Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Pulses
2.3. Cooking Methods
2.4. Analysis of Phenolic Acids
2.5. Analysis of Total Phenol Content
2.6. Antioxidant Assays
2.6.1. DPPH Scavenging Capacity
2.6.2. ABTS Scavenging Capacity
2.6.3. Oxygen Radical Absorbance Capacity (ORAC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Cooking on Phenolic Acids
3.2. Effect of Cooking on Total Phenolic Content
3.3. Effect of Cooking on Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leterme, P. Recommendations by health organizations for pulse consumption. Br. J. Nutr. 2002, 88, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, nutritional value, and health benefits of pulses. Cereal Chem. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M. Processing Pulses to Enhance Bioactive and Anti-Nutritional Attributes. A Mini-Review. 2016. Available online: http://canadianfoodbusiness.com/2016/06/20/processing-pulses-to-enhance-bioactive-and-anti-nutritional-attributes/ (accessed on 9 July 2020).
- Best, D. 10 things to know about pulses. CFW 2013, 58, 105–107. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Mirali, M.; Purves, R.W.; Vandenberg, A. Profiling the phenolic compounds of the four major seed coat types and their relation to color genes in lentil. J. Nat. Prod. 2017, 80, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Padhi, E.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Dueñas, M.; Sarmento, T.; Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Impact of cooking and germination on phenolic composition and dietary fibre fractions in dark beans (Phaseolus vulgaris L.) and lentils (Lens culinaris L.). LWT -Food Sci. Technol. 2016, 66, 72–78. [Google Scholar] [CrossRef]
