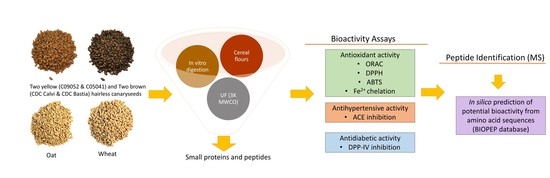
Health Promoting Bioactive Properties of Novel Hairless Canary Seed Flour after In Vitro Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis of Cereal Flours
2.3. Preparation of Cereal Flour Digestates
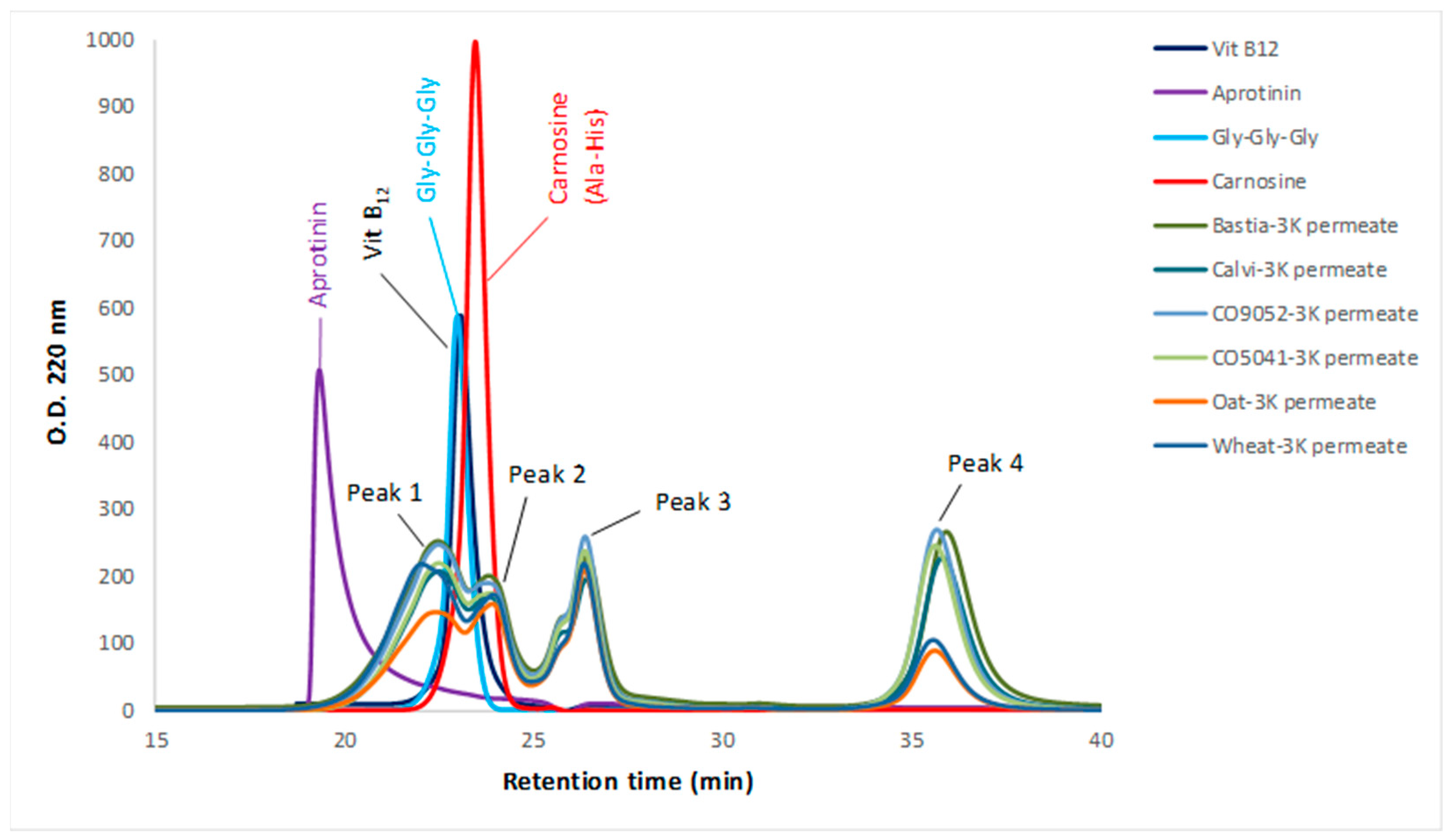
2.4. Characterization of the Molecular Weight Distribution of the 3K Permeates of Cereal Flours Digestates
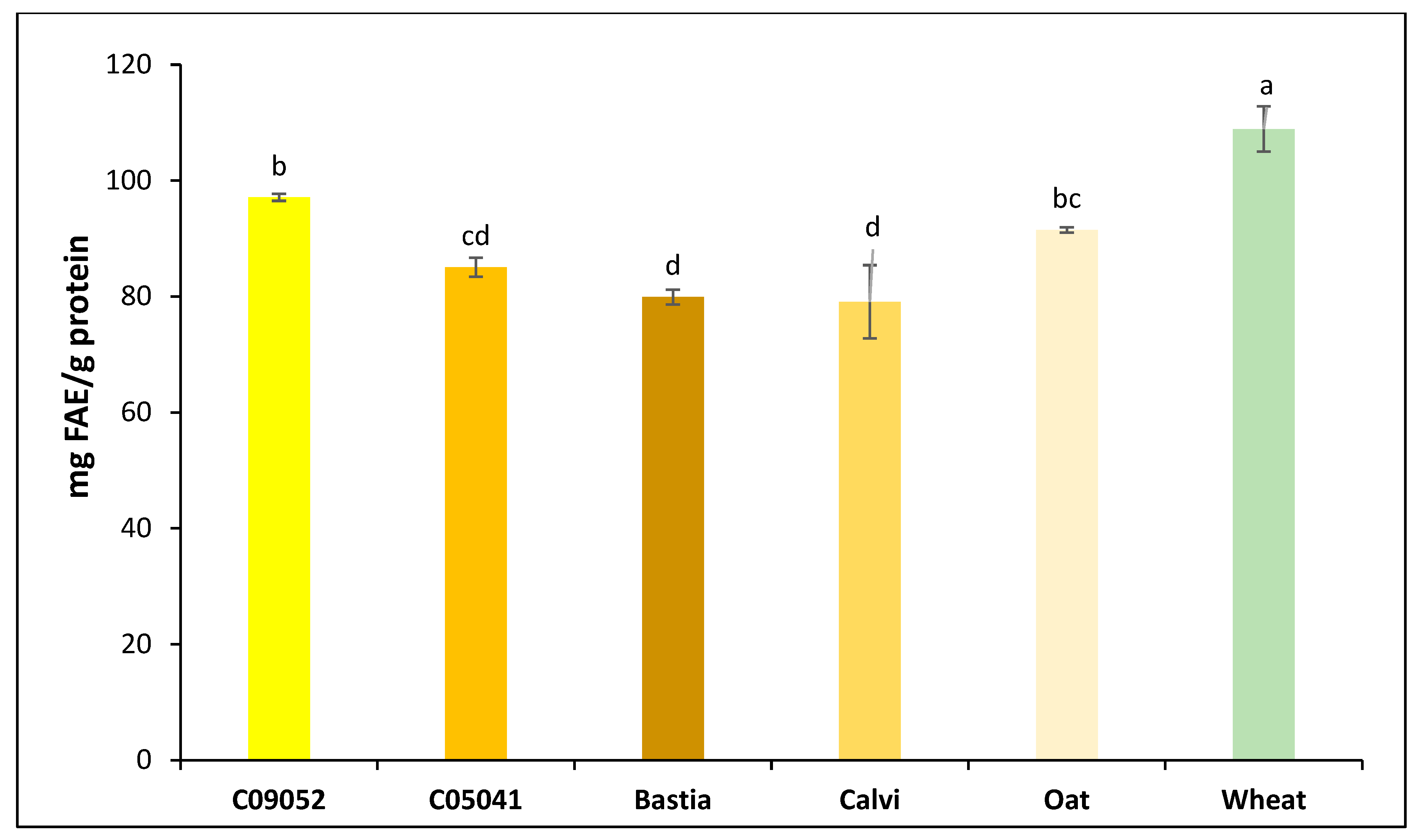
2.5. Determination of Total Polyphenol Content (TPC) in the 3K Permeates of Cereal Flours Digestates
2.6. Determination of the Antioxidant and Chelation Activities
2.6.1. Oxygen Radical Absorption Capacity (ORAC) Assay
2.6.2. DPPH Free-Radical Scavenging Capacity Assay
