Osmodehydrofreezing: An Integrated Process for Food Preservation during Frozen Storage
Abstract
1. Introduction
2. The Use of Alternative Osmotic Media in the Osmotic Dehydration Treatment, Prior to Freezing
3. Mass Transport and Heat Transfer Modeling during ODF
4. Complementary Treatments to Improve Osmotic Dehydration Effects
5. Quality Degradation during Frozen Storage of ODF Products: Kinetic Modeling of Important Quality Indices
5.1. Shelf Life Indices of Osmodehydrofrozen Foods
5.1.1. Vitamin C
5.1.2. Color
5.1.3. Structure and Texture
5.1.4. Drip Loss
5.1.5. Chlorophyll, Lycopene, Anthocyanins, and Other Pigments
5.1.6. Sensory Attributes
5.2. Shelf Life Modeling during Frozen Storage of ODF Food
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Van Buggenhout, S.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Use of pectinmethylesterase and calcium in osmotic dehydration and osmodehydrofreezing of strawberries. Eur. Food Res. Technol. 2008, 226, 1145–1154. [Google Scholar] [CrossRef]
- Torreggiani, D. Osmotic dehydration in fruit and vegetable processing. Food Res. Int. 1993, 26, 59–68. [Google Scholar] [CrossRef]
- Torreggiani, D.; Bertolo, G. Present and Future in Process Control and Optimization of Osmotic Dehydration: From Unit Operation to Innovative Combined Process: An Overview. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2004; Volume 48, pp. 173–238. [Google Scholar]
- Torreggiani, D.; Bertolo, G. Osmotic pre-treatments in fruit processing: Chemical, physical and structural effects. J. Food Eng. 2001, 49, 247–253. [Google Scholar] [CrossRef]
- Torreggiani, D.; Forni, E.; Erba, M.L.; Longoni, F. Functional properties of pepper osmodehydrated in hydrolyzed cheese whey permeate with or without sorbitol. Food Res. Int. 1995, 28, 161–166. [Google Scholar] [CrossRef]
- Xiao, M.; Bi, J.; Yi, J.; Zhao, Y.; Peng, J.; Zhou, L.; Chen, Q. Osmotic pretreatment for instant controlled pressure drop dried apple chips: Impact of the type of saccharides and treatment conditions. Dry. Technol. 2019, 37, 896–905. [Google Scholar] [CrossRef]
- Da Silva, A.F., Jr.; da Silva, W.P.; de Farias Aires, J.E.; Aires, K.L.C.A.F.; de Castro, D.S. Osmotic dehydration kinetics of banana slices considering variable diffusivities and shrinkage. Int. J. Food Prop. 2017, 20, 1313–1325. [Google Scholar] [CrossRef]
- Mokhtar, W.M.F.W.; Ghawi, S.K.; Niranjan, K. Dehydration of potato slices following brief dipping in osmotic solutions: Effect of conditions and understanding the mechanism of water loss. Dry. Technol. 2019, 37, 885–895. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Pantelaiaki, K.; Andreou, V.; Katsaros, G.J.; Taoukis, P.S. Osmotic pretreatment for the production of novel dehydrated tomatoes and cucumbers. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Mathematical Modelling of Osmotic Dehydration Kinetics of Apple Cubes. J. Food Process. Preserv. 2017, 41, e12895. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Mass Transfer in Osmotic Dehydration of Food Products: Comparison Between Mathematical Models. Food Eng. Rev. 2016, 8, 116–133. [Google Scholar] [CrossRef]
- De Farias Aires, J.E.; da Silva, W.P.; Aires, K.L.C.d.A.F.; da Silva, A.F., Jr.; Silva, C.M.D.P.d.S. Description of osmotic dehydration of apple using two-dimensional diffusion models considering shrinkage and variations in process parameters. Dry. Technol. 2017, 35, 815–826. [Google Scholar] [CrossRef]
- Pacheco-Angulo, H.; Herman-Lara, E.; García-Alvarado, M.A.; Ruiz-López, I.I. Mass transfer modeling in osmotic dehydration: Equilibrium characteristics and process dynamics under variable solution concentration and convective boundary. Food Bioprod. Process. 2016, 97, 88–99. [Google Scholar] [CrossRef]
- Zecchi, B.; Gerla, P. Effective diffusion coefficients and mass flux ratio during osmotic dehydration considering real shape and shrinkage. J. Food Eng. 2020, 274, 109821. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S. Mass transfer during osmotic dehydration of pineapple: Considering Fickian diffusion in cubical configuration. LWT Food Sci. Technol. 2004, 37, 43–47. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S. Mass Transfer During Osmotic Dehydration: Determination of Moisture and Solute Diffusion Coefficients from Concentration Profiles. Food Bioprod. Process. 2004, 82, 44–48. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Niranjan, K. Chapter 11—Recent Developments in Osmotic Dehydration. In Emerging Technologies for Food Processing, 2nd ed.; Sun, D.-W., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 181–212. [Google Scholar]
