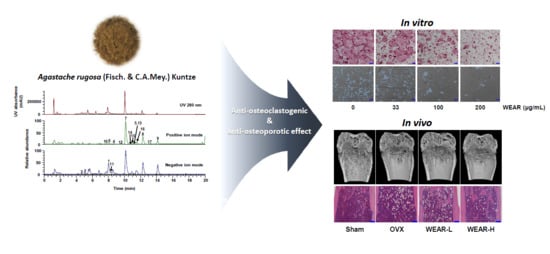
Water Extract of Agastache rugosa Prevents Ovariectomy-Induced Bone Loss by Inhibiting Osteoclastogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WEAR
2.3. Animal Experiment and Bone Analysis
2.4. Cell Viability Assay
2.5. Tartrate-Resistant Acid Phosphatase (TRAP) Assay and Resorption Assay
2.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
2.7. Western Blot Analysis
2.8. Ultrahigh-Performance Liquid Chromatography and Tandem Mass Spectrometry (UHPLC–MS/MS) Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. WEAR Attenuates OVX-Induced Bone Loss
3.2. WEAR Inhibits RANKL-Induced Osteoclast Differentiation
3.3. WEAR Inhibits RANKL-Induced Early Signaling Pathways
3.4. The Phytochemical Profile of WEAR
3.5. Phytochemicals of WEAR Inhibits RANKL-Induced Osteoclast Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMM | bone marrow-derived macrophage |
| M-CSF | macrophage colony-stimulating factor |
| OVX | ovariectomized |
| RANKL | receptor activator of nuclear kappa-B ligand |
| WEAR | water extract of A. rugosa |
References
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis--current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar] [PubMed]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.H.; Kang, S.Y.; Shin, J.S.; Ryu, S.M.; Lee, A.Y.; Choi, G.; Moon, B.C.; Jang, D.S.; Shim, S.H.; Lee, D.; et al. Chemical Constituents from the Aerial Parts of Agastache rugosa and Their Inhibitory Activities on Prostaglandin E2 Production in Lipopolysaccharide-Treated RAW 264.7 Macrophages. J. Nat. Prod. 2019, 82, 3379–3385. [Google Scholar] [CrossRef]
- Zielinska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Lee, H.K.; Shin, C.G.; Huh, H. HIV integrase inhibitory activity of Agastache rugosa. Arch. Pharm. Res. 1999, 22, 520–523. [Google Scholar] [CrossRef]
- Shin, S. Essential oil compounds from Agastache rugosa as antifungal agents against Trichophyton species. Arch. Pharm. Res. 2004, 27, 295–299. [Google Scholar] [CrossRef]
- Ha, H.; An, H.; Shim, K.S.; Kim, T.; Lee, K.J.; Hwang, Y.H.; Ma, J.Y. Ethanol extract of Atractylodes macrocephala protects bone loss by inhibiting osteoclast differentiation. Molecules 2013, 18, 7376–7388. [Google Scholar] [CrossRef] [Green Version]
- Sophocleous, A.; Idris, A.I. Rodent models of osteoporosis. Bonekey Rep. 2014, 3, 614. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Lee, C.J.; Lee, K.J.; Ma, J.Y. Water extract of Acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and function. Molecules 2014, 19, 3940–3954. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Shim, K.S.; Kim, T.; An, H.; Ma, J.Y. Water extract of Dryopteris crassirhizoma Attenuates Bone Loss by Suppressing Osteoclast Differentiation and Function. Evid. Based Complement. Alternat. Med. 2013, 2013, 852648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.A.; Hwang, Y.H.; Kim, T.; Lee, A.; Ha, H. Anti-Osteoporotic and Anti-Adipogenic Effects of the Water Extract of Drynaria roosii Nakaike in Ovariectomized Mice Fed a High-Fat Diet. Molecules 2019, 24, 3051. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.H.; Ma, J.Y. Preventive Effects of an UPLC-DAD-MS/MS Fingerprinted Hydroalcoholic Extract of Citrus aurantium in a Mouse Model of Ulcerative Colitis. Planta Med. 2018, 84, 1101–1109. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar] [PubMed]