2.6.3. ABTS Assay
2.6.4. Iron Chelating Activity (Fe2+) Assay
2.7. Determination of the Antihypertensive Activity by ACE Inhibition Assay
2.8. Determination of the Antidiabetic Activity by DPP-IV Inhibition Assay
2.9. Peptide Identification by Mass Spectroscopy and In Silico Analysis of Their Potential Bioactivity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Composition of Dehulled Canary Seed, Oat and Wheat Flours
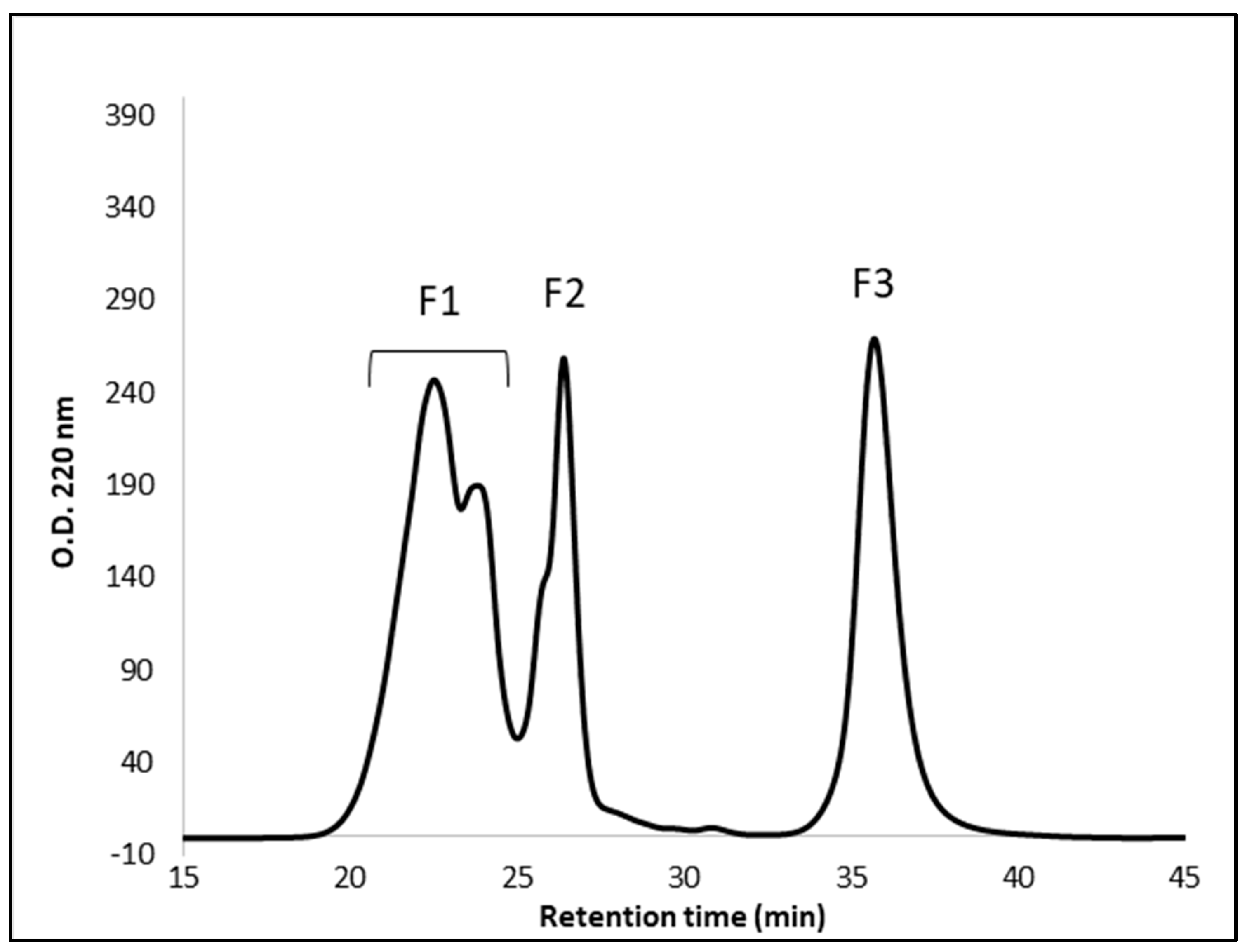
3.2. Molecular Weight Distribution of Peptides in the 3K Permeates of Cereal Flours Digestates
3.3. Bioactivity Assays
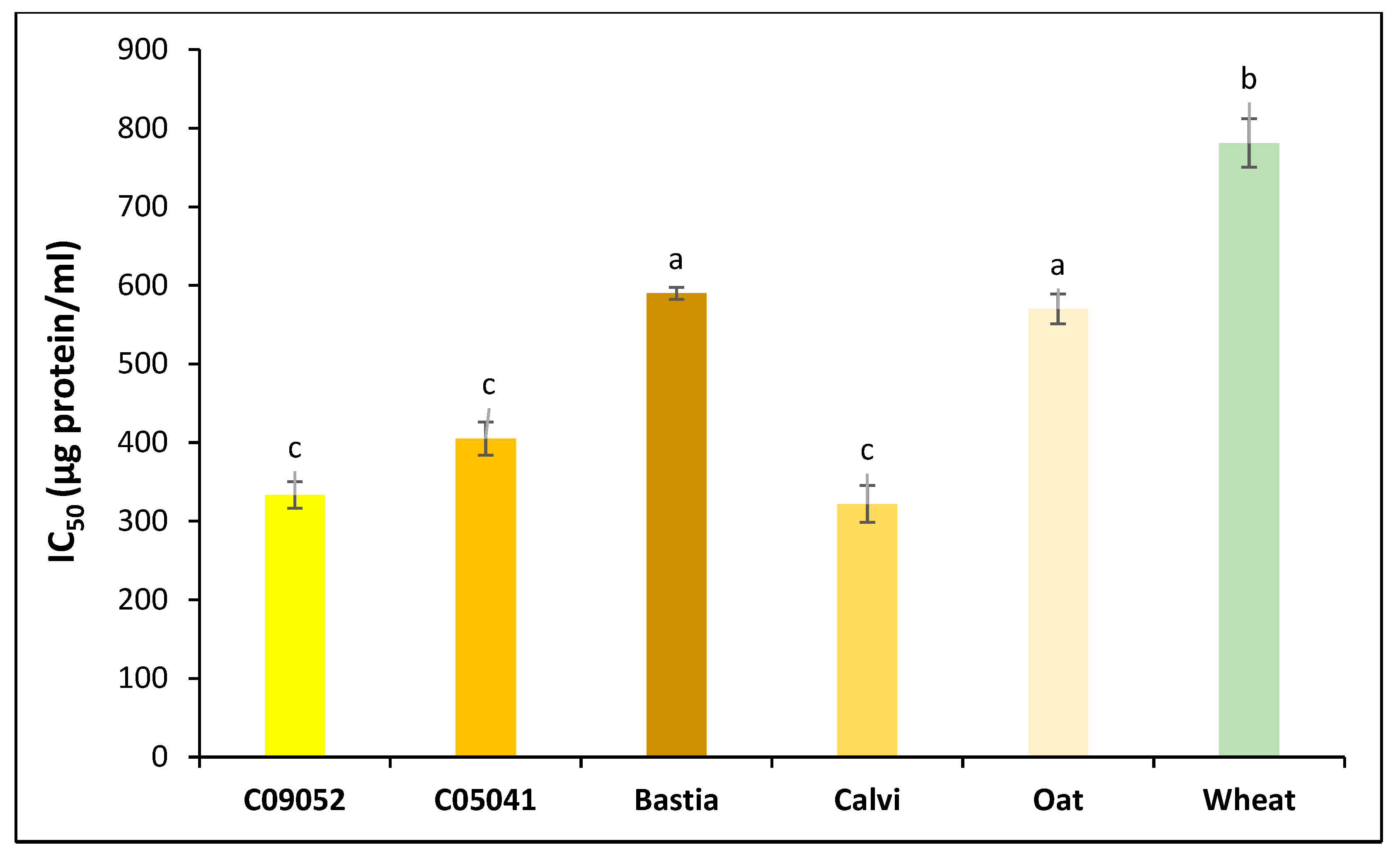
3.3.1. Antioxidant and Chelation Activity
3.3.2. Antihypertensive Activity