- Chiralt, A.; Martínez-Navarrete, N.; Martínez-Monzó, J.; Talens, P.; Moraga, G.; Ayala, A.; Fito, P. Changes in mechanical properties throughout osmotic processes: Cryoprotectant effect. J. Food Eng. 2001, 49, 129–135. [Google Scholar] [CrossRef]
- Forni, E.; Sormani, A.; Scalise, S.; Torreggiani, D. The influence of sugar composition on the colour stability of osmodehydrofrozen intermediate moisture apricots. Food Res. Int. 1997, 30, 87–94. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Cuadros, T.R.M.; Aguilera, J.M. Glass transitions and shrinkage during drying and storage of osmosed apple pieces. Food Res. Int. 1998, 31, 191–204. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Ding, Y.; Yuan, Y.-J.; Xiao, H.-W.; Zhou, C.-L.; Tan, M.-L.; Tang, X. Effect of osmotic dehydration on desorption isotherms and glass transition temperatures of mango. Int. J. Food Sci. Technol. 2018, 53, 2602–2609. [Google Scholar] [CrossRef]
- Sette, P.; Salvatori, D.; Schebor, C. Physical and mechanical properties of raspberries subjected to osmotic dehydration and further dehydration by air- and freeze-drying. Food Bioprod. Process. 2016, 100, 156–171. [Google Scholar] [CrossRef]
- Goula, A.M.; Lazarides, H.N. Modeling of mass and heat transfer during combined processes of osmotic dehydration and freezing (Osmo-Dehydro-Freezing). Chem. Eng. Sci. 2012, 82, 52–61. [Google Scholar] [CrossRef]
- Fernández, P.R.; Lovera, N.; Ramallo, L.A. Sucrose syrup reuse during one- and multi-stage osmotic dehydration of pineapple. J. Food Process. Eng. 2020, 43, e13399. [Google Scholar] [CrossRef]
- Aachary, A.A.; Prapulla, S.G. Value addition to spent osmotic sugar solution (SOS) by enzymatic conversion to fructooligosaccharides (FOS), a low calorie prebiotic. Innov. Food Sci. Emerg. Technol. 2009, 10, 284–288. [Google Scholar] [CrossRef]
- Kucner, A.; Papiewska, A.; Sójka, M.; Klewicki, R. Chemical and Microbiological Changes in Blueberries and in Hypertonic Solution during Osmotic Dehydration Employing Reused Concentrate. J. Food Process. Eng. 2013, 36, 608–618. [Google Scholar] [CrossRef]
- Marani, C.; Agnelli, M.; Mascheroni, R. Osmo-frozen fruits: Mass transfer and quality evaluation. J. Food Eng. 2007, 79, 1122–1130. [Google Scholar] [CrossRef]
- Ramallo, L.A.; Mascheroni, R.H. Dehydrofreezing of pineapple. J. Food Eng. 2010, 99, 269–275. [Google Scholar] [CrossRef]
- Said, L.B.H.; Bellagha, S.; Allaf, K. Dehydrofreezing of Apple Fruits: Freezing Profiles, Freezing Characteristics, and Texture Variation. Food Bioprocess Technol. 2015, 9, 252–261. [Google Scholar] [CrossRef]
- Said, L.B.H.; Bellagha, S.; Allaf, K. Measurements of texture, sorption isotherms and drying/rehydration kinetics of dehydrofrozen-textured apple. J. Food Eng. 2015, 165, 22–33. [Google Scholar] [CrossRef]
- Said, L.B.H.; Bellagha, S.; Allaf, K. Optimization of Instant Controlled Pressure Drop (DIC)-Assisted Dehydrofreezing Using Mechanical Texture Measurements Versus Initial Water Content of Apple. Food Bioprocess Technol. 2015, 8, 1102–1112. [Google Scholar] [CrossRef]
- James, C.; Purnell, G.; James, S.J. A Critical Review of Dehydrofreezing of Fruits and Vegetables. Food Bioprocess Technol. 2014, 7, 1219–1234. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.; Taoukis, P.S. Kinetic study of the effect of the osmotic dehydration pre-treatment with alternative osmotic solutes to the shelf life of frozen strawberry. Food Bioprod. Process. 2016, 99, 212–221. [Google Scholar] [CrossRef]
- De Gennaro, S.; Birch, G.G.; Parke, S.A.; Stancher, B. Studies on the physicochemical properties of inulin and inulin oligomers. Food Chem. 2000, 68, 179–183. [Google Scholar] [CrossRef]