- Ombra, M.; D’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M. Phenolic Composition and Antioxidant and Antiproliferative Activities of the Extracts of Twelve Common Bean (Phaseolus vulgaris L.) Endemic Ecotypes of Southern Italy before and after Cooking. Oxid. Med. Cell Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Zou, Y.; Chang, S.K.; Gu, Y.; Qian, S.Y. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Beninger, C.W.; Gu, L.; Prior, R.L.; Junk, D.C.; Vandenberg, A.; Bett, K.E. Changes in polyphenols of the seed coat during the after-darkening process in pinto beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 7777–7782. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Troszyńska, A. Antioxidant activity of extract of pea and its fractions of low molecular phenolics and tannins. Pol. J. Food Nutr. Sci. 2003, 12, 10–15. [Google Scholar]
- Ramdath, D.; Renwick, S.; Duncan, A.M. The role of pulses in the dietary management of diabetes. Can. J. Diabetes 2016, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Chen, G.; Yu, J.; Yang, L.; Gao, Y. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Aguilera, Y.; Estrella, I.; Benitez, V.; Esteban, R.; Martín-Cabrejas, M. Bioactive phenolic compounds and functional properties of dehydrated bean flours. Food Res. Int. 2011, 44, 774–780. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Effect of hydrothermal processing on changes of insoluble-bound phenolics of lentils. J. Funct. Foods 2017, 38, 716–722. [Google Scholar] [CrossRef]