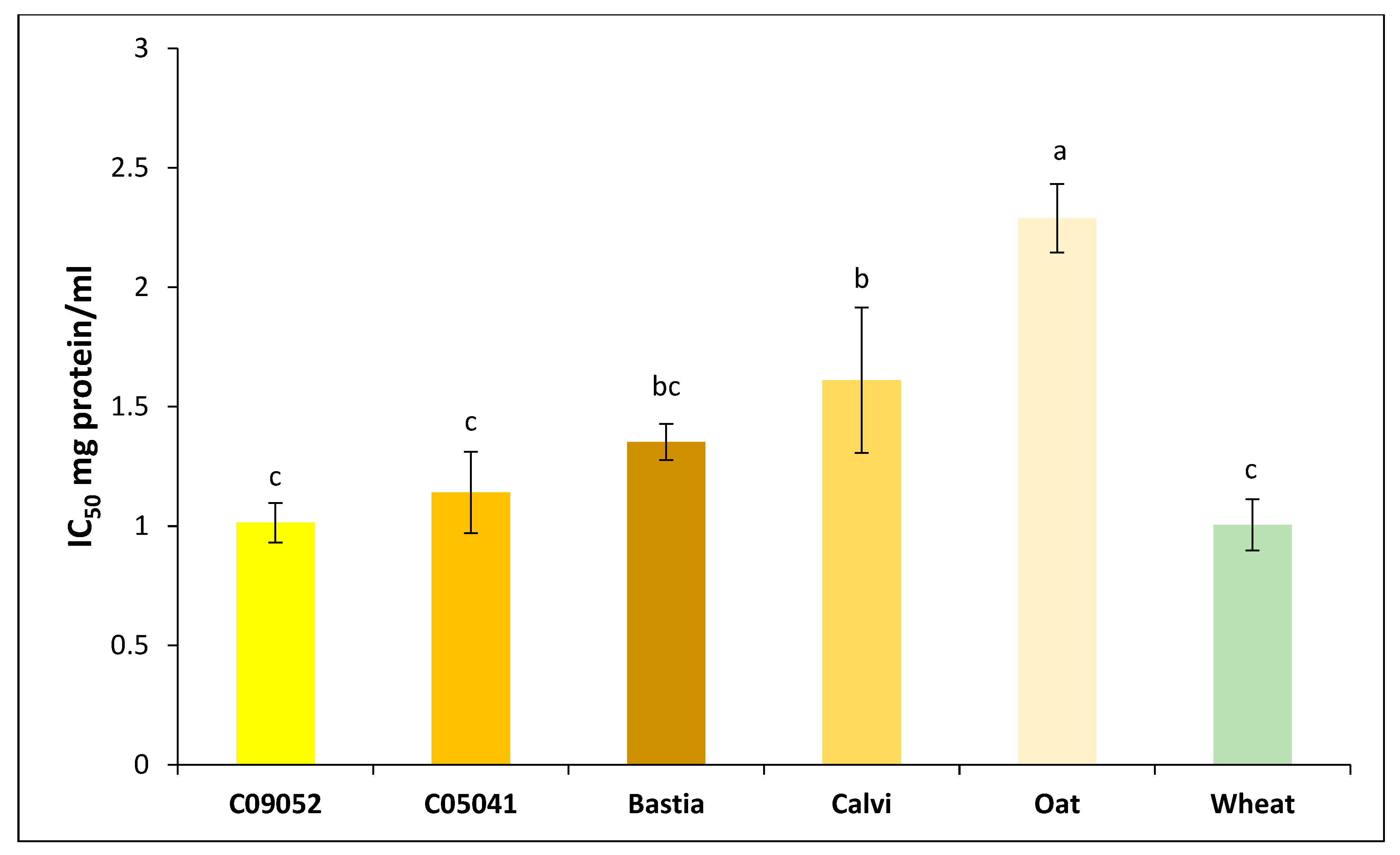
3.3.3. Antidiabetic Activity
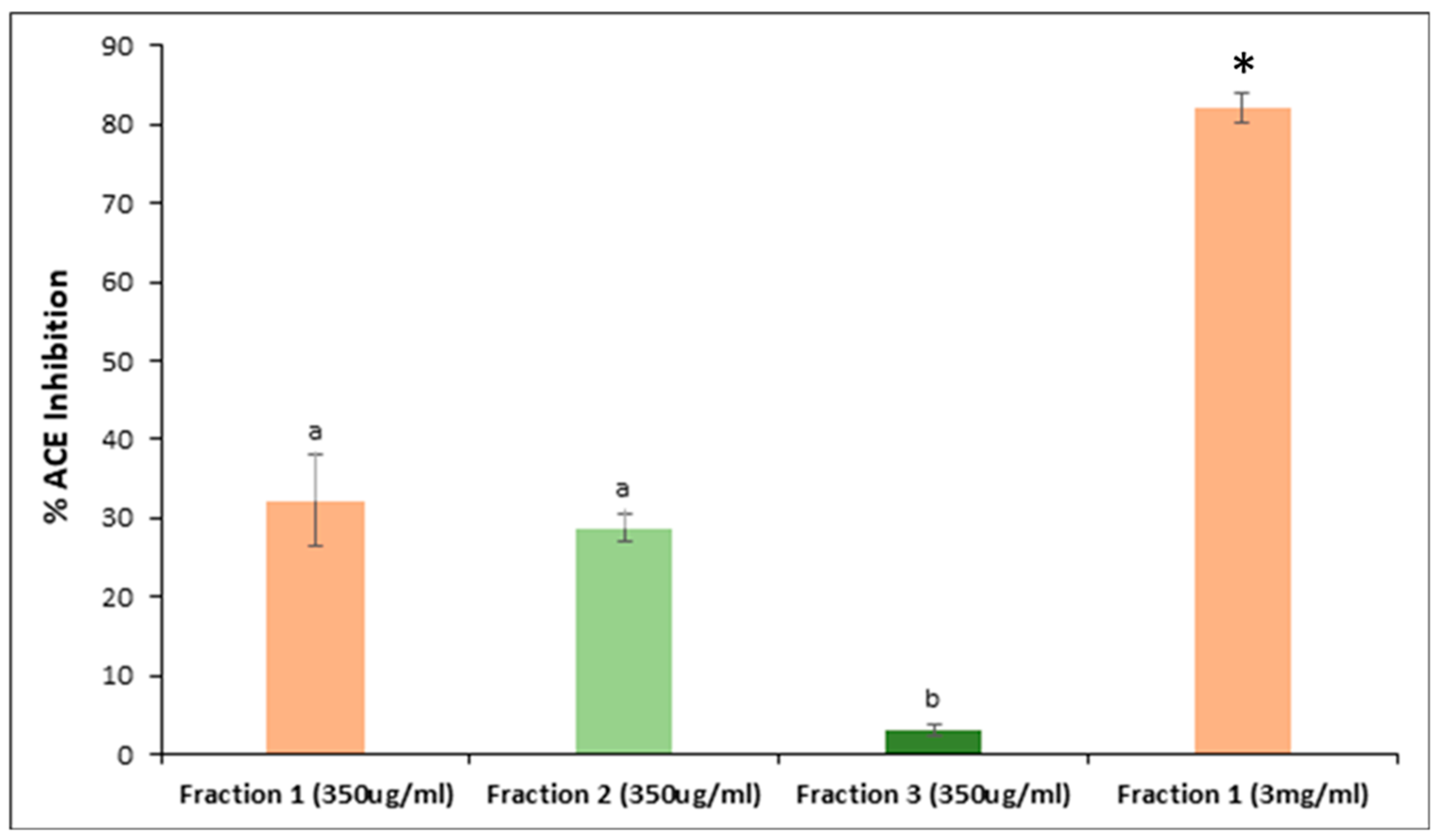
3.4. Peptide Fractionation and Measurement of ACE Inhibitor Activity
3.5. Peptide Identification and Potential Bioactivity
3.5.1. Peptide Identification
3.5.2. In Silico Prediction of Bioactive Peptide Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Health Canada. Novel Food Information—Glabrous Canary Seed (Phalaris canariensis L.). Available online: http://www.hc-sc.gc.ca/fn-an/gmf-agm/appro/canary-seed-lang-graine-alpiste-decision-eng.php#share (accessed on 15 March 2017).
- OEC. Which Countries Export Canary Seeds? 2017. Available online: https://oec.world/en/visualize/tree_map/hs92/export/show/all/100830/2017/ (accessed on 22 May 2020).
- FAO. Faostat Database. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 May 2020).
- Bhatt, T.; Coombs, M.; O’Neill, C. Biogenic silica fibre promotes carcinogenesis in mouse skin. Int. J. Cancer 1984, 34, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Hucl, P.; Matus-Cadiz, M.; Vandenberg, A.; Sosulski, F.W.; Abdel-Aal, E.S.M.; Hughes, G.R.; Slinkard, A.E. Cdc maria annual canarygrass. Can. J. Plant Sci. 2001, 81, 115–116. [Google Scholar] [CrossRef]
- FDA. Gras Notice No. 529: Seed of Phalaris canariensis L. (Canary Seed). Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=529&sort=GRN_No&order=DESC&startrow=1&type=basic&search=529 (accessed on 22 May 2020).
