007, 2–4, 521–558. [Google Scholar] [CrossRef]
- Stijepic, M.; Glusac, J.; Durdevic-Milosevic, D.; Pesic-Mikulec, D. Physicochemical characteristics of soy probiotic yoghurt with inulin additon during the refrigerated storage. Rom. Biotechnol. Lett. 2013, 18, 8077–8085. [Google Scholar]
- Salazar, N.; Prieto, A.; Leal, J.A.; Mayo, B.; Bada-Gancedo, J.C.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Production of exopolysaccharides by Lactobacillus and Bifidobacterium strains of human origin, and metabolic activity of the producing bacteria in milk. J. Dairy Sci. 2009, 92, 4158–4168. [Google Scholar] [CrossRef]
- Grygorczyk, A. A Novel Approach to Structure Generation for Texture Improvement in a Soymilk-Dairy Gel. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2012. [Google Scholar]
- Aboulfazli, F.; Baba, A.S.; Misran, M. The Rheology and Physical Properties of Fermented Probiotic Ice Creams Made with Dairy Alternatives. Int. J. Food Eng. 2015, 11, 493–504. [Google Scholar] [CrossRef]
- Brückner-gühmann, M.; Banovic, M.; Drusch, S. Food Hydrocolloids Towards an increased plant protein intake: Rheological properties, sensory perception and consumer acceptability of lactic acid fermented, oat-based gels. Food Hydrocoll. 2019, 96, 201–208. [Google Scholar] [CrossRef]
- Pujala, R.K. Dispersion Stability, Microstructure and Phase Transition of Anisotropic Nanodiscs; Springer: Berlin, Germany, 2014. [Google Scholar]
- Buldo, P.; Benfeldt, C.; Folkenberg, D.M.; Jensen, H.B.; Amigo, J.M.; Sieuwerts, S.; Thygesen, K.; van den Berg, F.; Ipsen, R. The role of exopolysaccharide-producing cultures and whey protein ingredients in yoghurt. LWT Food Sci. Technol. 2016, 72, 189–198. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yu, R.C.; Yang, H.Y.; Chou, C.C. Sugar and acid contents in soymilk fermented with lactic acid bacteria alone or simultaneously with bifidobacteria. Food Microbiol. 2003, 20, 333–338. [Google Scholar] [CrossRef]
- Meurman, J.; Antila, H.; Korhonen, A.; Salminen, S. Effect of Lactobacillus rhamnosus strain GG (ATCC 53103) on the growth of Streptococcus sobrinus in vitro. Eur. J. Oral Sci. 1995, 103. [Google Scholar] [CrossRef]
- Hedberg, M.; Hasslöf, P.; Sjöström, I.; Twetman, S.; Stecksén-Blicks, C. Sugar fermentation in probiotic bacteria—An in vitro study. Oral Microbiol. Immunol. 2008, 23, 482–485. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Beshkova, D.M.; Simova, E.D.; Frengova, G.I.; Simov, Z.I.; Dimitrov, Z.P. Production of volatile aroma compounds by kefir starter cultures. Int. Dairy J. 2003, 13, 529–535. [Google Scholar] [CrossRef]
