Influence of Plasma Treatment on the Polyphenols of Food Products—A Review
Abstract
1. Introduction
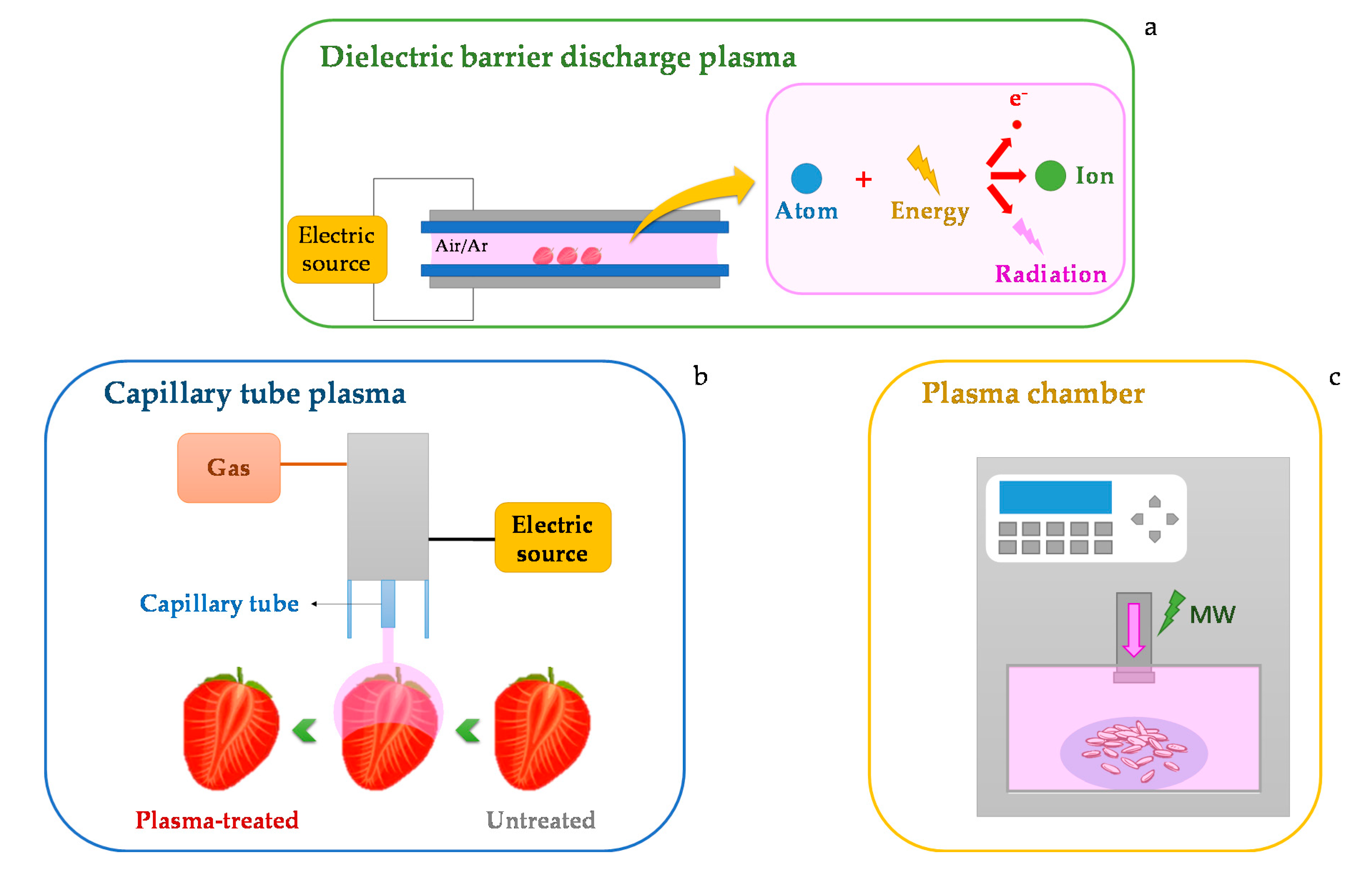
2. Fundaments, Main Applications, and Equipment Details of Plasma Technology
3. Effect of Plasma Treatment in Phenolic Compounds of Food
3.1. Uncut, Fresh Cut, and Processed Vegetables and Fruits
3.2. Beverages Rich in Phenolic Compounds
3.3. Germinated Seeds
4. Influence of Plasma on Enzymes Related to the Biosynthesis and Degradation of Phenolic Compounds
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misra, N.N.; Schlüter, O.; Cullen, P.J. Plasma in Food and Agriculture. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Elsevier: London, UK, 2016; pp. 1–16. ISBN 9780128013656. [Google Scholar]
- Bogue, J.; Collins, O.; Troy, A.J. Market analysis and concept development of functional foods. In Developing New Functional Food and Nutraceutical Products; Elsevier: London, UK, 2017; pp. 29–45. ISBN 9780128027790. [Google Scholar]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S.; Baldin, J.C.; Franco, D.; Domínguez, R.; Trindade, M.A. The use of natural antioxidants to replace chemical antioxidants in foods. In Strategies for Obtaining Healthier Foods; Lorenzo, J.M., Carballo, F.J., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 205–228. ISBN 9781536121599. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Schendel, R.R. Phenol content in sprouted grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Elsevier: London, UK, 2018; pp. 247–315. ISBN 9780128115251. [Google Scholar]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chemie Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: London, UK, 2018; pp. 295–323. [Google Scholar]
- Cullen, P.J.; Lalor, J.; Scally, L.; Boehm, D.; Milosavljević, V.; Bourke, P.; Keener, K. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 2018, 15, 1700085. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Understanding the role of plasma technology in food industry. Food Bioprocess Technol. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: A review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Kogelschatz, U. Atmospheric-pressure plasma technology. Plasma Phys. Control. Fusion 2004, 46, B63–B75. [Google Scholar] [CrossRef]
- Ragni, L.; Berardinelli, A.; Vannini, L.; Montanari, C.; Sirri, F.; Guerzoni, M.E.; Guarnieri, A. Non-thermal atmospheric gas plasma device for surface decontamination of shell eggs. J. Food Eng. 2010, 100, 125–132. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. Physiological and metabolomic analysis of cold plasma treated fresh-cut strawberries. J. Agric. Food Chem. 2019, 67, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Pedisić, S.; Režek Jambrak, A.; Herceg, Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016, 190, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Oh, Y.J.; Won, M.Y.; Lee, K.S.; Min, S.C. Microbial decontamination of onion powder using microwave-powered cold plasma treatments. Food Microbiol. 2017, 62, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Kim, H.J.; Jayasena, D.D.; Yong, H.I.; Alahakoon, A.U.; Park, S.; Park, J.; Choe, W.; Jo, C. Effect of atmospheric pressure plasma jet on the foodborne pathogens attached to commercial food containers. J. Food Sci. Technol. 2015, 52, 8410–8415. [Google Scholar] [CrossRef]