- Roberfroid, M. Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Crit. Rev. Food Sci. Nutr. 1993, 33, 103–148. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.H.; Brockbank, K.G.M. Culturing with trehalose produces viable endothelial cells after cryopreservation. Cryobiology 2012, 64, 240–244. [Google Scholar] [CrossRef]
- Lowithun, N.; Charoenrein, S. Influence of osmodehydrofreezing with different sugars on the quality of frozen rambutan. Int. J. Food Sci. Technol. 2009, 44, 2183–2188. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Taoukis, P.S. Stability of dehydrofrozen green peas pretreated with nonconventional osmotic agents. J. Food Sci. 2003, 68, 2002–2010. [Google Scholar] [CrossRef]
- Cichowska-Bogusz, J.; Figiel, A.; Stasiak-Różańska, L.; Witrowa-Rajchert, D. Modeling of Osmotic Dehydration of Apples in Sugar Alcohols and Dihydroxyacetone (DHA) Solutions. Foods 2019, 8, 20. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C.; Taoukis, P.S. Stability of dehydrofrozen tomatoes pretreated with alternative osmotic solutes. J. Food Eng. 2007, 78, 272–280. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C.; Taoukis, P.S. Kinetic modelling of the degradation of quality of osmo-dehydrofrozen tomatoes during storage. Food Chem. 2007, 103, 985–993. [Google Scholar] [CrossRef]
- Suutarinen, M.; Autio, K. 16—Improving the texture of frozen fruit: The case of berries. In Texture in Food; Kilcast, D., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 388–409. [Google Scholar]
- Gras, M.L.; Vidal, D.; Betoret, N.; Chiralt, A.; Fito, P. Calcium fortification of vegetables by vacuum impregnation. J. Food Eng. 2003, 56, 279–284. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Semenoglou, I.; Dimopoulos, G.; Tsironi, T.; Taoukis, P. Mathematical modelling of the effect of solution concentration and the combined application of pulsed electric fields on mass transfer during osmotic dehydration of sea bass fillets. Food Bioprod. Process. 2020, 121, 186–192. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C. Evaluation and modelling of osmotic pre-treatment of peach using alternative agents in a multiple-component solution. J. Sci. Food Agric. 2018, 99, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Dermesonlouoglou, E.K.; Giannakourou, M.C. Modelling dehydration of apricot in a non-conventional multi-component osmotic solution: Effect on mass transfer kinetics and quality characteristics. J. Food Sci. Technol. 2018, 55, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Lemus-Mondaca, R.; Zambra, C.; Marín, F.; Perez-Won, M.; Tabilo-Munizaga, G. Mass Transfer Kinetic and Quality Changes During High-Pressure Impregnation (HPI) of Jumbo Squid (Dosidicus gigas) Slices. Food Bioprocess Technol. 2018, 11, 1516–1526. [Google Scholar] [CrossRef]
- Junqueira, J.R.D.J.; Correa, J.; De Mendonça, K.S. Evaluation of the Shrinkage Effect on the Modeling Kinetics of Osmotic Dehydration of Sweet Potato (Ipomoea batatas (L.)). J. Food Process. Preserv. 2017, 41, e12881. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Braga, T.R.; Silva, E.O.; Rodrigues, S. Use of ultrasound for dehydration of mangoes (Mangifera indica L.): Kinetic modeling of ultrasound-assisted osmotic dehydration and convective air-drying. J. Food Sci. Technol. 2019, 56, 1793–1800. [Google Scholar] [CrossRef]
- Azuara, E.; Cortés, R.; García, H.S.; Beristain, C.I. Kinetic model for osmotic dehydration and its relationship with Fick’s second law. Int. J. Food Sci. Technol. 2007, 27, 409–418. [Google Scholar] [CrossRef]
- Shinde, B.; Ramaswamy, H. Kinetic modeling of microwave osmotic dehydration of mangoes under continuous flow medium spray conditions using sucrose and maltodextrin (10-18 DE) solute mixtures. Dry. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Hamedi, F.; Mohebbi, M.; Shahidi, F.; Azarpazhooh, E. Ultrasound-Assisted Osmotic Treatment of Model Food Impregnated with Pomegranate Peel Phenolic Compounds: Mass Transfer, Texture, and Phenolic Evaluations. Food Bioprocess Technol. 2018, 11, 1061–1074. [Google Scholar] [CrossRef]
- Peleg, M. An Empirical Model for the Description of Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Latocha, P.; Gondek, E. Mass Transfer in Osmotic Dehydration of Kiwiberry: Experimental and Mathematical Modelling Studies. Molecules 2018, 23, 1236. [Google Scholar] [CrossRef] [PubMed]