- Morales, P.; Feliu, I.; Fernández-García, E.; Nuñez, M. Volatile compounds produced in cheese by Enterobacteriaceae strains of dairy origin. J. Food Prot. 2004, 67, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, A.; Ortigosa, M.; García, S.; Ibáñez, F.C.; Torre, P. Comparison of free amino acids and volatile components in three fermented milks. Int. J. Dairy Technol. 2012, 65, 578–584. [Google Scholar] [CrossRef]
- Kaneko, D.; Igarashi, T.; Aoyama, K. Reduction of the off-flavor volatile generated by the yogurt starter culture including streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus in soymilk. J. Agric. Food Chem. 2014, 62, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Granata, L.A.; Morr, C.V. Improved acid, flavor and volatile compound production in a high protein and fiber soymilk yogurt-like product. J. Food Sci. 1996, 61, 331–336. [Google Scholar] [CrossRef]
- Yan Chun, L.; Song, H.L.; Li, X.; Wu, L.; Guo, S.T. Influence of Blanching and Grinding Process with Hot Water on Beany and Non-Beany Flavor in Soymilk. J. Food Sci. 2011, 76, 20–25. [Google Scholar] [CrossRef]
- Valero, E.; Villamiel, M.; Miralles, B.; Sanz, J.; Martínez-Castro, I. Changes in flavour and volatile components during storage of whole and skimmed UHT milk. Food Chem. 2001, 72, 51–58. [Google Scholar] [CrossRef]
- Salmenkallio-Marttila, M.; Heiniö, R.L.; Kaukovirta-Norja, A.; Poutanen, K. Flavor and Texture in Processing of New Oat Foods, 2nd ed.; AACC International, Inc.: Eagan, Minnesota, 2011; pp. 333–346. [Google Scholar] [CrossRef]
- Lee, S.M.; Oh, J.; Hurh, B.S.; Jeong, G.H.; Shin, Y.K.; Kim, Y.S. Volatile Compounds Produced by Lactobacillus paracasei During Oat Fermentation. J. Food Sci. 2016, 81, C2915–C2922. [Google Scholar] [CrossRef]
- Natrella, G.; Faccia, M.; Lorenzo, J.M.; De Palo, P.; Gambacorta, G. Short communication: Sensory characteristics and volatile organic compound profile of high-moisture mozzarella made by traditional and direct acidification technology. J. Dairy Sci. 2020, 103, 2089–2097. [Google Scholar] [CrossRef]
- Dan, T.; Wang, D.; Wu, S.; Jin, R.; Ren, W.; Sun, T. Profiles of Volatile Flavor Compounds in Milk Fermented with Different Proportional Combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules 2017, 22, 1633. [Google Scholar] [CrossRef]
- Achouri, A.; Boye, J.I.; Zamani, Y. Changes in soymilk quality as a function of composition and storage. J. Food Qual. 2007, 30, 731–744. [Google Scholar] [CrossRef]
- Kaczmarska, K.T.; Chandra-Hioe, M.V.; Frank, D.; Arcot, J. Aroma characteristics of lupin and soybean after germination and effect of fermentation on lupin aroma. LWT Food Sci. Technol. 2018, 87, 225–233. [Google Scholar] [CrossRef]
- Borse, B.B.; Rao, L.J.M.; Ramalakshmi, K.; Raghavan, B. Chemical composition of volatiles from coconut sap (neera) and effect of processing. Food Chem. 2007, 101, 877–880. [Google Scholar] [CrossRef]