- Pedrosa, M.; Cuadrado, C.; Burbano, C.; Muzquiz, M.; Cabellos, B.; Olmedilla-Alonso, B.; Asensio-Vegas, C. Effects of industrial canning on the proximate composition, bioactive compounds contents and nutritional profile of two Spanish common dry beans (Phaseolus vulgaris L.). Food Chem. 2015, 166, 68–75. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.; Hassapidou, M.; Andrikopoulos, N. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Abdel-Aal, E.; Ragaee, S.; Rabalski, I.; Warkentin, T.; Vandenberg, A. Nutrient content and viscosity of Saskatchewan-grown pulses in relation to their cooking quality. Can. J. Plant Sci. 2019, 99, 67–77. [Google Scholar] [CrossRef]
- Liu, Y.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E. Effect of different cooking methods and heating solutions on nutritionally-important starch fractions and flatus oligosaccharides in selected pulses. Cereal Chem. 2020. Under Review. [Google Scholar]
- Abdel-Aal, E.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Rabalski, I. Bioactive compounds and their antioxidant capacity in selected primitive and modern wheat species. Open Agric. 2008, 2, 7–14. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Rabalski, I. Antioxidant Properties of high-lutein grain-based functional foods in comparison with ferulic acid and lutein. Am. J. Biomed. Sci. 2013, 5, 109–125. [Google Scholar] [CrossRef]
- Sosulski, F.W.; Dabrowski, K.J. Composition of free and hydrolyzable phenolic acids in the flours and hulls of ten legume species. J. Agric. Food Chem. 1984, 32, 131–133. [Google Scholar] [CrossRef]