- Page, G.E. Factors Influencing the Maximum Rate of Air Drying Shelled Corn in Thin-Layers. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 1949. [Google Scholar]
- Cunha, L.M.; Oliveira, F.A.; Aboim, A.P.; Frias, J.M.; Pinheiro-Torres, A. Stochastic approach to the modelling of water losses during osmotic dehydration and improved parameter estimation. Int. J. Food Sci. Technol. 2001, 36, 253–262. [Google Scholar] [CrossRef]
- Aguirre-García, M.; Hernández-Carranza, P.; Cortés-Zavaleta, O.; Ruiz-Espinosa, H.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Mass transfer analysis of bioactive compounds in apple wedges impregnated with beetroot juice: A 3D modelling approach. J. Food Eng. 2020, 282, 110003. [Google Scholar] [CrossRef]
- Ramya, V.; Jain, N.K. A Review on Osmotic Dehydration of Fruits and Vegetables: An Integrated Approach. J. Food Process. Eng. 2016, 40, e12440. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Pourgouri, S.; Taoukis, P.S. Kinetic study of the effect of the osmotic dehydration pre-treatment to the shelf life of frozen cucumber. Innov. Food Sci. Emerg. Technol. 2008, 9, 542–549. [Google Scholar] [CrossRef]
- Agnelli, M.E.; Marani, C.M.; Mascheroni, R. Modelling of heat and mass transfer during (osmo) dehydrofreezing of fruits. J. Food Eng. 2005, 69, 415–424. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, F.; Zhua, D.; Zhao, N.; Liu, L. Effect of different sugars on the freezing characteristics of Kyoho grape. J. Texture Stud. 2018, 49, 604–611. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Liu, F.; Pang, X.-L.; Xiao, H.-W.; Wen, X.; Ni, Y.-Y. Effects of different osmo-dehydrofreezing treatments on the volatile compounds, phenolic compounds and physicochemical properties in mango (Mangifera indica L.). Int. J. Food Sci. Technol. 2016, 51, 1441–1448. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Hu, R.; Xiao, H.-W.; Yang, Y.; Liu, F.; Gan, Z.-L.; Ni, Y.-Y. Osmotic dehydration pretreatment for improving the quality attributes of frozen mango: Effects of different osmotic solutes and concentrations on the samples. Int. J. Food Sci. Technol. 2014, 49, 960–968. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.; Taoukis, P. Kinetic modelling of the quality degradation of frozen watermelon tissue: Effect of the osmotic dehydration as a pre-treatment. Int. J. Food Sci. Technol. 2007, 42, 790–798. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Freezing Characteristics and Storage Stability of Broccoli (Brassica oleracea L. var. botrytis L.) Under Osmodehydrofreezing and Ultrasound-Assisted Osmodehydrofreezing Treatments. Food Bioprocess Technol. 2013, 7, 1736–1744. [Google Scholar] [CrossRef]
- Li, J.; Chotiko, A.; Kyereh, E.; Zhang, J.; Liu, C.; Ortega, V.V.R.; Bankston, D.; Sathivel, S. Development of a Combined Osmotic Dehydration and Cryogenic Freezing Process for Minimizing Quality Changes During Freezing with Application to Fruits and Vegetables. J. Food Process. Preserv. 2016, 41, e12926. [Google Scholar] [CrossRef]
- Tsironi, T.; Taoukis, P.S. Effect of storage temperature and osmotic pre-treatment with alternative solutes on the shelf-life of gilthead seabream (Sparus aurata) fillets. Aquac. Fish. 2017, 2, 39–47. [Google Scholar] [CrossRef]
- Parniakov, O.; Bals, O.; Lebovka, N.; Vorobiev, E. Effects of pulsed electric fields assisted osmotic dehydration on freezing-thawing and texture of apple tissue. J. Food Eng. 2016, 183, 32–38. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Zachariou, I.; Andreou, V.; Taoukis, P.S. Quality assessment and shelf life modeling of pulsed electric field pretreated osmodehydrofrozen kiwifruit slices. Int. J. Food Stud. 2018, 7, 34–51. [Google Scholar] [CrossRef]
- Tregunno, N.B.; Goff, H.D. Osmodehydrofreezing of apples: Structural and textural effects. Food Res. Int. 1996, 29, 471–479. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, Y. Use of vacuum impregnation to develop high quality and nutritionally fortified frozen strawberries. J Food Process. Preserv. 2004, 28, 117–132. [Google Scholar] [CrossRef]