- Santos, J.E.R.; Villarino, B.J.; Zosa, A.R.; Dayrit, F.M. Analysis of volatile organic compounds in virgin coconut oil and their sensory attibutes. Philipp. J. Sci. 2011, 140, 161–171. [Google Scholar]
- Sides, A.; Robards, K.; Helliwell, S.; An, M. Changes in the volatile profile of oats induced by processing. J. Agric. Food Chem. 2001, 49, 2125–2130. [Google Scholar] [CrossRef]
- Hassan, F.A.; Abd El-Gawad, M.A.; Enab, A. Flavour compounds in cheese (Review). Int. J. Acad. Res. 2012, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Li, L. A new style of fermented tofu by Lactobacillus casei combined with salt coagulant. 3 Biotech 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Ahmad, N.; Li, L.; Yang, X.Q.; Ning, Z.X.; Randhawa, M.A. Improvements in the flavour of soy cheese. Food Technol. Biotechnol. 2008, 46, 252–261. [Google Scholar]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- van der Schaft, P.H.; ter Burg, N.; van den Bosch, S.; Cohen, A.M. Fed-batch production of 2-heptanone by Fusarium poae. Appl. Microbiol. Biotechnol. 1992, 36, 709–711. [Google Scholar] [CrossRef]
- Cognat, C.; Shepherd, T.; Verrall, S.R.; Stewart, D. Comparison of two headspace sampling techniques for the analysis of off-flavour volatiles from oat based products. Food Chem. 2012, 134, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, J.; Wang, F.; Song, H.; Huang, W.; Rayas-Duarte, P. Flavor Character of Bread with Oat Sourdough Fermented by Lactobacillus plantarum–Journal of Beijing Technology and Business University (Natural Science Edition). J. Beijing Technol. Bus. Univ. 2011, 29, 12–18. [Google Scholar]
- Dufosse, L.; Latrasse, A.; Spinnler, H.E. Importance of Lactones in Food Flavors—Structure, Distribution, Sensory Properties and Biosynthesis. Sci. Aliment. 1994, 14, 17–50. [Google Scholar]
- de Souza Oliveira, R.P.; Perego, P.; de Oliveira, M.N.; Converti, A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT Food Sci. Technol. 2012, 47, 358–363. [Google Scholar] [CrossRef]
- Dresselhuis, D.M.; de Hoog, E.H.; Cohen Stuart, M.A.; Vingerhoeds, M.H.; van Aken, G.A. The occurrence of in-mouth coalescence of emulsion droplets in relation to perception of fat. Food Hydrocoll. 2008, 22, 1170–1183. [Google Scholar] [CrossRef]

| Base | Composition |
|---|---|
| Soy | 95% soy milk, 5% sucrose |
| Oat | 30% w/w oat concentrate |
| Coconut | 93% coconut milk, 3% sucrose, 4% starch |
| Base | Protein (%) | Carbohydrates (%) | Main Sugar | Fat (%) |
|---|---|---|---|---|
| Soy | 3.7 | 5 | Sucrose | 2 |
| Oat | 4.5 | 18 | Glucose | 2.2 |
| Coconut | 1.49 | 3 | Sucrose | 17.67 |