- Muranyi, P.; Wunderlich, J.; Langowski, H.C. Modification of bacterial structures by a low-temperature gas plasma and influence on packaging material. J. Appl. Microbiol. 2010, 109, 1875–1885. [Google Scholar] [CrossRef]
- Puligundla, P.; Lee, T.; Mok, C. Inactivation effect of dielectric barrier discharge plasma against foodborne pathogens on the surfaces of different packaging materials. Innov. Food Sci. Emerg. Technol. 2016, 36, 221–227. [Google Scholar] [CrossRef]
- Li, Y.; Kojtari, A.; Friedman, G.; Brooks, A.D.; Fridman, A.; Ji, H.F. Decomposition of L-valine under nonthermal dielectric barrier discharge plasma. J. Phys. Chem. B 2014, 118, 1612–1620. [Google Scholar] [CrossRef]
- Setsuhara, Y.; Cho, K.; Shiratani, M.; Sekine, M.; Hori, M. Plasma interactions with aminoacid (L-alanine) as a basis of fundamental processes in plasma medicine. Curr. Appl. Phys. 2013, 13, S59–S63. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Surowsky, B.; Bußler, S.; Schlüter, O.K. Cold plasma interactions with food constituents in liquid and solid food matrices. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Elsevier: London, UK, 2016; pp. 179–203. ISBN 9780128013656. [Google Scholar]
- Amini, M.; Ghoranneviss, M. Effects of cold plasma treatment on antioxidants activity, phenolic contents and shelf life of fresh and dried walnut (Juglans regia L.) cultivars during storage. LWT Food Sci. Technol. 2016, 73, 178–184. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Frias, J.M.; Keener, K.M.; Cullen, P.J. The effects of nonthermal plasma on chemical quality of strawberries. Postharvest Biol. Technol. 2015, 110, 197–202. [Google Scholar] [CrossRef]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin preservation by microwave-powered cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Yang, Y.L. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Matan, N.; Puangjinda, K.; Phothisuwan, S.; Nisoa, M. Combined antibacterial activity of green tea extract with atmospheric radio-frequency plasma against pathogens on fresh-cut dragon fruit. Food Control 2015, 50, 291–296. [Google Scholar] [CrossRef]
- Ramazzina, I.; Berardinelli, A.; Rizzi, F.; Tappi, S.; Ragni, L.; Sacchetti, G.; Rocculi, P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015, 107, 55–65. [Google Scholar] [CrossRef]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Effect of low temperature plasma on the functional properties of basmati rice flour. J. Food Sci. Technol. 2016, 53, 2742–2751. