The Combined Effect of Pressure and Temperature on Kefir Production—A Case Study of Food Fermentation in Unconventional Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Kefir Fermentation under HP
2.3. pH Values and Titratable Acidity
2.4. Reducing Sugars Concentration
2.5. Activation Volume and Activation Energy Calculation
2.6. Organic Acids, Sugars, and Ethanol Determination
3. Results and Discussion
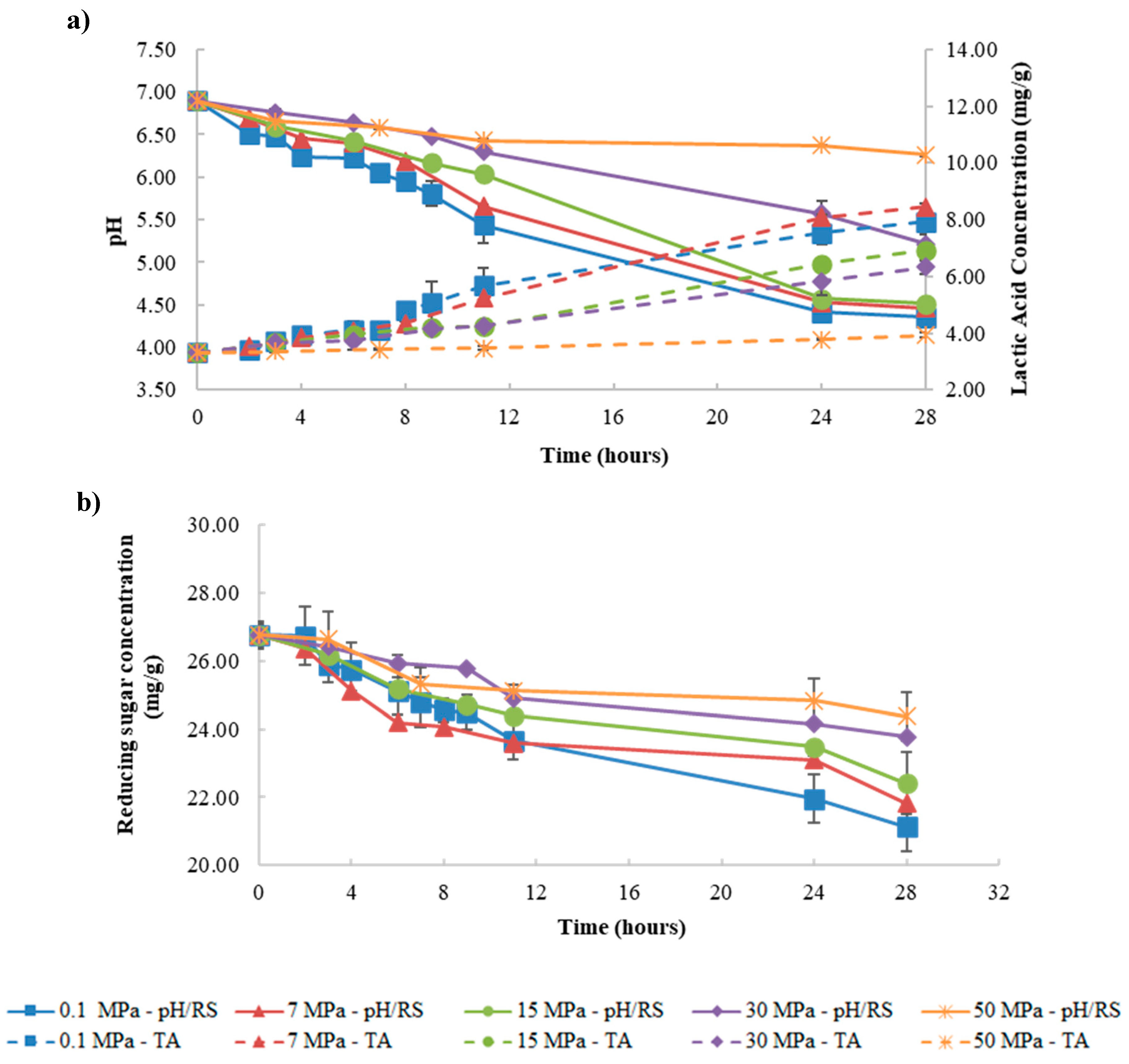
3.1. Effect of Pressure Fermentation on Kefir Production
3.1.1. Fermentation at Control Temperature
3.1.2. Fermentations at Temperatures above Control Temperature
3.1.3. Kinetic Analysis
Fermentation Rate Constants
Activation Volume
3.2. Effect of Fermentation Temperature on Kefir Production
3.2.1. Fermentation at 0.1, 15, and 50 MPa
3.2.2. Kinetic Analysis
Activation Energy
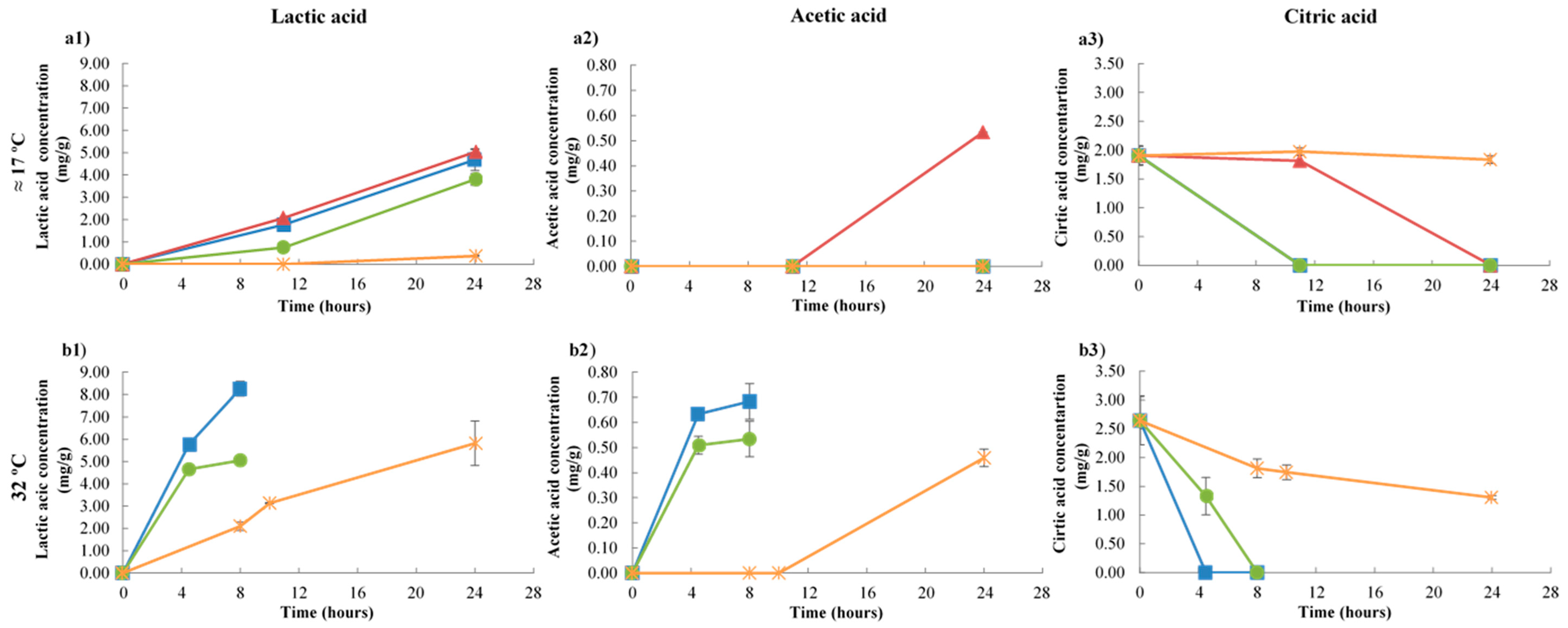
3.3. HPLC Analysis of Sugars, Organic Acids, and Ethanol During Kefir Fermentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kök-Taş, T.; Seydim, A.C.; Ozer, B.; Guzel-Seydim, Z.B. Effects of different fermentation parameters on quality characteristics of kefir. J. Dairy Sci. 2013, 96, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Marshall, V.M.; Cole, W.M. Methods for making kefir and fermented milks based on kefir. J. Dairy Res. 1985, 52, 451–456. [Google Scholar] [CrossRef]
- Otles, S.; Cagindi, O. Kefir: A probiotic dairy composition, nutritional and therapeutic aspects. Pak. J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibáñez, F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Pintado, M.E.; Da Silva, J.A.L.; Fernandes, P.B.; Malcata, F.X.; Hogg, T.A. Microbiological and rheological studies on Portuguese kefir grains. Int. J. Food Sci. Technol. 1996, 31, 15–26. [Google Scholar] [CrossRef]
- Irigoyen, A.; Ortigosa, M.; Torre, P.; Ibáñez, F.C. Influence of different technological parameters in the evolution of pH during fermentation of kefir. Milk Sci. Int. 2003, 58, 631–633. [Google Scholar]
- Dimitreli, G.; Antoniou, K.D. Effect of incubation temperature and caseinates on the rheological behaviour of kefir. Procedia Food Sci. 2011, 1, 583–588. [Google Scholar] [CrossRef][Green Version]