| Culture Name | Composition |
|---|---|
| YOFLEX® * YF-L01 DA (YF-L01) | Streptococcus thermophilus |
| YOFLEX® * YF-L02 DA (YF-L02) | Streptococcus thermophilus and Lactobacillus bulgaricus supplemented with Lactobacillus acidophilus, Lactobacillus paracasei, and Bifidobacterium |
| NU-TRISH® * BY-01 DA (BY-01) | Streptococcus thermophilus and Lactobacillus bulgaricus with Bifidobacterium, BB-12® |
| LGG® | Lactobacillus rhamnosus |
| Attribute | Definition | Indications |
|---|---|---|
| Gel firmness | Resistance to deformation of the product. | Slowly take a spoon of the product and place it on the untouched sample surface. Note how long it keeps its shape. |
| Ropiness | Sticky, glutinuous or soft nature of the product. | Dip the bottom of the spoon several times fast in the surface of the sample. A long string indicates high ropiness. |
| Astringency | Similar feeling to very unripe fruit. | If the sample dries out your mouth, it means high astringency. |
| Mouth coating | The extent to which the product coats the palate and teeth during mastication. | Distribute the product in your mouth and swallow it. If it leaves a coating in your mouth, it is high in mouth coating. |
| Mouth thickness | Sensation of sample consistency in mouth. | Evaluate the product’s resistance when swallowed with normal speed without tasting the sample. |
| Smoothness | The smoothness against the palate as it breaks up during mastication. | Perceive the smoothness of the sample by squeezing it between palate and tongue. |
| Acetic | Acidic smell of vinegar. | Hold your nose to perceive acidic flavor. |
| Cardboard | Aromatic associated with slightly oxidized fats, reminiscent of wet cardboard packaging. | Tasting of the sample. |
| Fatty/creamy | Feeling associated with heavy whipping cream. | Compare the product with the given full fat cream (38%) sample. |
| Foamy | Foam appearance of the sample. | Visual evaluation of the sample. |
| Powdery/chalky | Powder sensation in mouth. | Visual evaluation of the sample. |
| Pudding-like | Similar structure to a pudding. | Visual evaluation of the sample. |
| Shininess | How shiny the surface of the product looks like. | Visual evaluation of the sample. |
| Culture Combination | Base | ||
|---|---|---|---|
| Soy | Oat | Coconut | |
| YF-L01 | 7.09 ± 0.10 | 8.57 ± 0.07 | 5.32 ± 1.18 |
| YF-L02 | 6.64 ± 0.10 | 7.38 ± 0.03 | 5.11 ± 1.26 |
| BY-01 | 6.91 ± 0.10 | 7.94 ± 0.05 | 5.70 ± 1.16 |
| YF-L01+LGG® | 6.27 ± 0.07 | 7.27 ± 0.05 | 5.33 ± 1.40 |
| YF-L02+LGG® | 6.02 ± 0.10 | 6.65 ± 0.13 | 5.43 ± 1.18 |
| BY-01+LGG® | 6.05 ± 0.23 | 6.80 ± 0.09 | 5.93 ± 1.16 |
| Base | (Pa) | G* (Pa) |
|---|---|---|
| Soy | 24.1 ± 0.3 | 170.1 ± 3.3 |
| Oat | 44.8 ± 1.7 | 471.7 ± 40.9 |