- Kitajima, Y.; Doi, H.; Ono, Y.; Urata, Y.; Goto, S.; Kitajima, M.; Miura, K.; Li, T.S.; Masuzaki, H. Estrogen deficiency heterogeneously affects tissue specific stem cells in mice. Sci. Rep. 2015, 5, 12861. [Google Scholar] [CrossRef] [Green Version]
- Jakubas-Przewlocka, J.; Przewlocki, P.; Sawicki, A. Assessment of changes due to the long-term effect of estrogen and calcium deficiency in the trabecular bone structure in rats. Clin. Exp. Rheumatol. 2005, 23, 385–388. [Google Scholar]
- Marinozzi, F.; Marinozzi, A.; Bini, F.; Zuppante, F.; Pecci, R.; Bedini, R. Variability of morphometric parameters of human trabecular tissue from coxo-arthritis and osteoporotic samples. Ann. Ist. Super. Sanita 2012, 48, 19–25. [Google Scholar]
- Faienza, M.F.; Ventura, A.; Marzano, F.; Cavallo, L. Postmenopausal osteoporosis: The role of immune system cells. Clin. Dev. Immunol. 2013, 2013, 575936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schurmann, A.; et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Sanjay, A.; Neff, L.; Tanaka, S.; Horne, W.C.; Baron, R. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 2004, 279, 17660–17666. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ryu, J.; Ha, J.; Chang, E.J.; Kim, H.J.; Kim, H.M.; Kitamura, T.; Lee, Z.H.; Kim, H.H. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006, 13, 1879–1891. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Invest. 2004, 114, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Lee, Y.; Chang, E.J.; Kim, H.M.; Hong, S.P.; Lee, Z.H.; Ryu, J.; Kim, H.H. Suppression of osteoclastogenesis by N,N-dimethyl-D-erythro-sphingosine: A sphingosine kinase inhibition-independent action. Mol. Pharmacol. 2007, 72, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.W.; Baek, S.H.; Lee, S.H.; Kim, T.H.; Kim, S.Y. Fucoidan, a sulfated polysaccharide, inhibits osteoclast differentiation and function by modulating RANKL signaling. Int. J. Mol. Sci. 2014, 15, 18840–18855. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, B.M.; Ali, E.M.; Elsawy, H.; Badr, G.M.; Abdel-Moneim, A.M.; Alzahrani, A.M. The Coumarin Derivative 5′-Hydroxy Auraptene Suppresses Osteoclast Differentiation via Inhibiting MAPK and c-Fos/NFATc1 Pathways. BioMed. Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Verde, S.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Effects of gamma irradiation on cytotoxicity and phenolic compounds of Thymus vulgaris L. and Mentha × piperita L. Lwt-Food Sci. Technol. 2016, 71, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Qian, H.; Zhang, J.; Sun, J.; Ma, Z. Simultaneous quantification of eight organic acid components in Artemisia capillaris Thunb (Yinchen) extract using high-performance liquid chromatography coupled with diode array detection and high-resolution mass spectrometry. J. Food Drug Anal. 2018, 26, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Kolniak-Ostek, J.; Dziadas, M.; Oszmiański, J.; Matkowski, A.; Technologies, R. Characterization of polyphenols in Agastache rugosa leaves and inflorescences by UPLC–qTOF–MS following FCPC separation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 209–219. [Google Scholar] [CrossRef]