- Haque, A.; Richardson, R.K.; Morris, E.R. Effect of fermentation temperature on the rheology of set and stirred yogurt. Food Hydrocoll. 2001, 15, 593–602. [Google Scholar] [CrossRef]
- Tsevdou, M.S.; Taoukis, P.S. Effect of non-thermal processing by high ydrostatic pressure on the survival of probiotic microorganisms: Study on Bifidobacteria spp. Anaerobe 2011, 7, 456–458. [Google Scholar] [CrossRef]
- Vieira, A.P.P. Estudo da Conservação e Caracterização de um Iogurte Fermentado sob Pressão. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2019. [Google Scholar]
- Picard, A.; Daniel, I.; Montagnac, G.; Oger, P. In situ monitoring by quantitative Raman spectroscopy of alcoholic fermentation by Saccharomyces cerevisiae under high pressure. Extremophiles 2007, 11, 445–452. [Google Scholar] [CrossRef]
- Mota, M.J.; Lopes, R.P.; Sousa, S.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Lactobacillus reuteri growth and fermentation under high pressure towards the production of 1,3-propanediol. Food Res. Int. 2018, 113, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Delgadillo, I.; Saraiva, J.A. Probiotic yogurt production under high pressure and the possible use of pressure as an on/off switch to stop/start fermentation. Process Biochem. 2015, 50, 906–911. [Google Scholar] [CrossRef]
- Lopes, R.P.; Mota, M.J.; Sousa, S.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. Combined effect of pressure and temperature for yogurt production. Food Res. Int. 2019, 122, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.P.; Delgadillo, I.; Saraiva, J.A.; Gomes, A.M.; Pinto, C.A.; Lopes Da Silva, J.A. Physicochemical and microbial changes in yogurts produced under different pressure and temperature conditions. LWT 2019, 99, 423–430. [Google Scholar] [CrossRef]
- Bothun, G.D.; Knutson, B.L.; Berberich, J.A.; Strobel, H.J.; Nokes, S.E. Metabolic selectivity and growth of Clostridium thermocellum in continuous culture under elevated hydrostatic pressure. Appl. Microbiol. Biotechnol. 2004, 65, 149–157. [Google Scholar] [CrossRef]