- Siramard, S.; Charoenrein, S. Effect of ripening stage and infusion with calcium lactate and sucrose on the quality and microstructure of frozen mango. Int. J. Food Sci. Technol. 2014, 49, 2136–2141. [Google Scholar] [CrossRef]
- Lovera, N.N.; Ramallo, L.; Salvadori, V.O. Effects of different freezing methods on calcium enriched papaya (Carica papaya L.). J. Food Sci. Technol. 2018, 55, 2039–2047. [Google Scholar] [CrossRef]
- Blanda, G.; Cerretani, L.; Cardinali, A.; Bendini, A.; Lercker, G. Effect of frozen storage on the phenolic content of vacuum impregnated Granny Smith and Stark Delicious apple cvv. Eur. Food Res. Technol. 2007, 227, 961–964. [Google Scholar] [CrossRef]
- Terkmane, N.; Krea, M.; Moulai-Mostefa, N. Optimisation of inulin extraction from globe artichoke (Cynara cardunculus L. subsp. scolymus (L.) Hegi.) by electromagnetic induction heating process. Int. J. Food Sci. Technol. 2016, 51, 1997–2008. [Google Scholar] [CrossRef]
- Martinez-Monzo, J.; Martinez-Navarette, N.; Chiralt, A.; Fito, P. Combined Vacuum Impregnation Osmotic Dehydration in Fruit Cryoprotection. In Osmotic Dehydration and Vacuum Impregnation; Fito, P., Chiralt, A., Barat, J., Spiess, W.E.L., Behsnilian, D., Eds.; Technomic Publishing Co.: Lancaster, PA, USA, 2001. [Google Scholar]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Rosa, M.D.; Witrowa-Rajchert, D. Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, M.; Wang, W.; Bhandari, B. A novel method of osmotic-dehydrofreezing with ultrasound enhancement to improve water status and physicochemical properties of kiwifruit. Int. J. Refrig. 2020, 113, 49–57. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Cheng, X. Influence of Ultrasound-Assisted Osmotic Dehydration and Freezing on the Water State, Cell Structure, and Quality of Radish (Raphanus sativus L.) Cylinders. Dry. Technol. 2014, 32, 1803–1811. [Google Scholar] [CrossRef]
- Nuñez-Mancilla, Y.; Pérez-Won, M.; Uribe, E.; Vega-Gálvez, A.; Di Scala, K. Osmotic dehydration under high hydrostatic pressure: Effects on antioxidant activity, total phenolics compounds, vitamin C and colour of strawberry (Fragaria vesca). LWT Food Sci. Technol. 2013, 52, 151–156. [Google Scholar] [CrossRef]
- Nuñez-Mancilla, Y.; Pérez-Won, M.; Vega-Gálvez, A.; Arias, V.; Tabilo-Munizaga, G.; Briones-Labarca, V.; Lemus, R.A.; Di Scala, K. Modeling mass transfer during osmotic dehydration of strawberries under high hydrostatic pressure conditions. Innov. Food Sci. Emerg. Technol. 2011, 12, 338–343. [Google Scholar] [CrossRef]
- Sulistyawati, I.; Dekker, M.; Fogliano, V.; Verkerk, R. Osmotic dehydration of mango: Effect of vacuum impregnation, high pressure, pectin methylesterase and ripeness on quality. LWT Food Sci. Technol. 2018, 98, 179–186. [Google Scholar] [CrossRef]
- Dash, K.K.; Balasubramaniam, V.M.; Kamat, S. High pressure assisted osmotic dehydrated ginger slices. J. Food Eng. 2019, 247, 19–29. [Google Scholar] [CrossRef]
- Farr, D. High pressure technology in the food industry. Trends Food Sci. Technol. 1990, 1, 14–16. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Angersbach, A.; Knorr, D. Synergistic effect of high hydrostatic pressure pretreatment and osmotic stress on mass transfer during osmotic dehydration. J. Food Eng. 2000, 45, 25–31. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Bimpilas, A.; Andreou, V.; Katsaros, G.J.; Giannakourou, M.C.; Taoukis, P.S. Process Optimization and Kinetic Modeling of Quality of Fresh-Cut Strawberry Cubes Pretreated by High Pressure and Osmosis. J. Food Process. Preserv. 2016, 41, 13137. [Google Scholar] [CrossRef]
- Blanda, G.; Cerretani, L.; Cardinali, A.; Boselli, E.; Bendini, A. Mass transfer and phenolic profile of strawberries upon refrigerated osmodehydration Transferencia de masa y perfil fenólico de las fresas cuando son osmo-deshidratadas por refrigeración. CyTA J. Food 2010, 8, 129–138. [Google Scholar] [CrossRef]
- Mahato, S.; Zhu, Z.; Sun, D.-W. Glass transitions as affected by food compositions and by conventional and novel freezing technologies: A review. Trends Food Sci. Technol. 2019, 94, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Xiao, H.-W.; Ding, Y.; Nie, Y.; Zhang, Y.; Zhu, Z.; Tang, X.-M. Effect of osmotic dehydration pretreatment and glassy state storage on the quality attributes of frozen mangoes under long-term storage. J. Food Sci. Technol. 2017, 54, 1527–1537. [Google Scholar] [CrossRef]