- Dumaa, M.; Xiang, P.; Jinhai, Y.; Guolin, Z.; Yinggang, L. Chemical components of aerial parts of Lagochilus ilicifolius. Chin. J. Appl. Environ. Biol. 2012, 18, 924–927. [Google Scholar]
- Grayer, R.J.; Eckert, M.R.; Veitch, N.C.; Kite, G.C.; Marin, P.D.; Kokubun, T.; Simmonds, M.S.; Paton, A.J. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. Phytochemistry 2003, 64, 519–528. [Google Scholar] [CrossRef]
- Wang, S.F.; Leng, J.; Xu, Y.M.; Feng, M.L. Identification and determination of major constituents in a traditional Chinese medicine compound recipe Xiongdankaiming tablet using HPLC-PDA/ESI-MS(n) and HPLC-UV/ELSD. J. Zhejiang Univ. Sci. B 2013, 14, 604–614. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.C.; Liu, L.T.; Bian, J.J.; Yan, C.Q.; Ye, L.; Zhao, M.X.; Huang, Q.S.; Wang, W.; Liang, K.; Shi, Z.F.; et al. Identification of Multiple Constituents in Shuganjieyu Capsule and Rat Plasma after Oral Administration by Ultra-Performance Liquid Chromatography Coupled with Electrospray Ionization and Ion Trap Mass Spectrometry. Acta Chromatogr. 2018, 30, 95–102. [Google Scholar] [CrossRef]
- Huck, C.W.; Buchmeiser, M.R.; Bonn, G.K. Fast analysis of flavonoids in plant extracts by liquid chromatography-ultraviolet absorbance detection on poly(carboxylic acid)-coated silica and electrospray ionization tandem mass spectrometric detection. J. Chromatogr. A 2002, 943, 33–38. [Google Scholar] [CrossRef]
- Skowyra, M.; Calvo, M.I.; Gallego Iradi, M.G.; Azman, N.A.B.M.; Almajano Pablos, M.P. Characterization of phytochemicals in petals of different colours from Viola × wittrockiana Gams and their correlation with antioxidant activity. J. Agric. Sci. 2014, 6, 93–105. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Huang, X.; Zhao, F.L.; Tang, Y.L.; Yin, L. Study on the chemical markers of Caulis Lonicerae japonicae for quality control by HPLC-QTOF/MS/MS and chromatographic fingerprints combined with chemometrics methods. Anal. Methods 2015, 7, 2064–2076. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepulveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Lee, J.W.; Asai, M.; Jeon, S.K.; Iimura, T.; Yonezawa, T.; Cha, B.Y.; Woo, J.T.; Yamaguchi, A. Rosmarinic acid exerts an antiosteoporotic effect in the RANKL-induced mouse model of bone loss by promotion of osteoblastic differentiation and inhibition of osteoclastic differentiation. Mol. Nutr. Food Res. 2015, 59, 386–400. [Google Scholar] [CrossRef]
- Shao, S.; Fu, F.; Wang, Z.; Song, F.; Li, C.; Wu, Z.X.; Ding, J.; Li, K.; Xiao, Y.; Su, Y.; et al. Diosmetin inhibits osteoclast formation and differentiation and prevents LPS-induced osteolysis in mice. J. Cell. Physiol. 2019, 234, 12701–12713. [Google Scholar] [CrossRef]
- Puel, C.; Quintin, A.; Mathey, J.; Obled, C.; Davicco, M.J.; Lebecque, P.; Kati-Coulibaly, S.; Horcajada, M.N.; Coxam, V. Prevention of bone loss by phloridzin, an apple polyphenol, in ovariectomized rats under inflammation conditions. Calcif. Tissue Int. 2005, 77, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef]
- Kim, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef]