- Iwahashi, H.; Odani, M.; Ishidou, E.; Kitagawa, E. Adaptation of Saccharomyces cerevisiae to high hydrostatic pressure causing growth inhibition. FEBS Lett. 2005, 579, 2847–2852. [Google Scholar] [CrossRef]
- Bravim, F.; Lippman, S.I.; Da Silva, L.F.; Souza, D.T.; Fernandes, A.A.R.; Masuda, C.A.; Broach, J.R.; Fernandes, P.M.B. High hydrostatic pressure activates gene expression that leads to ethanol production enhancement in a Saccharomyces cerevisiae distillery strain. Appl. Microbiol. Biotechnol. 2013, 97, 2093–2107. [Google Scholar] [CrossRef][Green Version]
- Jankowska, A.; Reps, A.; Proszek, A.; Krasowska, M.; Porowski, S.; Szczepek, J. Influence of high pressure on some biochemical properties of kefir microflora (effect of HPP on kefir microflora). High Press. Res. 2003, 23, 87–92. [Google Scholar] [CrossRef]
- Mainville, I.; Montpetit, D.; Durand, N.; Farnworth, E.R. Deactivating the bacteria and yeast in kefir using heat treatment, irradiation and high pressure. Int. Dairy J. 2001, 11, 45–49. [Google Scholar] [CrossRef]
- Chandan, R.C.; Kilara, A. Manufacturing Yogurt and Fermented Milks, 1st ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006; p. 496. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Costa, M.P.D.; Frasao, B.D.S.; Lima, B.R.C.D.C.; Rodrigues, B.L.; Junior, C.A.C. Simultaneous analysis of carbohydrates and organic acids by HPLC-DAD-RI for monitoring goat’s milk yogurts fermentation. Talanta 2016, 152, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Horikoshi, K. Hydrostatic pressure promotes the acidification of vacuoles in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1995, 130, 307–312. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofmann, E.; Kopperschla, G.G. Phosphofructokinase from Yeast. Methods Enzym. 1982, 90, 49–60. [Google Scholar]