- Lim, M.; Wu, H.; Breckell, M.; Birch, J. Influence of the glass transition and storage temperature of frozen peas on the loss of quality attributes. Int. J. Food Sci. Technol. 2006, 41, 507–512. [Google Scholar] [CrossRef]
- Parreño, W.C.; Torres, M.D.A. Quality and Safety of Frozen Vegetables. In Handbook of Frozen Food Processing and Packaging; Sun, D.W., Ed.; Taylor & Francis Group LLC: Boca Raton, CA, USA, 2006; pp. 378–415. [Google Scholar]
- Giannakourou, M.C.; Taoukis, P.S. Kinetic modelling of vitamin C loss in frozen green vegetables under variable storage conditions. Food Chem. 2003, 83, 33–41. [Google Scholar] [CrossRef]
- Fito, P.; Chiralt, A.; Barat, J.M.; Spiess, W.E.L.; Behsnilian, D. Osmotic Dehydration and Vacuum Impregnation; Technomic Publising Co.: Lancaster, PA, USA, 2001. [Google Scholar]
- Robbers, M.; Singh, R.P.; Cunha, L.M. Osmotic-Convective Dehydrofreezing Process for Drying Kiwifruit. J. Food Sci. 1997, 62, 1039–1056. [Google Scholar] [CrossRef]
- Van Arsdel, W.B.; Copley, M.J.; Olson, R.L. Quality and Stability of Frozen Foods: Time, Temperature Tolerance and Its Significance; Wiley & Sons: New York, NY, USA, 1969; pp. 117–141. [Google Scholar]
- Kerr, W.L. Frozen food texture. In Handbook of Food Science, Technology and Engineering; Hui, Y.H., Ed.; CRC Press: Boca Raton, CA, USA, 2006; Volume 2. [Google Scholar]
- Olatidoye, O.; Sobowale, S.; Akinlua, O. Effect of osmodehydrofreezing on the quality attributes of frozen tomato. Electron. J. Environ. Agric. Food Chem. 2010, 9, 780–789. [Google Scholar]
- Talens, P.; Martínez-Navarrete, N.; Fito, P.; Chiralt, A. Changes in optical and mechanical properties during osmodehydrofreezing of kiwi fruit. Innov. Food Sci. Emerg. Technol. 2002, 3, 191–199. [Google Scholar] [CrossRef]
- Erba, M.L.; Forni, E.; Colonello, A.; Giangiacomo, R. Influence of sugar composition and air dehydration levels on the chemical-physical characteristics of osmodehydrofrozen fruit. Food Chem. 1994, 50, 69–73. [Google Scholar] [CrossRef]
- Ayala-Aponte, A.; Sánchez-Tamayo, M.I. Changes in liquid phase and mechanical properties during osmodehydrofreezing of papaya (Carica papaya L.). DYNA 2017, 84, 208–213. [Google Scholar] [CrossRef]
- Ayala-Aponte, A.; Cadena-G, M.I. The influence of osmotic pretreatments on melon (Cucumis melo L.) quality during frozen storage. DYNA 2014, 81, 81–86. [Google Scholar] [CrossRef]
- Shahidi, F.; Amidi, F.; Mohebbi, M.; Kouchaki, A. A perception to osmodehydrofreezing: A novel approach in cantaloupe processing. Acta Horticulturae 2007, 731, 463–466. [Google Scholar] [CrossRef]
- Torreggiani, D.; Maestrelli, A. Quality and Safety of Frozen Fruits. In Handbook of Frozen Food Processing and Packaging; Sun, D.W., Ed.; Taylor & Francis Group LLC: Boca Raton, CA, USA, 2006; pp. 417–440. [Google Scholar]
- Rizzolo, A.; Nani, R.C.; Viscardi, D.; Bertolo, G.; Torreggiani, D. Modification of glass transition temperature through carbohydrates addition and anthocyanin and soluble phenol stability of frozen blueberry juices. J. Food Eng. 2003, 56, 229–231. [Google Scholar] [CrossRef]
- Torreggiani, D.; Forni, E.; Guercilena, I.; Maestrelli, A.; Bertolo, G.; Archer, G.; Kennedy, C.; Bone, S.; Blond, G.; Contreras-Lopez, E.; et al. Modification of glass transition temperature through carbohydrates additions: Effect upon colour and anthocyanin pigment stability in frozen strawberry juices. Food Res. Int. 1999, 32, 441–446. [Google Scholar] [CrossRef]
- Peleg, M. On the use of the WLF model in polymers and foods. Crit. Rev. Food Sci. Nutr. 1992, 32, 59–66. [Google Scholar] [CrossRef]
- Peleg, M.; Engel, R.; Gonzalez-Martinez, C.; Corradini, M.G. Non-Arrhenius and non-WLF kinetics in food systems. J. Sci. Food Agric. 2002, 82, 1346–1355. [Google Scholar] [CrossRef]
- Dellino, G.; Mari, R.; Meloni, C. Waste Reduction in Fresh Food Supply Chains: An Operations Research Approach. In Food Waste Reduction and Valorisation: Sustainability Assessment and Policy Analysis; Morone, P., Papendiek, F., Tartiu, V.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 235–259. [Google Scholar]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef]