- Abdel-Aal, E.-S.M.; Hucl, P.; Shea Miller, S.; Patterson, C.A.; Gray, D. Microstructure and nutrient composition of hairless canary seed and its potential as a blending flour for food use. Food Chem. 2011, 125, 410–416. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an Fao Expert Consultation; FAO: Rome, Italy, 2013. [Google Scholar]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [PubMed]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef]
- Cavazos, A.; Gonzalez de Mejia, E. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Thacker, P.A. Performance and carcass characteristics of growing-finishing pigs fed diets containing graded levels of canaryseed. Can. J. Anim. Sci. 2003, 83, 89–93. [Google Scholar] [CrossRef]
- Newkirk, R.W.; Ram, J.I.; Hucl, P.; Patterson, C.A.; Classen, H.L. A study of nutrient digestibility and growth performance of broiler chicks fed hairy and hairless canary seed (Phalaris canariensis L.) products. Poult. Sci. 2011, 90, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Misaki, C.; Pierre, H.; Susantha, G.; Ann, P.C. Performance, health and tissue weights of broiler chickens fed graded levels of hairless hulled yellow and brown canary seed (Phalaris canariensis L.). Can. J. Anim. Sci. 2014, 94, 669–678. [Google Scholar]
- Magnuson, B.A.; Patterson, C.A.; Hucl, P.; Newkirk, R.W.; Ram, J.I.; Classen, H.L. Safety assessment of consumption of glabrous canary seed (Phalaris canariensis L.) in rats. Food Chem. Toxicol. 2014, 63, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P.J.; Sosulski, F.W. Structural and compositional characteristics of canaryseed (Phalaris canariensis L.). J. Agric. Food Chem. 1997, 45, 3049–3055. [Google Scholar] [CrossRef]
- Estrada-Salas, P.A.; Montero-Moran, G.M.; Martinez-Cuevas, P.P.; Gonzalez, C.; Barba de la Rosa, A.P. Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. J. Agric. Food Chem. 2014, 62, 427–433. [Google Scholar] [CrossRef]
- Valverde, M.E.; Orona-Tamayo, D.; Nieto-Rendón, B.; Paredes-López, O. Antioxidant and antihypertensive potential of protein fractions from flour and milk substitutes from canary seeds (Phalaris canariensis L.). Plant Foods Hum. Nutr. 2017, 72, 20–25. [Google Scholar] [CrossRef]
- William, H. Official Methods of Analysis of AOAC INTERNATIONAL. In Official Method 992.15, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AACC Approved Methods of Analysis. Method 30-25.01. In Crude Fat in Wheat, Corn, and Soy Flour, Feeds, and Mixed Feeds, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC Approved Methods of Analysis. Method 08-03.01. In Ash-Rapid (2-hour, 600°) Method, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC Approved Methods of Analysis. Method 44-15.02. In Moisture—Air-Oven Methods, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Achouri, A.; Boye, J.I.; Belanger, D.; Chiron, T.; Yaylayan, V.A.; Yeboah, F.K. Functional and molecular properties of calcium precipitated soy glycinin and the effect of glycation with k-carrageenan. Food Res. Int. 2010, 43, 1494–1504. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Tomer, D.P.; McLeman, L.D.; Ohmine, S.; Scherer, P.M.; Murray, B.K.; O’Neill, K.L. Comparison of the total oxyradical scavenging capacity and oxygen radical absorbance capacity antioxidant assays. J. Med. Food 2007, 10, 337–344. [Google Scholar] [CrossRef]
- Garrett, A.R.; Murray, B.K.; Robison, R.A.; O’Neill, K.L. Measuring antioxidant capacity using the orac and tosc assays. In Advanced Protocols in Oxidative Stress II. Methods in Molecular Biology (Methods and Protocols); Humana Press: Totowa, NJ, USA, 2010; Volume 594, pp. 251–262. [Google Scholar]
- Orona-Tamayo, D.; Valverde, M.E.; Nieto-Rendón, B.; Paredes-López, O. Inhibitory activity of chia (Salvia hispanica L.) protein fractions against angiotensin i-converting enzyme and antioxidant capacity. LWT Food Sci. Technol. 2015, 64, 236–242. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Wang, X.; Gu, Z.; Beta, T. Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis L.) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin i-converting enzyme inhibitory activity of chickpea and pea protein hydrolysates. Food Res. Int. 2010, 43, 1642–1649. [Google Scholar] [CrossRef]
- Rui, X.; Boye, J.I.; Simpson, B.K.; Prasher, S.O. Angiotensin i-converting enzyme inhibitory properties of phaseolus vulgaris bean hydrolysates: Effects of different thermal and enzymatic digestion treatments. Food Res. Int. 2012, 49, 739–746. [Google Scholar] [CrossRef]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-González, S.; Montero-Morán, G.M.; Díaz-Gois, A.; González de Mejia, E.; Barba de la Rosa, A.P. In vitro inhibition of dipeptidyl peptidase iv by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase iv inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by ms/ms and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Fekadu Gemede, H. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Alfieri, M.; Redaelli, R. Oat phenolic content and total antioxidant capacity during grain development. J. Cereal Sci. 2015, 65, 39–42. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, L.; Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. Technol. 2010, 45, 7–16. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P.; Patterson, C.A.; Gray, D. Phytochemicals and heavy metals content of hairless canary seed: A variety developed for food use. J. Food Sci. Technol. 2011, 44, 904–910. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Y.; Patterson, C.A.; Beta, T. The analysis of phenolic constituents in glabrous canaryseed groats. Food Chem. 2011, 127, 10–20. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Becerra, L.; Lugo-Cervantes, E. Antioxidant activity of hydrolysates and peptide fractions of glutelin from cocoa (Theobroma cacao L.) seed. CyTA J. Food 2017, 15, 489–496. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa willd.) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Ghosal, M.; Gupta, S.K.; Ghosh, A.; Mandal, P. In-vitro free-radical scavenging potential of oligopeptides derived from wheat and mung bean. Int. J. Pharm. Pharm. Sci. 2015, 8, 428–432. [Google Scholar]