| Coconut | 35.8 ± 3.5 | 232.9 ± 69.4 |
| Culture Combination | Base | |
|---|---|---|
| Soy | Oat | |
| YF-L01 | 1239.6 ± 83.8 | 3849 ± 110.1 |
| YF-L02 | 1286 ± 43 | 3693.5 ± 71.8 |
| BY-01 | 1275.7 ± 39.6 | 4505.4 ± 63.2 |
| YF-L01+LGG® | 1357.4 ± 58.8 | 4019.7 ± 317 |
| YF-L02+LGG® | 1327.6 ± 75.3 | 4015.5 ± 104.5 |
| BY-01+LGG® | 1346.1 ± 72.5 | 3987.3 ± 466 |
| Shear Rate (s−1) | Base | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOY | OAT | Coconut | ||||||||||
| Textural Attributes | Textural Attributes | Textural Attributes | ||||||||||
| Gel Firmness | Mouth Thickness | Ropiness | Creaminess | Gel Firmness | Mouth Coating | Smoothness | Gel Firmness | Mouth Thickness | Mouth Coating | |||
| 0.3 | 0.0 | −0.3 | 0.0 | 0.4 | 0.9 * | −0.3 | −0.4 | 0.8 * | 0.8 * | 0.7 * | ||
| 15.3 | 0.9 * | 0.4 | −0.5 | 0.1 | 1.0 * | −0.7 * | −0.7 * | 0.9 * | 0.8 * | 0.8 * | ||
| 30.2 | 0.6 * | 0.5 | −0.5 | 0.3 | 1.0 * | −0.6 * | −0.6 * | 0.9 * | 0.8 * | 0.8 * | ||
| 45.2 | 0.6 * | 0.4 | −0.5 | 0.4 | 1.0 * | −0.6 * | −0.6 * | 0.8 * | 0.8 * | 0.8 * | ||
| 60.2 | 0.6 * | 0.5 | −0.5 | 0.3 | 1.0 * | −0.7 * | −0.6 * | 0.8 * | 0.8 * | 0.7 * | ||
| 75.2 | 0.5 | 0.5 | −0.5 | 0.3 | 1.0 * | −0.6 * | −0.6 * | 0.8 * | 0.8 * | 0.7 * | ||
| 90.2 | 0.6 * | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.6 * | −0.5 | 0.8 * | 0.8 * | 0.7 * | ||
| 105.0 | 0.6 * | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.6 * | −0.5 | 0.8 * | 0.8 * | 0.7 * | ||
| 120.0 | 0.5 | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.6 * | −0.5 | 0.8 * | 0.8 * | 0.6 * | ||
| 135.0 | 0.5 | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.5 | −0.4 | 0.8 * | 0.8 * | 0.6 * | ||
| 150.0 | 0.5 | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.5 | −0.4 | 0.8 * | 0.8 * | 0.6 * | ||
| 165.0 | 0.5 | 0.6 * | −0.4 | 0.3 | 0.9 * | −0.5 | −0.4 | 0.8 * | 0.8 * | 0.6 * | ||
| 180.0 | 0.6 * | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.5 | −0.4 | 0.8 * | 0.8 * | 0.7 * | ||
| 195.0 | 0.5 | 0.6 * | −0.5 | 0.3 | 0.9 * | −0.4 | −0.3 | 0.8 * | 0.8 * | 0.8 * | ||
| 210.0 | 0.5 | 0.6 * | −0.5 | 0.3 | 0.8 * | −0.4 | −0.3 | 0.8 * | 0.9 * | 0.8 * | ||
| 225.0 | 0.5 | 0.6 * | −0.4 | 0.3 | 0.8 * | −0.4 | −0.3 | 0.8 * | 0.9 * | 0.8 * | ||
| 240.0 | 0.5 | 0.7 * | −0.4 | 0.2 | 0.7 * | −0.4 | −0.2 | 0.8 * | 0.9 * | 0.8 * | ||
| 255.0 | 0.6 * | 0.7 * | −0.4 | 0.2 | 0.8 * | −0.3 | −0.2 | 0.9 * | 0.9 * | 0.8 * | ||
| 270.0 | 0.6 * | 0.6 * | −0.4 | 0.2 | 0.8 * | −0.3 | −0.2 | 0.9 * | 0.9 * | 0.8 * | ||
| 285.0 | 0.6 * | 0.6 * | −0.5 | 0.2 | 0.8 * | −0.3 | −0.2 | 0.9 * | 0.9 * | 0.8 * | ||
| 300.0 | 0.6 * | 0.6 * | −0.5 | 0.2 | 0.8 * | −0.3 | −0.1 | 0.9 * | 0.9 * | 0.8 * | ||
| Shear Rate (s−1) | Base | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOY | OAT | Coconut | ||||||||||