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.; Ding, T. Understanding the impact of nonthermal plasma on food constituents and microstructure—A review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- de Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Application of a dielectric barrier discharge atmospheric cold plasma (DBD-ACP) for Eshcerichia coli inactivation in apple juice. J. Food Sci. 2018, 83, 401–408. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Garofulić, I.E.; Jambrak, A.R.; Milošević, S.; Dragović-Uzelac, V.; Zorić, Z.; Herceg, Z. The effect of gas phase plasma treatment on the anthocyanin and phenolic acid content of sour cherry Marasca (Prunus cerasus var. Marasca) juice. LWT Food Sci. Technol. 2015, 62, 894–900. [Google Scholar] [CrossRef]
- Paixão, L.M.N.; Fonteles, T.V.; Oliveira, V.S.; Fernandes, F.A.N.; Rodrigues, S. Cold plasma effects on functional compounds of siriguela juice. Food Bioprocess Technol. 2019, 12, 110–121. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT Food Sci. Technol. 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Mehta, D.; Sharma, N.; Bansal, V.; Sangwan, R.S.; Yadav, S.K. Impact of ultrasonication, ultraviolet and atmospheric cold plasma processing on quality parameters of tomato-based beverage in comparison with thermal processing. Innov. Food Sci. Emerg. Technol. 2019, 52, 343–349. [Google Scholar] [CrossRef]
- Silveira, M.R.; Coutinho, N.M.; Esmerino, E.A.; Moraes, J.; Fernandes, L.M.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Senaka Ranadheera, C.; et al. Guava-flavored whey beverage processed by cold plasma technology: Bioactive compounds, fatty acid profile and volatile compounds. Food Chem. 2019, 279, 120–127. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Dasan, B.G.; Boyaci, I.H. Effect of cold atmospheric plasma on inactivation of Escherichia coli and physicochemical properties of apple, orange, tomato juices, and sour cherry nectar. Food Bioprocess Technol. 2018, 11, 334–343. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Lukić, K.; Vukušić, T.; Tomašević, M.; Ćurko, N.; Gracin, L.; Ganić, K.K. The impact of high voltage electrical discharge plasma on the chromatic characteristics and phenolic composition of red and white wines. Innov. Food Sci. Emerg. Technol. 2019, 53, 70–77. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Q.; Zhang, L.; Chen, Z.; Han, Y.; Gu, Z. Physiological and biochemical metabolism of germinating broccoli seeds and sprouts. J. Agric. Food Chem. 2012, 60, 209–213. [Google Scholar] [CrossRef]
- Nakamura, K.; Koyama, M.; Ishida, R.; Kitahara, T.; Nakajima, T.; Aoyama, T. Characterization of bioactive agents in five types of marketed sprouts and comparison of their antihypertensive, antihyperlipidemic, and antidiabetic effects in fructose-loaded SHRs. J. Food Sci. Technol. 2016, 53, 581–590. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Food Engineering Series; Springer: New York, NY, USA, 2017; pp. 403–432. [Google Scholar]
- FDA. Outbreaks of Foodborne Illness. Available online: https://www.fda.gov/food/recalls-outbreaks-emergencies/outbreaks-foodborne-illness (accessed on 9 July 2020).