- Durak, A.; Baraniak, B.; Jakubczyk, A.; Swieca, M. Biologically active peptides obtained by enzymatic hydrolysis of adzuki bean seeds. Food chem. 2013, 141, 2177–2183. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Santhanam, R.K.; Wang, C.; Gao, X.; Chen, Y.; Wang, C.; Xu, L.; Chen, H. Bioactive peptide with antioxidant and anticancer activities from black soybean [glycine max (l.) merr.] byproduct: Isolation, identification and molecular docking study. Eur. Food Res. Technol. 2019, 245, 677–689. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using dpph assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Proestos, C.; Zoumpoulakis, P.; Sinanoglou, V. Determination of plant bioactive compounds. Antioxidant capacity and antimicrobial screening. Focus. Mod. Food Ind. 2013, 2, 26–35. [Google Scholar]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Jung, W.S.; Chun, S.C.; Yu, C.Y.; Ma, K.H.; Gwag, J.G.; Chung, I.M. A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties. Eur. Food Res. Technol. 2006, 224, 259–270. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Goñi, I. Contribution of cereals to dietary fibre and antioxidant intakes: Toward more reliable methodology. J. Cereal Sci. 2009, 50, 291–294. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Lim, Y.Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef]
- Mäkinen, S.; Kelloniemi, J.; Pihlanto, A.; Mäkinen, K.; Korhonen, H.; Hopia, A.; Valkonen, J.P.T. Inhibition of angiotensin converting enzyme i caused by autolysis of potato proteins by enzymatic activities confined to different parts of the potato tuber. J. Agric. Food Chem. 2008, 56, 9875–9883. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Adebiyi, A.P.; Doyen, A.; Li, H.; Bazinet, L.; Aluko, R.E. Low molecular weight flaxseed protein-derived arginine-containing peptides reduced blood pressure of spontaneously hypertensive rats faster than amino acid form of arginine and native flaxseed protein. Food Chem. 2012, 132, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.C.; Serra, C.P.; Viana Júnior, N.S.; Oliveira, A.B.; Côrtes, S.F.; Lombardi, J.A. Angiotensin-converting enzyme inhibition by brazilian plants. Fitoterapia 2007, 78, 353–358. [Google Scholar] [CrossRef]
- Wang, F.; Yu, G.; Zhang, Y.; Zhang, B.; Fan, J. Dipeptidyl peptidase iv inhibitory peptides derived from oat (Avena sativa L.), buckwheat (Fagopyrum esculentum), and highland barley (Hordeum vulgare trifurcatum (L.) Trofim) proteins. J. Agric. Food Chem. 2015, 63, 9543–9549. [Google Scholar] [CrossRef]
- Lin, S.-R.; Chang, C.-H.; Tsai, M.-J.; Cheng, H.; Chen, J.-C.; Leong, M.K.; Weng, C.-F. The perceptions of natural compounds against dipeptidyl peptidase iv in diabetes: From in silico to in vivo. Ther. Adv. Chronic Dis. 2019, 10, 2040622319875305. [Google Scholar] [CrossRef] [PubMed]
- Breuer, D.; Nowak, J.; Ivakov, A.; Somssich, M.; Persson, S.; Nikoloski, Z. System-wide organization of actin cytoskeleton determines organelle transport in hypocotyl plant cells. Proc. Natl. Acad. Sci. USA 2017, 114, E5741–E5749. [Google Scholar] [CrossRef]
- Hahn, A.; Vonck, J.; Mills, D.J.; Meier, T.; Kühlbrandt, W. Structure, mechanism, and regulation of the chloroplast atp synthase. Science 2018, 360. [Google Scholar] [CrossRef]
- Dorigo, B.; Schalch, T.; Bystricky, K.; Richmond, T.J. Chromatin fiber folding: Requirement for the histone h4 n-terminal tail. J. Mol. Biol. 2003, 327, 85–96. [Google Scholar] [CrossRef]
- Aluko, R.E. Food protein-derived renin-inhibitory peptides: In vitro and in vivo properties. J. Food Biochem. 2019, 43, e12648. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Malomo, S.A.; Girgih, A.T.; Aluko, R.E. Blood pressure lowering effects of australian canola protein hydrolysates in spontaneously hypertensive rats. Food Res. Int. 2014, 55, 281–287. [Google Scholar] [CrossRef]
- Girgih, A.T.; Alashi, A.; He, R.; Malomo, S.; Aluko, R.E. Preventive and treatment effects of a hemp seed (Cannabis sativa L.) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2014, 53, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Ciau-Solís, N.A.; Acevedo-Fernández, J.J.; Betancur-Ancona, D. In vitro renin–angiotensin system inhibition and in vivo antihypertensive activity of peptide fractions from lima bean (Phaseolus lunatus L.). J. Sci. Food Agric. 2018, 98, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Lin, Y.-S.; Hou, W.-C.; Aluko, R.E. Kinetics of the inhibition of renin and angiotensin i-converting enzyme by flaxseed protein hydrolysate fractions. J. Funct. Foods 2009, 1, 199–207. [Google Scholar] [CrossRef]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.; Khosla, C. Prolyl endopeptidases. Cell. Mol. Life Sci. 2007, 64, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Wang, T.Y.; Hung, C.C.; Hsieh, Y.L.; Hsu, K.C. Isolation of prolyl endopeptidase inhibitory peptides from a sodium caseinate hydrolysate. Food Funct. 2016, 7, 565–573. [Google Scholar] [CrossRef]
- Polgár, L. The prolyl oligopeptidase family. Cell. Mol. Life Sci. 2002, 59, 349–362. [Google Scholar] [CrossRef]
- Cheng, S.; Tu, M.; Liu, H.; Zhao, G.; Du, M. Food-derived antithrombotic peptides: Preparation, identification, and interactions with thrombin. Crit. Rev. Food Sci. Nutr. 2019, 59, S81–S95. [Google Scholar] [CrossRef]
- de Roos, B.; Zhang, X.; Rodriguez Gutierrez, G.; Wood, S.; Rucklidge, G.J.; Reid, M.D.; Duncan, G.J.; Cantlay, L.L.; Duthie, G.G.; O’Kennedy, N. Anti-platelet effects of olive oil extract: In vitro functional and proteomic studies. Eur. J. Nutr. 2011, 50, 553–562. [Google Scholar] [CrossRef]
- Qiao, M.; Tu, M.; Chen, H.; Mao, F.; Yu, C.; Du, M. Identification and in silico prediction of anticoagulant peptides from the enzymatic hydrolysates of Mytilus edulis proteins. Int. J. Mol. Sci. 2018, 19, 2100. [Google Scholar] [CrossRef] [PubMed]