| Textural Attributes | Textural Attributes | Textural Attributes | ||||||||||
| Gel Firmness | Mouth Thickness | Ropiness | Creaminess | Gel Firmness | Mouth Coating | Smoothness | Gel Firmness | Mouth Thickness | Mouth Coating | |||
| 0.5 | 0.8 * | 0.4 | −0.5 | 0.3 | 0.9 * | −0.7 * | −0.7 * | 0.9 * | 0.8 * | 0.9 * | ||
| 0.9 | 0.8 * | 0.3 | −0.5 | 0.3 | 0.9 * | −0.7 * | −0.7 * | 0.9 * | 0.8 * | 0.9 * | ||
| 1.5 | 0.8 * | 0.3 | −0.5 | 0.3 | 0.9 * | −0.7 * | −0.7 * | 0.9 * | 0.8 * | 0.9 * | ||
| 2.6 | 0.9 * | 0.3 | −0.5 | 0.3 | 0.9 * | −0.8 * | −0.7 * | 0.9 * | 0.8 * | 0.9 * | ||
| 4.6 | 0.9 * | 0.2 | −0.5 | 0.3 | 0.9 * | −0.8 * | −0.7 * | 0.9 * | 0.8 * | 0.9 * | ||
| 8.0 | 0.8 * | 0.0 | −0.4 | 0.3 | 0.9 * | −0.8 * | −0.8 * | 0.9 * | 0.8 * | 0.9 * | ||
| Base | Culture Combination | Glucose | Fructose | Sucrose | Maltose |
|---|---|---|---|---|---|
| Soy | YF-L01 | 0.00 | 0.74 | 50.55 | 0.00 |
| YF-L02 | 0.00 | 0.74 | 50.55 | 0.00 | |
| BY-01 | 0.00 | 0.74 | 50.55 | 0.00 | |
| YF-L01+LGG® | 0.00 | 0.74 | 50.55 | 0.00 | |
| YF-L02+LGG® | 0.00 | 0.74 | 50.55 | 0.00 | |
| BY-01+ LGG® | 0.00 | 0.74 | 50.55 | 0.00 | |
| Unfermented soy | 0.00 | 0.00 | 55.81 | 0.00 | |
| Oat | YF-L01 | 92.38 | 4.06 | 4.34 | 24.15 |
| YF-L02 | 92.38 | 4.06 | 4.34 | 24.15 | |
| BY-01 | 92.38 | 4.06 | 4.34 | 24.15 | |
| Unfermented oat | 94.55 | 3.93 | 5.77 | 24.08 | |
| Coconut | YF-L01 | <LOD | 1.50 | 48.35 | <LOD |
| YF-L02 | <LOD | 1.35 | 47.45 | <LOD | |
| BY-01 | <LOD | 1.65 | 49.05 | <LOD | |
| YF-L01+LGG® | <LOD | 1.20 | 47.40 | <LOD | |
| YF-L02+LGG® | 0.60 | 0.55 | 47.70 | <LOD | |
| BY-01+LGG® | <LOD | 0.60 | 48.25 | <LOD | |
| Unfermented coconut | 0.27 | 1.13 | 27.64 | 0.00 |
| Base | Culture Combination | Lactic Acid | Acetic Acid | Tartaric Acid | Citric Acid | Malic Acid |
|---|---|---|---|---|---|---|
| Soy | YF-L01 | 3.50 | 0.00 | 3.50 | 0.00 | 0.00 |
| YF-L02 | 3.50 | 0.00 | 3.50 | 0.00 | 0.00 | |
| BY-01 | 3.50 | 0.00 | 3.50 | 0.00 | 0.00 | |
| YF-L01+LGG® | 3.50 | 2.00 | 3.50 | 0.00 | 0.00 | |
| YF-L02+LGG® | 3.50 | 2.00 | 3.50 | 0.00 | 0.00 | |
| BY-01+LGG® | 3.50 | 2.00 | 3.50 | 0.00 | 0.00 | |
| Unfermented soy | 0.10 | 0.00 | 0.00 | 0.85 | 0.00 | |
| Oat | YF-L01 | 3.40 | 0.10 | 0.00 | 0.18 | 0.05 |
| YF-L02 | 3.40 | 0.10 | 0.00 | 0.18 | 0.05 | |
| BY-01 | 3.40 | 0.10 | 0.00 | 0.18 | 0.05 | |
| Unfermented oat | 0.03 | 0.00 | 0.00 | 0.22 | 0.27 | |
| Coconut | YF-L01 | 2.90 | 0.25 | <LOD | 0.30 | 1.55 |
| YF-L02 | 3.25 | 0.25 | <LOD | 0.25 | 1.00 | |
| BY-01 | 2.80 | 0.50 | <LOD | 0.30 | 1.45 | |
| YF-L01+LGG® | 4.25 | 0.25 | <LOD | 0.30 | <LOD | |
| YF-L02+LGG® | 4.50 | 0.25 | <LOD | 0.30 | <LOD | |
| BY-01+LGG® | 4.35 | 0.50 | <LOD | 0.30 | <LOD | |