- Siah, S.; Wood, J.; Agboola, S.; Konczak, I.; Blanchard, C. Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chem. 2014, 142, 461–468. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Alshikh, N.; de Camargo, A.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, S.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef]
- Chaieb, N.; González, J.L.; López-Mesas, M.; Bouslama, M.; Valiente, M. Polyphenols content and antioxidant capacity of thirteen faba bean (Vicia faba L.) genotypes cultivated in Tunisia. Food Res. Int. 2011, 44, 970–977. [Google Scholar] [CrossRef]
- Nithiyanantham, S.; Selvakumar, S.; Siddhuraju, P. Total phenolic content and antioxidant activity of two different solvent extracts from raw and processed legumes, Cicer arietinum L. and Pisum sativum L. J. Food Compos. Anal. 2012, 27, 52–60. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008, 110, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Pacifico, S.; Piccolella, S.; Di Giuseppe, A.; Mezzacapo, M.; Ragucci, S.; Di Maro, A. Valle Agricola lentil, an unknown lentil (Lens culinaris Medik.) seed from Southern Italy as a novel antioxidant and prebiotic source. Food Funct. 2015, 6, 3155–3164. [Google Scholar] [CrossRef]
- Han, H.; Baik, B. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Malcolmson, L.; Han, J. Pulse processing and untilization of pulse ingredients in foods. In Health Benefits of Pulses; Dahl, W.J., Ed.; Springer Nature: Basel, Switzerland, 2019; Chapter 9; pp. 129–149. [Google Scholar] [CrossRef]