| No | Rt (min) | Calculated (m/z) | Estimated (m/z) | Adducts | Error (ppm) | Formula | MS/MS Fragments (m/z) | Identifications [References] |
|---|---|---|---|---|---|---|---|---|
| Phenolics | ||||||||
| 1 | 8.00 | 359.0772 | 359.0765 | [M-H]- | −1.9548 | C18H16O8 | 135.0435, 161.0228, 179.0337, 197.0443 | Rosmarinic acid [7,33] |
| 2 | 5.19 | 353.0878 | 353.0872 | [M-H]- | −1.8460 | C16H18O9 | 93.0326, 173.0439, 191.0547 | Chlorogenic acid [34,35] |
| 3 | 4.67 | 353.0878 | 353.0873 | [M-H]- | −1.5003 | C16H18O9 | 135.0437, 173.0438, 179.0334, 191.0547 | Neochlorogenic acid [34,35] |
| 4 | 8.68 | 349.1258 | 349.1258 | [M+Na]+ | 0.0672 | C16H22O7 | 349.1258 | Citrusin C [7,36] |
| 5 | 11.37 | 315.0863 | 315.0862 | [M+H]+ | −0.2137 | C17H14O6 | 163.0390 | Nepetoidin B [7,37] |
| Flavonoids | ||||||||
| 6 | 8.12 | 463.1235 | 463.1234 | [M+H]+ | −0.1661 | C22H22O11 | 286.0473, 301.0707 | Diosmetin-7-O-β-d-glucopyranoside [7,38] |
| 7 | 10.15 | 447.1286 | 447.1284 | [M+H]+ | −0.3400 | C22H22O10 | 285.0756 | Tilianin [7,39] |
| 8 | 12.27 | 489.1391 | 489.1392 | [M+H]+ | 0.0412 | C24H24O11 | 285.0755 | Isoagastachoside [7] |
| 9 | 14.12 | 285.0758 | 285.0758 | [M+H]+ | 0.0882 | C16H12O5 | 242.0584, 285.0756 | Acacetin [7,40] |
| 10 | 7.70 | 433.1129 | 433.1125 | [M+H]+ | −0.8570 | C21H20O10 | 271.0599 | Apigetrin [7,41] |
| 11 | 8.33 | 435.1297 | 435.1288 | [M-H]- | −2.1059 | C21H24O10 | 167.0333, 179.0334, 273.0761, 297.0728 | Phlorizin [7,42] |
| 12 | 9.53 | 287.0550 | 287.0550 | [M+H]+ | −0.1667 | C15H10O6 | 287.0550 | Luteolin [7,43] |
| 13 | 11.13 | 301.0707 | 301.0707 | [M+H]+ | 0.0621 | C16H12O6 | 286.0474 | Diosmetin [7,38] |
| 14 | 10.76 | 271.0601 | 271.0601 | [M+H]+ | −0.0402 | C15H10O5 | 271.0599 | Apigenin [7,39] |
| 15 | 10.95 | 533.1290 | 533.1288 | [M+H]+ | −0.2341 | C25H24O13 | 285.0757 | Acacetin-7-O-(6″-O-malonyl)-β-d-glucopyranoside [7] |
| 16 | 11.56 | 489.1391 | 489.1393 | [M+H]+ | 0.2284 | C24H24O11 | 285.0756 | Acacetin-7-O-(3″-O-acetyl)-β-d-glucopyranoside [7] |
| 17 | 13.12 | 575.1395 | 575.1395 | [M+H]+ | −0.0063 | C27H26O14 | 285.0756 | Acacetin-7-O-(2″-O-acetyl-6″-O-malonyl)-β-d-glucopyranoside [7] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-A.; Hwang, Y.-H.; Kim, T.; Yang, H.; Lee, J.; Seo, Y.H.; Park, J.-I.; Ha, H. Water Extract of Agastache rugosa Prevents Ovariectomy-Induced Bone Loss by Inhibiting Osteoclastogenesis. Foods 2020, 9, 1181. https://doi.org/10.3390/foods9091181
Jang S-A, Hwang Y-H, Kim T, Yang H, Lee J, Seo YH, Park J-I, Ha H. Water Extract of Agastache rugosa Prevents Ovariectomy-Induced Bone Loss by Inhibiting Osteoclastogenesis. Foods. 2020; 9(9):1181. https://doi.org/10.3390/foods9091181
Chicago/Turabian StyleJang, Seon-A, Youn-Hwan Hwang, Taesoo Kim, Hyun Yang, Jun Lee, Young Hye Seo, Jae-Il Park, and Hyunil Ha. 2020. "Water Extract of Agastache rugosa Prevents Ovariectomy-Induced Bone Loss by Inhibiting Osteoclastogenesis" Foods 9, no. 9: 1181. https://doi.org/10.3390/foods9091181
APA StyleJang, S.-A., Hwang, Y.-H., Kim, T., Yang, H., Lee, J., Seo, Y. H., Park, J.-I., & Ha, H. (2020). Water Extract of Agastache rugosa Prevents Ovariectomy-Induced Bone Loss by Inhibiting Osteoclastogenesis. Foods, 9(9), 1181. https://doi.org/10.3390/foods9091181