- Randeniya, L.K.; de Groot, G.J.J.B. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, T.; Panngom, K.; Hong, Y.J.; Pengkit, A.; Park, D.H.; Kang, M.H.; Lee, S.H.; Im, J.S.; Kim, J.S.; et al. Assessment of the effects of nitrogen plasma and plasma-generated nitric oxide on early development of Coriandum sativum. Plasma Process. Polym. 2015, 12, 1164–1173. [Google Scholar] [CrossRef]
- Kim, J.W.; Puligundla, P.; Mok, C. Effect of corona discharge plasma jet on surface-borne microorganisms and sprouting of broccoli seeds. J. Sci. Food Agric. 2017, 97, 128–134. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effects of nonthermal plasma treatment on decontamination and sprouting of radish (Raphanus sativus L.) seeds. Food Bioprocess Technol. 2017, 10, 1093–1102. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effect of corona discharge plasma jet treatment on decontamination and sprouting of rapeseed (Brassica napus L.) seeds. Food Control 2017, 71, 376–382. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, H.C.; Chen, Y.K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.k.; Choi, E.H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.; Larondelle, Y.; Evers, D. Dietary antioxidants and oxidative stress from a human and plant perspective: A review. Curr. Nutr. Food Sci. 2010, 6, 2–12. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; García-Carmona, F. Enzymatic and nonenzymatic degradation of polyphenols. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability; Wiley-Blackwell: Oxford, UK, 2009; pp. 101–129. ISBN 9780813803203. [Google Scholar]
- Han, Y.; Cheng, J.H.; Sun, D.W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef] [PubMed]
| Food | Gas (Applier and Energy Source) | Treatment Conditions | Point(s) of Assay | Effect of Plasma Treatment on Phenolic Compounds | Reference |
|---|---|---|---|---|---|
| Fresh and dried walnuts (uncut) | Ar (capillary tube, electric source) | Voltage (15 kV); frequency (12 kHz); gas flow (1 L/min); and time (3, 5, 7, 9, and 11 min) | After treatment | No effect of treatment time or storage time on TPC | [28] |
| Strawberries (uncut) | Air (dielectric barrier discharge, electric source) | Voltage discharge (60 and 80 kV) and time (1 and 5 min) | After treatment | No effect on anthocyanin content | [29] |
| Strawberries (fresh cut) | Air (dielectric barrier discharge, electric source) | Voltage (45 kV) and time (1 min) | 7 days at 4 °C | Increase TPC, flavonoid, and anthocyanin contents up to day 5 | [17] |
| Mandarin (uncut) | N2, (chamber, MW source) | MW power (900 W), frequency (2.45 GHz), vacuum, and time (10 min) | 7 at 25 °C and 28 days at 4 °C | No effect on TPC of flesh; slight increase on peel | [30] |
| Blueberries (uncut) | Air (capillary tube, electric source) | Power (549 W), frequency (47 kHz), gas flow (4 ft3/m), and time (15–120 s) | After treatment | Reduction of anthocyanin content as treatment time increased | [31] |
| Blueberry (uncut) | Air (dielectric barrier discharge, electric source) | Voltage (36 V), current (1.8 A), and time (up to 10 min) | 20 days at 25 °C | Highest anthocyanin levels were obtained using 6 and 8 min; effect lasted for 20 days | [32] |
| Dragon fruit (fresh cut) | Ar (capillary tube, RF source) | RF power (40 W) and time (60 s) | After treatment | No effect on TPC | [33] |