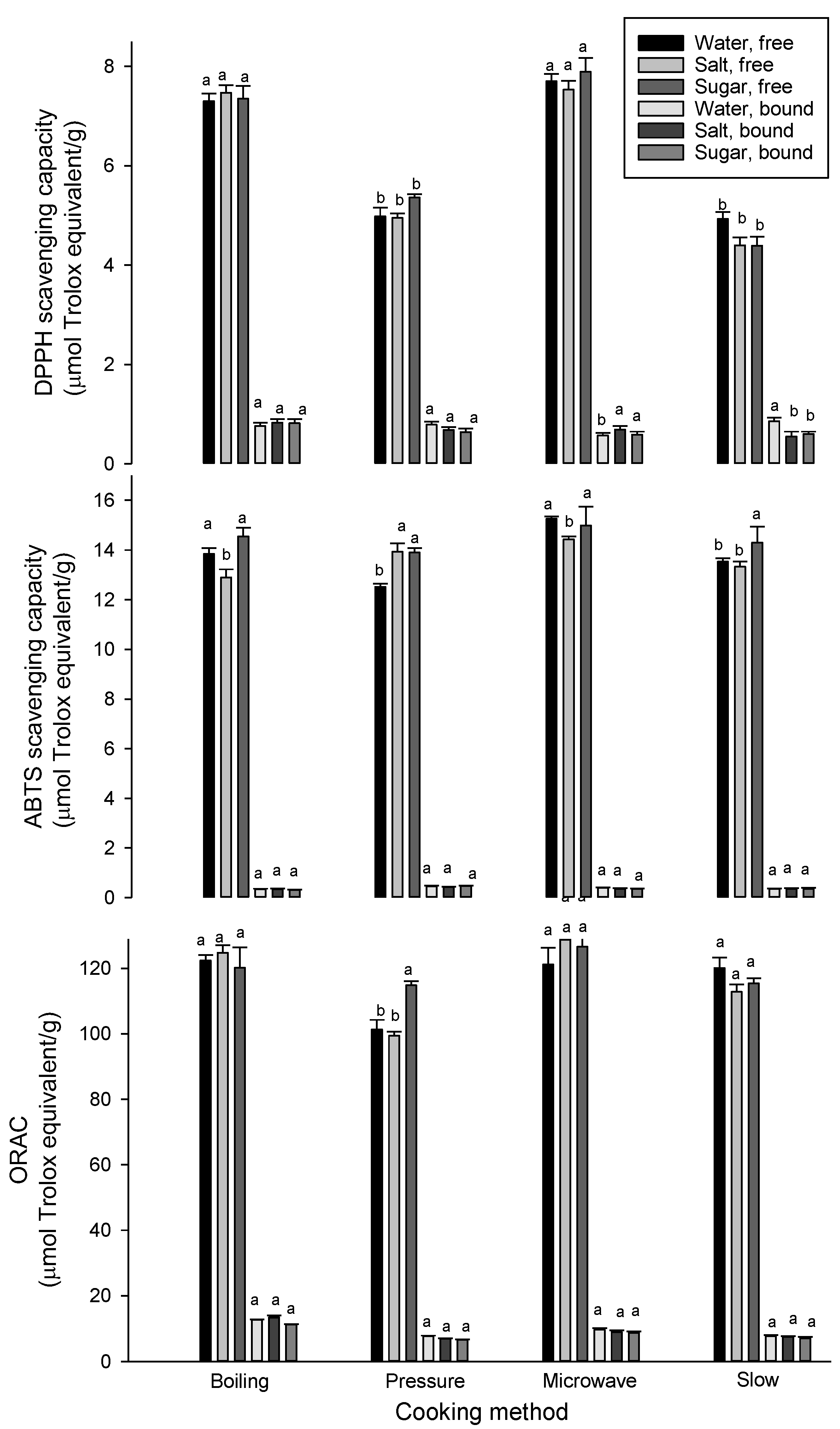
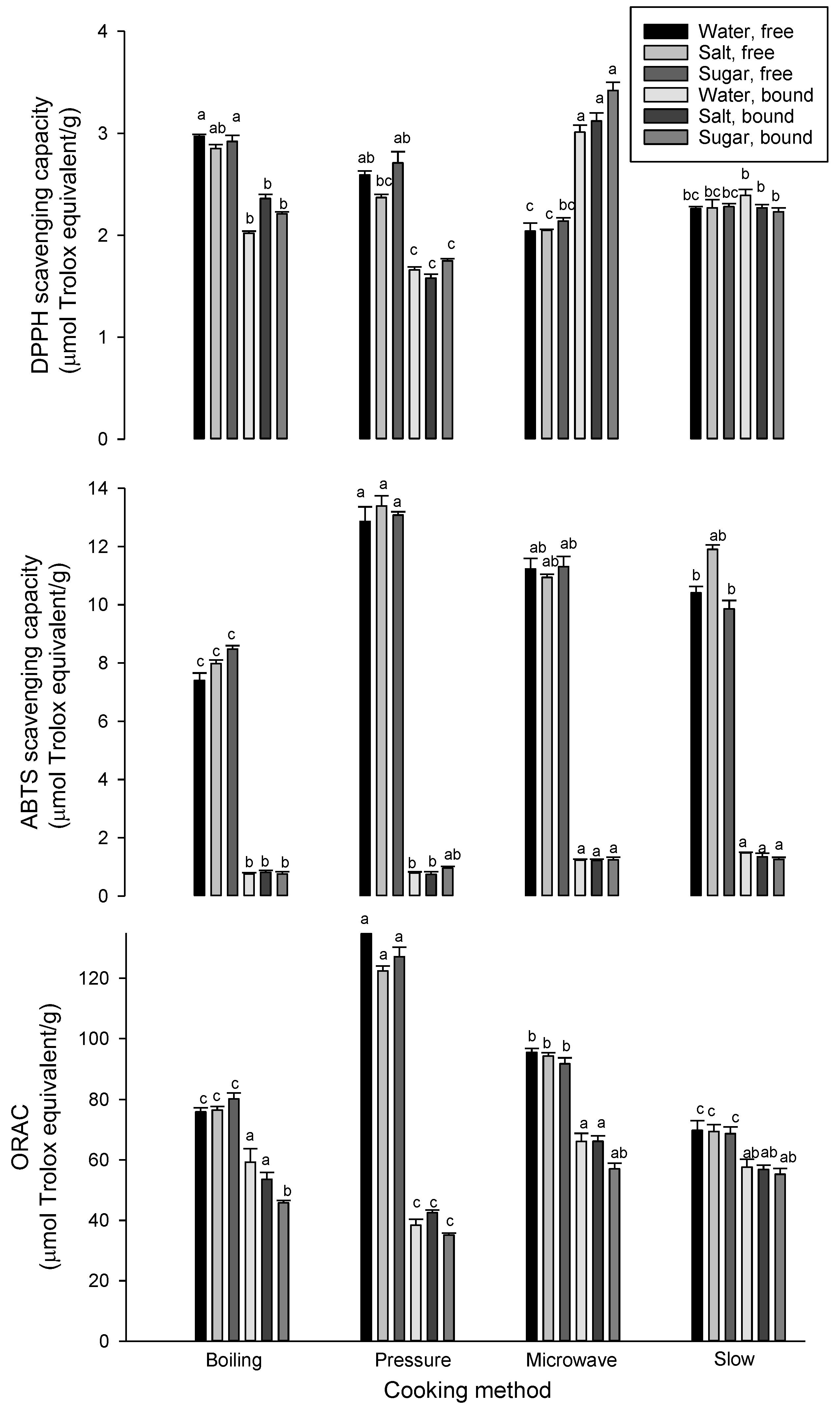
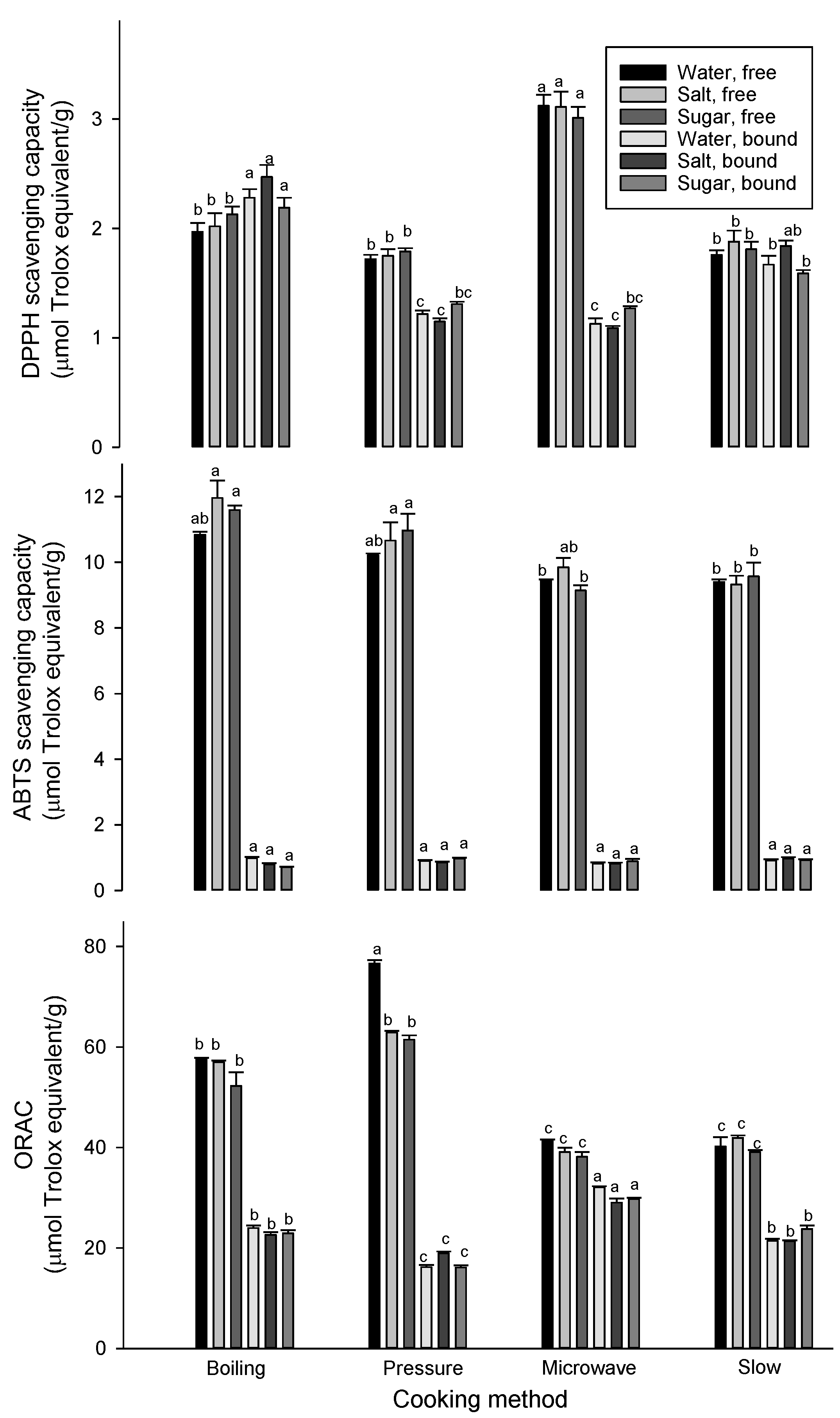
| Cooking Method | Solution | Free phenolic Acids | Bound Phenolic Acids | ||||
|---|---|---|---|---|---|---|---|
| p-Hydroxybenzoic | p-Coumaric | Ferulic | Sinapic | p-Coumaric | Ferulic | ||
| Boiling | Water | 1.24 ± 0.03 | 0.56 ± 0.02 | 2.98 ± 0.12 | 3.75 ± 0.13 | 13.81 ± 0.06 | 11.21 ± 0.12 |
| Salt | 1.06 ± 0.04 | 0.35 ± 0.04 | 2.96 ± 0.15 | 3.00 ± 0.09 | 11.59 ± 0.09 | 9.79 ± 0.11 | |
| Sugar | 1.40 ± 0.02 | 0.30 ± 0.04 | 2.70 ± 0.10 | 3.55 ± 0.27 | 9.93 ± 0.02 | 8.39 ± 0.04 | |
| Pressure | Water | 1.53 ± 0.04 | 0.48 ± 0.05 | 2.50 ± 0.14 | 3.03 ± 0.09 | 9.80 ± 0.05 | 8.43 ± 0.12 |
| Salt | 1.16 ± 0.07 | 0.44 ± 0.02 | 3.47 ± 0.19 | 3.44 ± 0.07 | 9.96 ± 0.05 | 7.11 ± 0.03 | |
| Sugar | 1.12 ± 0.05 | 0.53 ± 0.03 | 2.97 ± 0.20 | 3.15 ± 0.18 | 10.41 ± 0.06 | 6.74 ± 0.03 | |
| Microwave | Water | 1.34 ± 0.03 | 0.57 ± 0.11 | 3.51 ± 0.10 | 3.35 ± 0.08 | 11.52 ± 0.08 | 9.27 ± 0.04 |
| Salt | 1.38 ± 0.10 | 0.42 ± 0.05 | 2.50 ± 0.17 | 3.47 ± 0.10 | 10.89 ± 0.01 | 9.25 ± 0.02 | |
| Sugar | 1.10 ± 0.06 | 0.40 ± 0.05 | 2.60 ± 0.05 | 3.66 ± 0.33 | 11.23 ± 0.05 | 8.79 ± 0.06 | |
| Slow | Water | 1.27 ± 0.11 | 0.35 ± 0.02 | 2.29 ± 0.06 | 3.62 ± 0.13 | 9.91 ± 0.07 | 8.88 ± 0.04 |
| Salt | 1.03 ± 0.03 | 0.31 ± 0.04 | 2.64 ± 0.13 | 2.93 ± 0.17 | 9.76 ± 0.05 | 8.39 ± 0.07 | |
| Sugar | 1.06 ± 0.05 | 0.49 ± 0.04 | 2.93 ± 0.42 | 3.46 ± 0.12 | 9.39 ± 0.06 | 8.61 ± 0.03 | |
| Raw | 1.03 ± 0.04 | 0.63 ± 0.04 | 1.16 ± 0.07 | 1.67 ± 0.07 | 13.93 ± 0.04 | 11.33 ± 0.04 | |