| Unfermented coconut | 0.15 | 0.15 | 0.0 | 0.47 | 1.89 |
| Base | Culture Combination | Acetaldehyde | Diacetyl | Acetoin | Acetone | 3-Methyl-Butanal | Ethanol |
|---|---|---|---|---|---|---|---|
| Soy | YF-L01 | 2.73 ± 0.06 | 4.05 ± 0.17 | 15.38 ± 0.13 | 1.47 ± 0.02 | n/s | 0.71 ± 0.09 |
| YF-L02 | 2.52 ± 0.08 | 7.06 ± 0.48 | 13.51 ± 1.11 | 1.48 ± 0.08 | n/s | 2.08 ± 0.04 | |
| BY-01 | 2.84 ± 0.01 | 3.54 ± 0.02 | 9.39 ± 1.68 | 1.55 ± 0.04 | n/s | 4.1 ± 0.16 | |
| YF-L01 + LGG® | 1.19 ± 0.02 | 3.19 ± 0.12 | 19.71 ± 1.45 | 1.31 ± 0 | n/s | 1.23 ± 0.2 | |
| YF-L02 + LGG® | 0.58 ± 0.02 | 3.89 ± 0.15 | 26.24 ± 0.4 | 1.54 | n/s | 2.59 ± 0.02 | |
| BY-01 + LGG® | 1.77 ± 0.08 | 2.98 ± 0.2 | 18.79 ± 1.54 | 1.65 ± 0.08 | n/s | 5.55 ± 0.3 | |
| Oat | YF-L01 | 1.08 ± | 4.27 ± 0.02 | 40.19 ± 0.7 | 0.3 ± 0.05 | 1.21 ± 0.04 | 92.8 ± 3.18 |
| YF-L02 | 1.13 ± 0.01 | 39.35 ± 0.71 | 42.44 ± 0.32 | 0.37 ± 0.01 | 0.19 ± 0.01 | 17.28 ± 0.26 | |
| BY-01 | 2.53 ± 0.06 | 8.34 ± 0.09 | 41.89 ± 3.31 | 0.35 ± 0.01 | 0.1 ± 0.11 | 8.15 ± 2.33 | |
| YF-L01 + LGG® | 0.25 | 16.7 ± 0.01 | 97.86 ± 0.55 | 0.27 ± 0.03 | 0.29 ± 0.01 | 24.54 ± 0.65 | |
| YF-L02 + LGG® | 0.22 ± 0.01 | 17.53 ± 0.07 | 81.41 ± 3.05 | 0.32 ± 0.09 | 0.15 | 18.28 ± 0.25 | |
| BY-01 + LGG® | 0.29 ± 0.03 | 15.74 ± 0.07 | 87.57 ± 2.01 | 0.29 ± 0.02 | 0.06 | 16.8 ± 0.36 | |
| Coconut | YF-L01 | 3.42 ± 0.03 | 3.27 ± 0.05 | 50.5 ± 0.34 | 0.42 ± 0.03 | n/s | 71.32 ± 1.61 |
| YF-L02 | 3.1 ± 0.03 | 18.23 ± 0.09 | 48.19 ± 1.02 | 0.44 ± 0.06 | n/s | 71.81 ± 0.12 | |
| BY-01 | 3.92 ± 0.03 | 3.56 ± 0.1 | 39.92 ± 5.88 | 0.42 ± 0.08 | n/s | 71.69 ± 1.39 | |
| YF-L01 + LGG® | 2.01 ± 0.03 | 13.86 ± 0.64 | 67.95 ± 1.47 | 0.51 ± 0.05 | n/s | 73.53 ± 1.71 | |
| YF-L02 + LGG® | 0.62 ± 0.03 | 15.88 ± 0.48 | 71.77 ± 1.04 | 0.55 | n/s | 71.97 ± 3.14 | |
| BY-01 + LGG® | 0.41 ± 0.02 | 13.41 ± 0.64 | 69.05 ± 0.72 | 0.57 ± 0.03 | n/s | 72.53 ± 3.01 |
| Culture Combination | Ketones | Esters | Terpene | Aldehyde | Lactones | Furan | |||
|---|---|---|---|---|---|---|---|---|---|
| 2-Heptanone | 2,3-Pentanedione | EthylOctanoate | EthylDecanoate | Limonene | 2-MethylButanal | -Decalactone | -Octalactone | 2-Pentyl-Furan | |
| YF-L01 | 1.5 ± 2.1 | 8467.5 ± 5478 | 14 | 27.5 ± 23.3 | 246 ± 11.3 | 6 ± 4.2 | 60.5 ± 78.5 | 38 ± 45.3 | 7613 ± 7905.5 |
| YF-L02 | 22 ± 2.8 | 11,280.5 ± 68.6 | 1621 ± 380.4 | 972.5 ± 293.4 | 241.5 ± 41.7 | 29 ± 2.8 | 450.5 ± 160.5 | 307.5 ± 177.5 | 46,420.5 ± 4297.1 |
| BY-01 | 2.5 ± 3.5 | 4012 ± 2547 | 4.5 | 13.5 ± 3.5 | 54.5 ± 36.1 | 1.5 ± 0.7 | 4.5 ± 2.1 | 4 ± 2.8 | 22,223 ± 16,525.1 |
| YF-L01 + LGG® | 6 | 5439 ± 753.8 | 12.5 ± 2.1 | 14.5 ± 6.4 | 112.5 ± 13.4 | 1.5 ± 0.7 | 27 ± 29.7 | 6 ± 4.2 | 46,280.5 ± 6639 |