| Apples (fresh cut) | Air (dielectric barrier discharge, electric source) | Power (150 W), frequency (12.7 kHz), gas flow (1.5 L/min), and time (30 and 120 min) | After treatment | Reduced TPC; reduction of some procyanidin dimers and trimers (120 min) | [34] |
| Kiwi (fresh cut) | Air (dielectric barrier discharge, electric source) | Voltage (15 kV) and time (20 and 40 min) | 4 days at 10 °C | No effect on hydrophilic fraction of phenolics; slight reduction on hydrophobic fraction of phenolics after 4 days | [35] |
| Pitaya (fresh cut) | Air (dielectric barrier discharge, electric source) | Voltage (60 kV) and time (5 min) | 48 h at 15 °C | Slight increase on selected phenolic and expression of genes related to polyphenol synthesis during storage | [36] |
| Onion powder | He (chamber, MW source) | MW intensity (400 W), frequency (2.45 GHz), gas flow (1 L/min), pressure (0.7 kPa), and time (40 min) | 28 days at 4 and 25 °C | No effect on quercetin content | [19] |
| Basmati rice flour | Air (dielectric barrier discharge, RF source) | RF power (30 and 40 W), frequency (13.56 MHz), and time (5 and 10 min) | After treatment | Increase TPC content by reducing time and power | [37] |
| Food | Gas (Applier and Energy Source) | Treatment Conditions | Effect | Reference |
|---|---|---|---|---|
| Camu-camu juice | Air (dielectric barrier discharge, electric source) | Frequency (200–960 Hz) and time (15 min) | Reduced phenolic and monomeric anthocyanin contents as frequency was improved | [39] |
| Apple juice | Air (dielectric barrier discharge, electric source) | Power (30, 40, and 50 W) and time (40 s) | Reduction on TPC as the power increased | [40] |
| White grape juice | Air (dielectric barrier discharge, electric source) | Voltage (80 kV) and time (1–4 min) | Reduced TPC and flavonoid contents; increased flavonol | [41] |
| Pomegranate juice | Ar (capillary tube, electric source) | Power (4 W); sample (3, 4, and 5 cm3); gas flow (0.75, 1, and 1.25 dm3/min); and time (3, 5, and 7 min) | Increased TPC similarly to pasteurization | [42] |
| Sour cherry Marasca juice | Ar (capillary tube, electric source) | Power (4 W); sample (2, 3, and 4 mL); gas flow (0.75, 1, and 1.25 L/min); and time (3, 4, and 5 min) | Increased anthocyanin and TPC | [43] |
| Siriguela juice | N2 (capillary tube, radiofrequency source) | Gas flow (10, 20, and 30 mL/min) and time (5, 10, and 15 min) | Increased TPC | [44] |
| Cashew apple juice | N2 (capillary tube, radiofrequency source) | Gas flow (10, 30, and 50 mL/min) and time (5, 10, and 15 min) | Increasing effect was dependent of compound class | [45] |
| Tomato-based beverage | Air (dielectric barrier discharge, electric source) | Voltage (60 kV), frequency (50 Hz), and time (10 and 15 min) | Increased TPC and individual polyphenols using treatment for 10 min | [46] |
| Guava-flavored whey beverage | N2 (capillary tube, radiofrequency source) | Power (400 W); frequency (50 kHz); gas flow (10, 20, and 30 mL/min); and time (5, 10, and 15 min) | Higher phenolic content than pasteurized sample | [47] |
| Blueberry juice | Ar and O2 (capillary tube, electric source) | Voltage (11 kV); frequency (1000 Hz); O2 content (0%, 0.5%, and 1%); and time (2, 4, and 6 min) | Increased TPC as treatment time was increased and O2 content in gas was reduced | [48] |