- Aertsen, A.; Vanoirbeek, K.; De Spiegeleer, P.; Sermon, J.; Hauben, K.; Farewell, A.; Nystrom, T.; Michiels, W.C. Heat shock protein-mediated resistance to high hydrostatic pressure in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Apar, D.K.; Demirhan, E.; Özel, B.; Özbek, B. Kefir grain biomass production: Influence of different culturing conditions and examination of growth kinetic models. J. Food Process. Eng. 2017, 40, e12332. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ghaly, M.F.; El-Naggar, A.K. Some physicochemical analyses of kefir produced under different fermentation conditions. J. Sci. Ind. Res. 2011, 70, 365–372. [Google Scholar]
- Magalhães, K.T.; Pereira, G.V.D.M.; Dias, D.R.; Schwan, R.F. Microbial communities and chemical changes during fermentation of sugary Brazilian kefir. World J. Microbiol. Biotechnol. 2010, 26, 1241–1250. [Google Scholar]
- Rea, M.C.; Lennartsson, T.; Dillon, P.; Drinan, F.D.; Reville, W.J.; Heapes, M.; Cogan, T.M. Irish kefir-like grains: Their structure, microbial composition and fermentation kinetics. J. Appl. Bacteriol. 1996, 81, 83–94. [Google Scholar] [CrossRef]
- Beshkova, D.M.; Simova, E.D.; Frengova, G.I.; Simov, Z.I.; Dimitrov, Z.P. Production of volatile aroma compounds by kefir starter cultures. Int. Dairy J. 2003, 13, 529–535. [Google Scholar] [CrossRef]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef]


| Temperature (°C) | Pressure (MPa) | Fermentation Rate Constant (k, h−1) | ||
|---|---|---|---|---|
| H+ Concentration | Titratable Acidity | Reducing Sugar Concentration | ||
| 17 | 0.1 | 0.272 P(−)/(3.14) T | 0.050 (−)/(3.16) | 0.011 |
| 7 | 0.230 (1.18)/(−) | 0.040 (1.25)/(−) | 0.015 (0.73) | |
| 15 | 0.222 (1.23)/(3.47) | 0.027 (1.85)/(5.89) | 0.009 (1.22) | |
| 30 | 0.138 (1.97)/(−) | 0.023 (2.17)/(−) | 0.004 (2.75) | |
| 50 | 0.042 (6.48)/(9.95) | 0.006 (8.33)/(12.17) | 0.003 (3.67) | |
| 25 | 0.1 | 0.638 (−)/(1.34) | 0.099 (−)/(1.60) | NE § |
| 15 | 0.572 (1.12)/(1.35) | 0.116 (0.85)/(1.37) | ||
| 50 | 0.233 (2.74)/(1.79) | 0.040 (2.48)/(1.83) | ||
| 32 | 0.1 | 0.854 | 0.158 | NE |
| 15 | 0.771 (1.11)/(−) | 0.159 (0.99)/(−) | ||
| 50 | 0.418 (2.04)/(−) | 0.073 (2.16)/(−) | ||
| Physicochemical Parameter | Temperature (°C) | Activation Volume (cm3/mol)–R2 |
|---|---|---|
| H+ concentration | 17 | 83.86–0.89 |
| 25 | 52.46–0.96 | |
| 32 | 37.76–0.97 | |
| Titratable acidity | 17 | 96.88–0.94 |
| 25 | 50.45–0.82 | |
| 32 | 42.33–0.91 | |