- Sabbione, A.C.; Nardo, A.E.; Añón, M.C.; Scilingo, A. Amaranth peptides with antithrombotic activity released by simulated gastrointestinal digestion. J. Funct. Foods 2016, 20, 204–214. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Scilingo, A.; Añón, M.C. Potential antithrombotic activity detected in amaranth proteins and its hydrolysates. LWT Food Sci. Technol. 2015, 60, 171–177. [Google Scholar] [CrossRef]
- Yu, G.; Wang, F.; Zhang, B.; Fan, J. In vitro inhibition of platelet aggregation by peptides derived from oat (Avena sativa L.), highland barley (Hordeum vulgare Linn. Var. Nudum Hook. F.), and buckwheat (Fagopyrum esculentum Moench) proteins. Food Chem. 2016, 194, 577–586. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef]
- Liu, Z.; Udenigwe, C.C. Role of food-derived opioid peptides in the central nervous and gastrointestinal systems. J. Food Biochem. 2019, 43, e12629. [Google Scholar] [CrossRef]
- Yoshikawa, M. Exorphins. In Handbook of Biologically Active Peptides, 2nd ed.; Kastin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1570–1576. [Google Scholar]
- Garg, S.; Apostolopoulos, V.; Nurgali, K.; Mishra, V.K. Evaluation of in silico approach for prediction of presence of opioid peptides in wheat. J. Funct. Foods 2018, 41, 34–40. [Google Scholar] [CrossRef]
- Kecel-Gunduz, S.; Celik, S.; Ozel, A.E.; Akyuz, S. The conformational and vibrational behavior of the inhibitory neuropeptide derived from beta-endorphin. J. Biomol. Struct. Dyn. 2017, 35, 585–602. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, M.; Marmiroli, N. Bioactive peptides in plant-derived foodstuffs. J. Proteom. 2016, 147, 140–155. [Google Scholar] [CrossRef]
- Guijarro-Díez, M.; García, M.C.; Marina, M.L.; Crego, A.L. Lc-esi-tof ms method for the evaluation of the immunostimulating activity of soybeans via the determination of the functional peptide soymetide. J. Agric. Food Chem. 2013, 61, 3611–3618. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; de la Rosa, A.P.B.; León-Galván, M.F.; de Lumen, B.O.; de León-Rodríguez, A.; de Mejía, E.G. Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Jaziri, M.h.; Migliore-Samour, D.; Casabianca-Pignède, M.-R.; Keddad, K.; Morgat, J.L.; Jollès, P. Specific binding sites on human phagocytic blood cells for gly-leu-phe and val-glu-pro-ile-pro-tyr, immunostimulating peptides from human milk proteins. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1992, 1160, 251–261. [Google Scholar] [CrossRef]
- Tsuruki, T.; Yoshikawa, M. Anti-alopecia effect of gly-leu-phe, an immunostimulating peptide derived from α-lactalbumin. Biosci. Biotechnol. Biochem. 2005, 69, 1633–1635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, R.J. Bioactive peptides in milk and their biological and health implications. Food Rev. Int. 1998, 14, 1–16. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur, D.; Hernandez-Escalante, V. Bioavailability of bioactive peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]

| Cereal Cultivars | Proximate Analysis of Flours | |||||
|---|---|---|---|---|---|---|
| Ash (%) d.b. 1 | Moisture (%) | Oil (%) d.b. | Protein (%) d.b. | Total Carbohydrate (%) d.b. | Total Polyphenol (TPC) mg FAE/g | |
| Bastia Brown | 2.46 ± 0.03 a | 8.94 ± 0.62 b | 6.39 ± 0.16 b | 22.33 ± 0.26 a | 59.88 ± 0.90 c | 1.44 ± 0.03 b |
| Calvi Brown | 2.44 ± 0.06 a | 8.29 ± 0.16 b | 6.29 ± 0.14 b | 21.55 ± 0.10 a | 61.44 ± 0.38 c | 1.42 ± 0.01 b |
| C05041 Yellow | 2.43 ± 0.02 a | 8.90 ± 0.26 b | 6.03 ± 0.35 bc | 21.53 ± 0.25 a | 61.11 ± 0.65 c | 1.34 ± 0.06 b |
| C09052 Yellow | 2.44 ± 0.08 a | 8.58 ± 0.11 b | 5.61 ± 0.04 c | 21.97 ± 0.56 a | 61.41 ± 0.38 c | 1.47 ± 0.01 b |
| Oat (Turcotte) | 2.14 ± 0.01 b | 7.08 ± 0.10 c | 8.44 ± 0.06 a | 14.30 ± 0.12 c | 65.19 ± 0.59 b | 2.04 ± 0.15 a |
| Wheat (Snowbird) | 1.97 ± 0.02 c | 10.70 ± 0.47 a | 1.26 ± 0.01 d | 16.35 ± 0.07 b | 70.60 ± 0.57 a | 0.65 ± 0.05 c |
| Protein Peptide Sequence | MW (Da) | Protein Accession Number | Organism |
|---|---|---|---|
| Predicted protein | 41,787.00 | F2D4P0_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| AVFPSIVGRPR | 1197.71 | ||
| DLYANTVLSGGTTMYPGIADR | 2214.07 | ||
| FPSIVGRPR | 1027.60 | ||
| GYSFTTTAER | 1131.53 | ||
| HQGVMVGMGQK | 1170.57 | ||
| IWHHTFYNELR | 1514.75 | ||
| KDLYANTVLSGGTTMYPGIADR | 2342.17 | ||
| SYELPDGQVITIGNER | 1789.89 | ||
| VFPSIVGRPR | 1126.67 | ||
| WHHTFYNELR | 1401.67 | ||
| Predicted protein | 42,047.70 | F2DZG9_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| AVFPSIVGRPR | 1197.71 | ||
| FPSIVGRPR | 1027.60 | ||
| GYSFTTTAER | 1131.53 | ||
| HQGVMVGMGQK | 1170.57 | ||
| IWHHTFYNELR | 1514.75 | ||
| QEYDESGPSIVHR | 1515.70 | ||
| VAPEEHPVLLTEAPLNPK | 1953.06 | ||
| VFPSIVGRPR | 1126.67 | ||
| WHHTFYNELR | 1401.67 | ||
| Uncharacterized protein | 42,333.10 | A0A287LZV9_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| AVFPSIVGRPR | 1197.71 | ||
| FPSIVGRPR | 1027.60 | ||
| GYSFTTTAER | 1131.53 | ||
| IWHHTFYNELR | 1514.75 | ||
| TTGIVMDSGDGVSHTVPIYEGFTLPHAIIR | 3182.61 | ||
| VFPSIVGRPR | 1126.67 | ||
| WHHTFYNELR | 1401.67 | ||
| Tubulin alpha chain | 50,071.70 | F2E847_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| AFVHWYVGEGMEEGEFSEAR | 2345.01 | ||
| AVFVDLEPTVIDEVR | 1700.91 | ||
| DVNAAIATIK | 1014.58 | ||
| QLFHPEQLITGK | 1409.77 | ||
| TIGGGDDSFNTFFSETGAGK | 2006.89 | ||
| VGINYQPPTVVPGGDLAK | 1823.99 | ||
| Elongation factor 1-alpha | 50,936.00 | A0A287P673_HORVV | Hordeum vulgare subsp. Vulgare (barley) |
| IGGIGTVPVGR | 1024.61 | ||
| LPLQDVYK | 974.55 | ||
| QTVAVGVIK | 913.57 | ||
| STTTGHLIYK | 1119.60 | ||
| THINIVVIGHVDSGK | 1587.88 | ||
| Tubulin beta chain | 50,691.10 | F2D8W7_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| FPGQLNADLR | 1129.60 | ||
| IMNTFSVVPSPK | 1334.70 | ||
| ISEQFTAMFR | 1228.60 | ||
| KLAVNMVPFPR | 1270.73 | ||
| LAVNMVPFPR | 1142.63 | ||
| Uncharacterized protein | 71,301.20 | A0A287NGJ6_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| HGSLGFLPR | 982.54 | ||
| Tubulin alpha chain | 40,676.70 | F2DQT3_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| DVNAAIATIK | 1014.58 | ||