| Orange, tomato, apple, and sour cherry juices | Dry air (capillary tube, electric source) | Frequency (25 kHz), power (650 W), and time (30–120 s) | The highest increase was obtained with 90 and 120 s | [49] |
| Prebiotic orange juice | Air (dielectric barrier discharge, electric source) | Voltage (70 kV), frequency (50 Hz), exposure (direct or indirect), and time (15–60 s) | No effect after direct exposure treatment; indirect exposure induced the loss of phenolic compounds as treatment time increased | [50] |
| Red and white wines | Ar (capillary tube inserted in liquid, electric source) | Gas flow (4 L/min); frequency (60, 90, and 120 Hz); and time (3, 5, and 10 min) | Reduced TPC, anthocyanin, and tannin contents as frequency and time were increased | [51] |
| Food | Gas (Applier and Energy Source) | Treatment Conditions | Point(s) of Assay | Effect of Plasma Treatment on Phenolic Compounds | Reference |
|---|---|---|---|---|---|
| Broccoli seeds (Brassica oleracea var. kialica plen. Mill.) | Air (capillary tube, electric source) | Voltage (20 kV); frequency (58 kHz); and time (1, 2, and 3 min) | 24 h at 25 °C | No significant effect | [60] |
| Radish seeds (Raphanus sativus L.) | Air (capillary tube, electric source) | Voltage (20 kV); current (1.5 A); frequency (58 kHz); and time (1, 2, and 3 min) | 4 days at 25 °C | No significant effect | [61] |
| Rapeseed seeds (Brassica napus L.) | Air (capillary tube, electric source) | Voltage (20 kV); frequency (58 kHz); and time (1, 2, and 3 min) | 4 days at 25 °C | No significant effect | [62] |
| Brown rice (Oryza sativa L. var. Koshihikari) | Air (dielectric barrier discharge-like apparatus, electric source) | Voltage (1, 2, and 3 kV); current (1.2 mA); and plasma time (10 min) | 12, 18, and 24 h at 25 °C | Increased TPC using 2 and 3 kV after 18 h of imbibition | [63] |
| Brown rice (Oryza sativa L.) | Ar (dielectric barrier discharge, RF source) | Power (100–200 W), gas flow (18–24 mL/min), and time (25–300 s) | 4 days at 25–28 °C | Anticipated and increased the rise of TPC during germination | [64] |
| Coriandrum sativum L. seeds | N2 (capillary tube, MW source) | Power (400 W), frequency (2.45 GHz), gas flow (10 L/min), and time (1 and 3 min) | 2 and 4 weeks | Highest increased was obtained after 1 min | [59] |
| Spinach seeds (Spinacia oleracea L.) | N2 and air (dielectric barrier discharge, electric source) | Voltage (6 kV), current (14 mA), gas flow (1.5 L/min), and time (up to 5 min) | 5 weeks | Highest increase was obtained using N2 for 3 min | [65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munekata, P.E.S.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Influence of Plasma Treatment on the Polyphenols of Food Products—A Review. Foods 2020, 9, 929. https://doi.org/10.3390/foods9070929
Munekata PES, Domínguez R, Pateiro M, Lorenzo JM. Influence of Plasma Treatment on the Polyphenols of Food Products—A Review. Foods. 2020; 9(7):929. https://doi.org/10.3390/foods9070929
Chicago/Turabian StyleMunekata, Paulo E. S., Rubén Domínguez, Mirian Pateiro, and José M. Lorenzo. 2020. "Influence of Plasma Treatment on the Polyphenols of Food Products—A Review" Foods 9, no. 7: 929. https://doi.org/10.3390/foods9070929
APA StyleMunekata, P. E. S., Domínguez, R., Pateiro, M., & Lorenzo, J. M. (2020). Influence of Plasma Treatment on the Polyphenols of Food Products—A Review. Foods, 9(7), 929. https://doi.org/10.3390/foods9070929