| YF-L02 + LGG® | 0 | 11,269.5 ± 67.2 | 13.5 ± 0.7 | 21.5 ± 0.7 | 48.5 ± 7.8 | 8 ± 1.4 | 2 ± 1.4 | 9 ± 2.8 | 4886.5 ± 437.7 |
| BY-01 + LGG® | 15.5 ± 2.1 | 14,537 ± 1619.3 | 1045.5 ± 227 | 832.5 ± 160.5 | 79 ± 1.4 | 22.5 ± 3.5 | 623.5 ± 142.1 | 605.5 ± 234.1 | 25,638 ± 4307.7 |
| Culture Combination | Ketones | Ester | Terpene | Aldehyde | Alcohol | |||
|---|---|---|---|---|---|---|---|---|
| 2,3-Pentanedione | 2-Methyl-3-Thiolanone | Acetone | Ethyl Acetate | Limonene | Furfural | 1-Hexanol | 2-Propanol | |
| YF-L01 | 54,062.5 ± 3853 | 6 ± 1.4 | 1150.5 ± 160.5 | 1498 ± 424.3 | 17.5 ± 0.7 | 10 ± 2.8 | 59 ± 53.7 | 724 ± 73.5 |
| YF-L02 | 11,712 | 18 | 350 | 1095 | 41 | 12 | 120 | 115 |
| BY-01 | 43,894.5 ± 386.8 | 15 ± 1.4 | 178.5 ± 98.3 | 408.5 ± 272.2 | 24 ± 1.4 | 19 ± 1.4 | 47 ± 22.6 | 6 ± 1.4 |
| YF-L01 + LGG® | 430 ± 29.7 | 8 ± 1.4 | 265 ± 100.4 | 447.5 ± 0.7 | 17 ± 4.2 | 12.5 ± 2.1 | 14.5 ± 2.1 | 122.5 ± 46 |
| YF-L02 + LGG® | 154 ± 24 | 43 ± 18.4 | 30 ± 11.3 | 751.5 ± 101.1 | 24.5 ± 7.8 | 9.5 ± 3.5 | 21.5 ± 7.8 | 8.5 ± 3.5 |
| BY-01 + LGG® | 210.5 ± 64.3 | 96.5 ± 40.3 | 104.5 ± 118.1 | 2204.5 ± 439.1 | 20.5 ± 2.1 | 8.5 ± 3.5 | 44 ± 19.8 | 14 ± 18.4 |
| Culture Combination | Ketone | Terpene | Lactone | Alcohol | |||
|---|---|---|---|---|---|---|---|
| 2-Pentanone | 2-3-Pentanedione | Diacetyl | -Pinene | -Nonalactone | Butyrolactone | 1-Butanol | |
| YF-L01 | 10.5 ± 0.7 | 89 ± 9.9 | 11,375.5 ± 675.3 | 77.5 ± 12 | 2153 ± 144.2 | 12.5 ± 2.1 | 45.5 ± 7.8 |
| YF-L02 | 8.5 ± 0.7 | 65.5 ± 3.5 | 49,102 ± 5395.2 | 64 ± 2.8 | 1883.5 ± 156.3 | 12 ± 2.8 | 43.5 ± 2.1 |
| BY-01 | 8 | 55 | 9851 | 54 | 1381 | 8 | 32 |
| YF-L01+LGG® | 26.5 ± 4.9 | 23.5 ± 4.9 | 32,402.5 ± 5052.3 | 69.5 ± 2.1 | 2211.5 ± 266.6 | 18 ± 2.8 | 45.5 ± 5.7 |
| YF-L02+LGG® | 28.5 ± 11.3 | 17.5 ± 1.4 | 47,037.5 ± 1850.5 | 86.5 ± 16.3 | 2403.5 ± 84.9 | 22 ± 4.9 | 55 ± 7.8 |
| BY-01+LGG® | 39 ± 2.8 | 15 ± 1.4 | 39,865 ± 1390.2 | 66 ± 1.4 | 2153 ± 257.4 | 13.5 ± 0.7 | 39.5 ± 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masiá, C.; Jensen, P.E.; Buldo, P. RETRACTED: Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2020, 9, 1182. https://doi.org/10.3390/foods9091182
Masiá C, Jensen PE, Buldo P. RETRACTED: Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods. 2020; 9(9):1182. https://doi.org/10.3390/foods9091182
Chicago/Turabian StyleMasiá, Carmen, Poul Erik Jensen, and Patrizia Buldo. 2020. "RETRACTED: Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials" Foods 9, no. 9: 1182. https://doi.org/10.3390/foods9091182
APA StyleMasiá, C., Jensen, P. E., & Buldo, P. (2020). RETRACTED: Effect of Lactobacillus rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods, 9(9), 1182. https://doi.org/10.3390/foods9091182