| LISQVISSLTASLR | 1486.88 | ||
| Predicted protein (Fragment) | 22,495.80 | F2EJ22_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| LKFPLPHR | 1006.62 | ||
| Predicted protein (Fragment) | 40,285.70 | F2DYG9_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| FATEAAITILR | 1204.69 | ||
| Predicted protein | 71,916.00 | F2E5M4_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| GVPQIEVTFDLDANGILNVSAVDK | 2514.29 | ||
| T-complex protein 1 subunit eta | 27,260.50 | A0A287TMA9_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| SLHDAIMIVR | 1153.64 | ||
| Uncharacterized protein | 69,103.00 | A0A287QLL8_HORVV | Hordeum vulgare subsp. vulgare (barley) |
| DAGVIAGINVLR | 1196.70 | ||
| ATP synthase subunit alpha | 55,307.40 | A0A1C9ZNX9_HORVS | Hordeum vulgare subsp. Spontaneum (barley) |
| GIRPAINVGLSVSR | 1437.85 | ||
| Histone H4 | 11,367.70 | M7ZMQ6_TRIUA | Triticum urartu (Red wild einkorn) |
| DNIQGITKPAIR | 1324.75 | ||
| ISGLIYEETR | 1179.62 | ||
| KTVTAMDVVYALK | 1437.80 | ||
| MDVVYALK | 937.50 | ||
| TVTAMDVVYALK | 1325.70 | ||
| VFLENVIR | 988.58 | ||
| Uncharacterized protein | 59,868.80 | A0A1D5UUD3_WHEAT | Triticum estivum (Wheat) |
| ESTLHLVLR | 1066.62 | ||
| Histone H2A | 17,470.80 | A0A0C4BKM5_WHEAT | Triticum estivum (Wheat) |
| AGLQFPVGR | 943.53 | ||
| ATP synthase subunit beta, chloroplastic | 53,842.70 | A0A075W706_AEGBI | Aegilops bicornis (Spach goat grass) |
| IGLFGGAGVGK | 974.56 |
| Peptide Sequence | Potential Bioactivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AVFPSIVGRPR | ACE Inhibitor | DPP-IV Inhibitor | |||||||
| DLYANTVLSGGTTMYPGIADR | ACE inhibitor | DPP-IV Inhibitor | Antiamnestic | Antithrombotic | Opioid | Antioxidant | Hypotensive | ||
| FPSIVGRPR | ACE inhibitor | DPP-IV Inhibitor | |||||||
| GYSFTTTAER | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| HQGVMVGMGQK | ACE inhibitor | DPP-IV Inhibitor | Immunostimulating | Neuropeptide | |||||
| IWHHTFYNELR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| KDLYANTVLSGGTTMYPGIADR | ACE inhibitor | DPP-IV Inhibitor | Antiamnestic | Antithrombotic | Opioid | Antioxidant | Hypotensive | ||
| SYELPDGQVITIGNER | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Neuropeptide | |||||
| VFPSIVGRPR | ACE inhibitor | DPP-IV Inhibitor | |||||||
| WHHTFYNELR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| QEYDESGPSIVHR | ACE inhibitor | DPP-IV Inhibitor | Antiamnestic | Antithrombotic | |||||
| VAPEEHPVLLTEAPLNPK | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | ||||||
| TTGIVMDSGDGVSHTVPIYEGFTLPHAIIR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| AFVHWYVGEGMEEGEFSEAR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| AVFVDLEPTVIDEVR | ACE inhibitor | DPP-IV Inhibitor | |||||||
| DVNAAIATIK | ACE inhibitor | DPP-IV Inhibitor | |||||||
| QLFHPEQLITGK | ACE inhibitor | DPP-IV Inhibitor | |||||||
| TIGGGDDSFNTFFSETGAGK | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| VGINYQPPTVVPGGDLAK | ACE inhibitor | DPP-IV Inhibitor | Antiamnestic | Antithrombotic | |||||
| IGGIGTVPVGR | ACE inhibitor | DPP-IV Inhibitor | |||||||
| LPLQDVYK | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | ||||||
| QTVAVGVIK | ACE inhibitor | DPP-IV Inhibitor | |||||||
| STTTGHLIYK | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | ||||||
| THINIVVIGHVDSGK | ACE inhibitor | DPP-IV Inhibitor | |||||||
| FPGQLNADLR | ACE inhibitor | DPP-IV Inhibitor | Antiamnestic | Antithrombotic | Hypotensive | Neuropeptide | |||
| IMNTFSVVPSPK | ACE inhibitor | DPP-IV Inhibitor | |||||||
| ISEQFTAMFR | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| KLAVNMVPFPR | ACE inhibitor | DPP-IV Inhibitor | |||||||
| LAVNMVPFPR | ACE inhibitor | DPP-IV Inhibitor | |||||||
| HGSLGFLPR | ACE inhibitor | DPP-IV Inhibitor | Immunostimulating | ||||||
| LISQVISSLTASLR | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| LKFPLPHR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| FATEAAITILR | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| GVPQIEVTFDLDANGILNVSAVDK | ACE inhibitor | DPP-IV Inhibitor | |||||||
| SLHDAIMIVR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | ||||||
| DAGVIAGINVLR | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| GIRPAINVGLSVSR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| DNIQGITKPAIR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| ISGLIYEETR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | ||||||
| KTVTAMDVVYALK | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| MDVVYALK | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| TVTAMDVVYALK | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| VFLENVIR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| ESTLHLVLR | ACE inhibitor | DPP-IV Inhibitor | Antioxidant | Hypotensive | |||||
| AGLQFPVGR | ACE inhibitor | DPP-IV Inhibitor | Hypotensive | ||||||
| IGLFGGAGVGK | ACE inhibitor | DPP-IV Inhibitor | Immunostimulating | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, E.; L’Hocine, L.; Achouri, A.; Pitre, M.; Karboune, S. Health Promoting Bioactive Properties of Novel Hairless Canary Seed Flour after In Vitro Gastrointestinal Digestion. Foods 2020, 9, 932. https://doi.org/10.3390/foods9070932
Mason E, L’Hocine L, Achouri A, Pitre M, Karboune S. Health Promoting Bioactive Properties of Novel Hairless Canary Seed Flour after In Vitro Gastrointestinal Digestion. Foods. 2020; 9(7):932. https://doi.org/10.3390/foods9070932
Chicago/Turabian StyleMason, Emily, Lamia L’Hocine, Allaoua Achouri, Mélanie Pitre, and Salwa Karboune. 2020. "Health Promoting Bioactive Properties of Novel Hairless Canary Seed Flour after In Vitro Gastrointestinal Digestion" Foods 9, no. 7: 932. https://doi.org/10.3390/foods9070932
APA StyleMason, E., L’Hocine, L., Achouri, A., Pitre, M., & Karboune, S. (2020). Health Promoting Bioactive Properties of Novel Hairless Canary Seed Flour after In Vitro Gastrointestinal Digestion. Foods, 9(7), 932. https://doi.org/10.3390/foods9070932