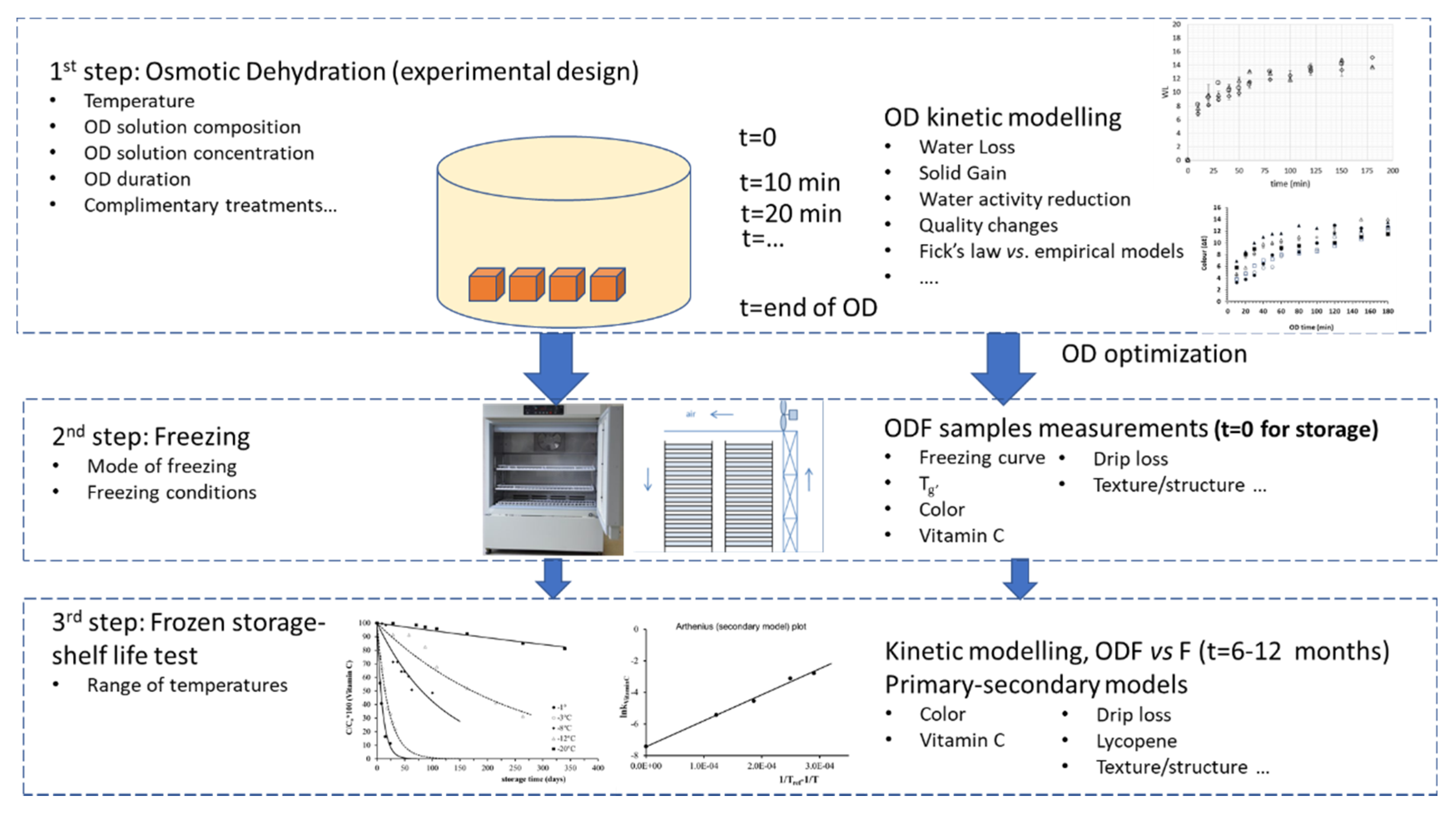
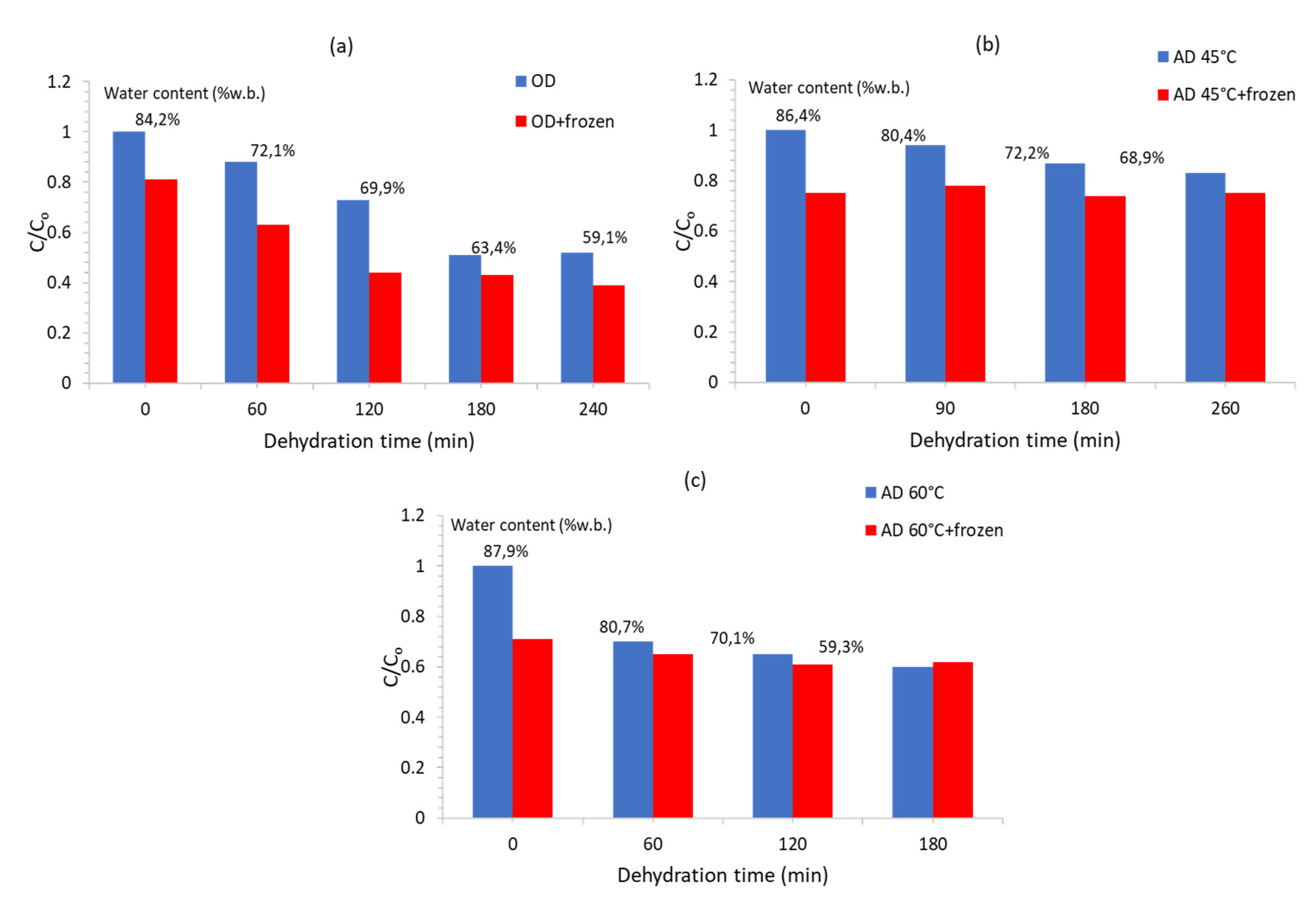
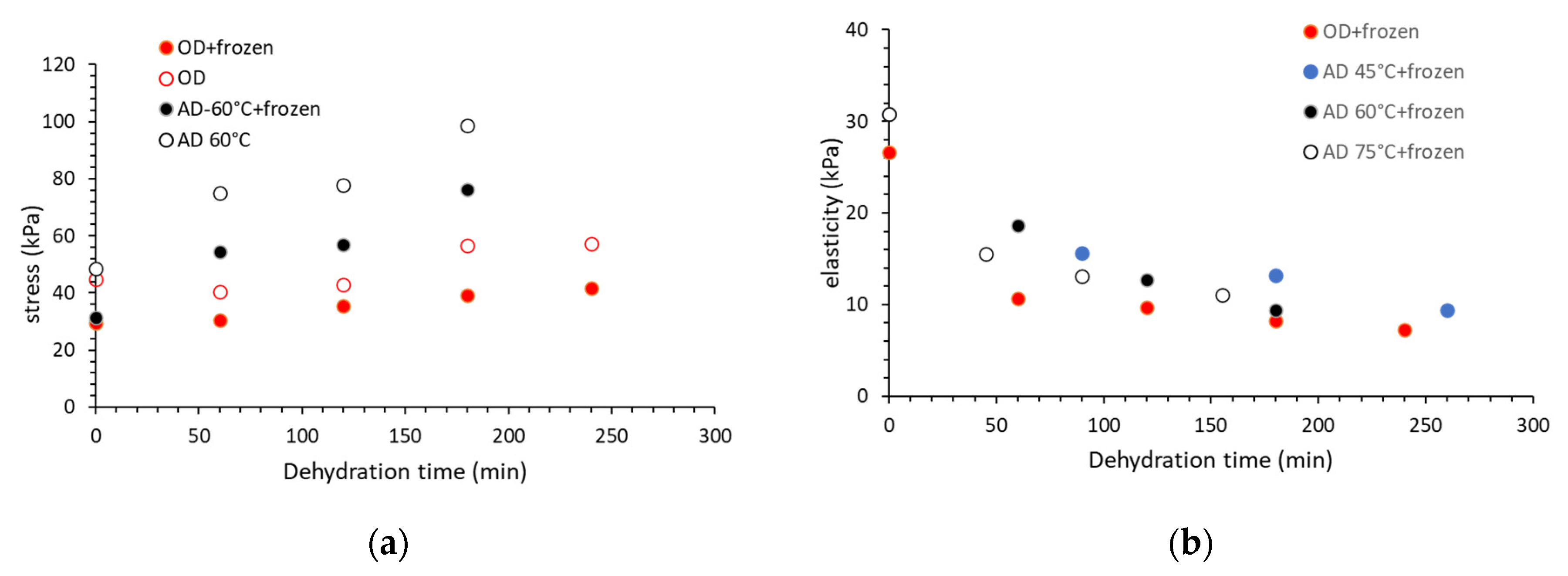
| Carbohydrate/Salt | Product, (Concentration (% W.T), OD Temperature and Duration) | Reference |
|---|---|---|
| Glucose | Koho grape (40%, 20 °C, 60 min) | [62] |
| Mangoes (45%, 30 °C, 2 h) | [63,64] | |
| Strawberry (56.5%, 35 °C, 150 min) | [33] | |
| Tomato (56.5%, 35 °C, 60 min) | [40] | |
| Watermelon (50%, 35 °C, 60 min) | [65] | |
| Trehalose | Koho grape (40%, 20 °C, 60 min) | [62] |
| Broccoli (40%, 35 °C, 120 min) | [66] | |
| Rambutan (50%, 30 °C, 5 h) | [37] | |
| Lactose | Koho grape (20%, 20 °C, 60 min) | [62] |
| Maltodextrin | Tomato cubes (55%, 35 °C, 24 h) | [67] |
| Marinated gilthead seabream (40%, 15 °C, 6 h) | [68] | |
| Strawberry (56.5%, 35 °C, 150 min) | [33] | |
| Cucumber (56.5%, 15 °C−35 °C−55 °C for 360, 300, and 180 min, respectively) | [60] | |
| Tomato (56.5%, 35 °C, 60 min) | [40,41] | |
| Watermelon (50%, 35 °C, 60 min) | [65] | |
| Glycerol | Apple slices (0, 20, 40, 60 wt. %, 180 min) | [69] |
| Apricot (40–60%, 25–45 °C, 0–240 min) | [47] | |
| Cucumber (50%, 35 °C, 90 min) | [9] | |
| Peach (40–60%, 25–45 °C, 0–240 min) | [46] | |
| Kiwifruit (30%, 35–55 °C, 0–240 min) | [70] | |
| Tomato (50%, 35 °C, 90 min) | [9] | |
| Sorbitol | Apple slices (50 wt. %, 50 °C, 40 min) | [71] |
| Apricot (65%, 45 °C, 120 min) | [19] | |
| Maltose | Mangoes (45%, 30 °C, 2 h) | [63,64] |
| Apricot (65%, 45 °C, 120 min) | [19] | |
| High fructose corn syrup (HFCS) | Strawberries (50%, 25 °C, 45 min, vacuum pressure of 50 mmHg) | [72] |
| Oligofructose | Strawberry (56.5%, 35 °C, 150 min) | [33] |
| Cucumber (56.5%, 15 °C−35 °C−55 °C for 360, 300, and 180 min, respectively) | [60] | |
| Tomato (56.5%, 35 °C, 60 min) | [40] | |
| Watermelon (50%, 35 °C, 60 min) | [65] | |
| Green peas (56.5%, 35 °C, 4 h or 5 °C, 12 h) | [38] | |
| Maltitol | Rambutan (50%, 30 °C, 5 h) | [37] |
| Green peas (56.5%, 35 °C, 4 h or 5 °C, 12 h) | [38] | |
| Calcium lactate | Mangoes (1%, 25 °C, 1 h) | [73] |
| Papaya (1.5%, 45 °C, 4 and 8 h) | [74] | |
| Calcium gluconate | Papaya (1.5%, 45 °C, 4 h) | [74] |
| Mixture of calcium gluconate and calcium lactate | Strawberries (12%, 25 °C, 45 min, vacuum pressure of 50 mmHg) | [72] |
| NaCl/CaCl2 | Tomato (3.5 and 1.5%, 35 °C, 60 min) | [40,41] |
| Cucumber (3.5 and 1.5%, 15 °C−35 °C−55 °C for 360, 300, and 180 min, respectively) | [60] | |
| Ascorbic acid | Apricot (1%, 45 °C, 120 min) | [19] |
| Apple (1%, 25 °C, 30 min, vacuum pressure of 100 mbar) | [75] |
| Model Name | Mathematical Expression | Explanation of Symbols | Reference |
|---|---|---|---|
| Azuara | WL∞ and SG∞ are the terms that express the water loss/solid gain at equilibrium, and s1 and s2 are parameters that can be defined as relative rate constants for water loss and solids gain, respectively | [51] | |
| Crank (based on Fick’s second law for diffusion) | For spherical shape: For slabs | MR and SR are the diffused moisture and solute ratio, respectively; M and S are the moisture and solute content, respectively; the subscripts o, t, and ∞ represent the relevant values at time 0, t, and at equilibrium, respectively; Dew and Des (m2/s) are the effective coefficients of water and solute diffusivity, respectively; r (m) is the average radius of sphere; and L(m) is half the thickness of a slab (when OD occurs in both sides of the slab) | [44] |
| Peleg | M is the moisture at time t and M0 is the initial moisture (both in dry weight basis). The moisture sorption curves approach the equilibrium asymptotically using the parameters k1’ and k2’. is considered to be WL and SG, k1 and k2 are the Peleg’s constants for WL and SG. | [54] | |
| Page | A and B are Page’s constants. | [56] | |
| Weibull | τ is the scale parameter of the Weibull’s mode that is associated with the process rate (the time required for or to reach the value 1-e−1), and β is the shape parameter. | [57] | |