| Reducing sugar concentration | 17 | 77.72–0.88 |
| Physicochemical Parameter | Pressure (MPa) | Activation Energy (kJ/mol)–R2 |
|---|---|---|
| H+ Concentration | 0.1 | 56.83–0.95 |
| 15 | 61.90–0.94 | |
| 50 | 114.1–0.95 | |
| Titratable Acidity | 0.1 | 56.63–0.99 |
| 15 | 88.48–0.91 | |
| 50 | 124.2–0.94 |
| Conditions | Lactose (mg/g) | Substrate Consumption (%) | Glucose (mg/g) | Galactose (mg/g) | |||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 0 h | 24 h | 0 h | 24 h | ||
| 0.1 MPa | 32.49 ± 1.47 | 27.09 ± 2.14 | 17 | 0.99 ± 0.01 | 0.99 ± 0.04 | BQL ‡ | BQL |
| 7 MPa | 29.37 ± 1.65 | 10 | 1.03 ± 0.10 | 0.31 ± 0.05 | |||
| 15 MPa | 28.37 ± 2.64 | 13 | 0.94 ± 0.12 | 0.22 ± 0.02 | |||
| 50 MPa | 29.32 ± 0.58 | 10 | 1.16 ± 0.16 | 0.41 ± 0.10 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.C.; Lemos, Á.T.; Lopes, R.P.; Mota, M.J.; Inácio, R.S.; Gomes, A.M.P.; Sousa, S.; Delgadillo, I.; Saraiva, J.A. The Combined Effect of Pressure and Temperature on Kefir Production—A Case Study of Food Fermentation in Unconventional Conditions. Foods 2020, 9, 1133. https://doi.org/10.3390/foods9081133
Ribeiro AC, Lemos ÁT, Lopes RP, Mota MJ, Inácio RS, Gomes AMP, Sousa S, Delgadillo I, Saraiva JA. The Combined Effect of Pressure and Temperature on Kefir Production—A Case Study of Food Fermentation in Unconventional Conditions. Foods. 2020; 9(8):1133. https://doi.org/10.3390/foods9081133
Chicago/Turabian StyleRibeiro, Ana C., Álvaro T. Lemos, Rita P. Lopes, Maria J. Mota, Rita S. Inácio, Ana M. P. Gomes, Sérgio Sousa, Ivonne Delgadillo, and Jorge A. Saraiva. 2020. "The Combined Effect of Pressure and Temperature on Kefir Production—A Case Study of Food Fermentation in Unconventional Conditions" Foods 9, no. 8: 1133. https://doi.org/10.3390/foods9081133
APA StyleRibeiro, A. C., Lemos, Á. T., Lopes, R. P., Mota, M. J., Inácio, R. S., Gomes, A. M. P., Sousa, S., Delgadillo, I., & Saraiva, J. A. (2020). The Combined Effect of Pressure and Temperature on Kefir Production—A Case Study of Food Fermentation in Unconventional Conditions. Foods, 9(8), 1133. https://doi.org/10.3390/foods9081133