| Polynomial model | Y represents the selected response variables (for example, WL, SG, aw, quality attributes measured during OD) and Xi are the factor variables [i.e., the process parameters, for example, OD solution concentration, process temperature and time, and so on] αο is is the constant, αi represents the linear, αii the quadratic, and αij is the interaction effects of the factors. | [46,47,76] |
| Food Product | Quality Index | Storage Temperature | Primary Model | Shelf Life Calculations Based on Kinetic Information Provided | Reference |
|---|---|---|---|---|---|
| Kiwifruit (untreated, OD, and PEF-OD) | Vitamin C (in mg L-ascorbic acid/100 g fresh material) | −5, −10, −15, and −25 °C | First order | Shelf life at −18 °C (untreated, OD, and PEF-OD): 200, 450, and 500 days | [70] |
| Color | First order (where ΔE is expressed by Equation (6)) | Shelf life at −18 °C (untreated, OD, and PEF-OD): 90, 540, and 400 days | [70] | ||
| Overall sensory acceptance (scoring in a 1–9 descriptive intensity scale) | Zero order | Shelf life at −18 °C (untreated, OD, and PEF-OD): 280, 410, and 310 days | [70] | ||
| Marine cultured gilthead seabream *2 | 2-Thiobarbituric acid reactive substances, TBARS (in mg malonaldehyde/kg muscle). | 15, 10, 5, 2.5, 0, −1, and −3 °C | Shelf life at −3 °C (untreated and OD): 21 and 40 days | [68] | |
| Pseudomonas growth (in CFU/g) | Baranyi growth model | Shelf life at −3 °C (untreated and OD): 25 and 48 days | [68] | ||
| Total volatile basic nitrogen, TVBN (in mg N/100 g) | Shelf life at −3 °C (untreated and OD): 40 and 60 days | [68] | |||
| Overall sensory acceptance (scoring in a 1–9 descriptive intensity scale) | Shelf life at −3 °C (untreated and OD): 21 and 38 days | [68] | |||
| Strawberry | Vitamin C (in mg L-ascorbic acid/100 g fresh material) | −5, −8, −12, and −16 °C | Shelf life at −16 °C (untreated and OD with glucose, maltodextrin, and oligofructose): 74, 198, 237, and 594 days | [33] | |
| Color | (where ΔC is expressed by Equation (5)) | Shelf life at −16 °C (untreated and OD with glucose, maltodextrin, and oligofructose): 216, 495, 577, and 770 days | [33] | ||
| Cucumber | Color | −5, −8, −12, and −15 °C | First order (where ΔC expressed by Equation (5)) | Shelf life at −18 °C (untreated, OD with maltodextrin and oligofructose): 237, 402, and 331 days | [60] |
| Tomato | Color | −5, −8, −12, −15, and −20 °C | Shelf life at −18 °C (untreated, OD with maltodextrin): 133 and 161 days * | [41] | |
| Vitamin C (in mg L-ascorbic acid/100 g fresh material) | First order | Shelf life at −18 °C (untreated, OD with maltodextrin): 87 and 173 days * | [41] | ||
| Lycopene content (in mg/100 g initial content) | First order | Shelf life at −15 °C (untreated, OD with maltodextrin): 155 and 210 days * | [41] | ||
| Watermelon | Color | −5, −8, −12, −15, and −20 °C | [65] | ||
| Lycopene content (in mg/100 g initial content) | First order | Shelf life at −12 °C (untreated, OD with oligofructose): 142 and 209 days | [65] | ||
| Green peas | Color | −3, −5, −8, −12, −16, −18, and −24 °C | First order | Shelf life at −18 °C (untreated, OD with oligofructose, maltitol, mixture of oligofructose/trehalose): 223, 396, 325, and 357 days * | [38] |
| Vitamin C (in mg L-ascorbic acid/100 g fresh material) | First order | Shelf life at −18 °C (untreated, OD with oligofructose, maltitol, mixture of oligofructose/trehalose): 216, 347, 888, and 456 days * | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakourou, M.C.; Dermesonlouoglou, E.K.; Taoukis, P.S. Osmodehydrofreezing: An Integrated Process for Food Preservation during Frozen Storage. Foods 2020, 9, 1042. https://doi.org/10.3390/foods9081042
Giannakourou MC, Dermesonlouoglou EK, Taoukis PS. Osmodehydrofreezing: An Integrated Process for Food Preservation during Frozen Storage. Foods. 2020; 9(8):1042. https://doi.org/10.3390/foods9081042
Chicago/Turabian StyleGiannakourou, Maria C., Efimia K. Dermesonlouoglou, and Petros S. Taoukis. 2020. "Osmodehydrofreezing: An Integrated Process for Food Preservation during Frozen Storage" Foods 9, no. 8: 1042. https://doi.org/10.3390/foods9081042
APA StyleGiannakourou, M. C., Dermesonlouoglou, E. K., & Taoukis, P. S. (2020). Osmodehydrofreezing: An Integrated Process for Food Preservation during Frozen Storage. Foods, 9(8), 1042. https://doi.org/10.3390/foods9081042