| LSD at p < 0.05 | 0.25 | 0.16 | 0.55 | 0.39 | 1.75 | 1.66 | |
| Cooking Method | Solution | Free Phenolic Acids | Bound Phenolic Acids | |||||
|---|---|---|---|---|---|---|---|---|
| p-Hydroxybenzoic | p-Coumaric | Ferulic | Protocatechuic | p-Coumaric | Ferulic | Vanillic | ||
| Boiling | Water | 0.98 ± 0.09 | 1.65 ± 0.15 | 0.87 ± 0.04 | 5.88 ± 0.14 | 9.49 ± 0.15 | 9.10 ± 0.15 | 4.11 ± 0.03 |
| Salt | 1.02 ± 0.10 | 1.47 ± 0.02 | 0.87 ± 0.05 | 5.92 ± 0.13 | 9.19 ± 0.11 | 9.23 ± 0.11 | 4.44 ± 0.04 | |
| Sugar | 1.16 ± 0.11 | 1.19 ± 0.03 | 0.64 ± 0.03 | 5.59 ± 0.16 | 9.21 ± 0.17 | 9.21 ± 0.11 | 4.01 ± 0.00 | |
| Pressure | Water | 1.38 ± 0.12 | 1.68 ± 0.13 | 0.73 ± 0.05 | 5.67 ± 0.16 | 9.63 ± 0.10 | 8.81 ± 0.03 | 4.11 ± 0.03 |
| Salt | 1.33 ± 0.08 | 1.32 ± 0.06 | 0.69 ± 0.01 | 5.61 ± 0.45 | 9.71 ± 0.05 | 8.93 ± 0.01 | 4.10 ± 0.04 | |
| Sugar | 1.38 ± 0.13 | 1.28 ± 0.15 | 0.68 ± 0.10 | 5.12 ± 0.29 | 9.59 ± 0.03 | 8.73 ± 0.01 | 4.13 ± 0.07 | |
| Microwave | Water | 1.23 ± 0.15 | 1.02 ± 0.02 | 1.44 ± 0.08 | 5.55 ± 0.43 | 9.91 ± 0.05 | 8.69 ± 0.07 | 4.51 ± 0.03 |
| Salt | 1.23 ± 0.11 | 1.42 ± 0.11 | 1.38 ± 0.02 | 5.51 ± 0.18 | 10.13 ± 0.12 | 8.81 ± 0.00 | 4.54 ± 0.04 | |
| Sugar | 1.21 ± 0.09 | 1.04 ± 0.05 | 1.25 ± 0.04 | 5.50 ± 0.84 | 9.75 ± 0.11 | 9.03 ± 0.07 | 4.33 ± 0.00 | |
| Slow | Water | 1.33 ± 0.12 | 1.21 ± 0.09 | 1.01 ± 0.02 | 5.57 ± 0.26 | 10.22 ± 0.12 | 8.61 ± 0.11 | 4.42 ± 0.03 |
| Salt | 1.23 ± 0.09 | 1.40 ± 0.09 | 1.03 ± 0.07 | 5.54 ± 0.18 | 9.81 ± 0.12 | 8.49 ± 0.07 | 4.41 ± 0.04 | |
| Sugar | 1.25 ± 0.13 | 1.15 ± 0.07 | 1.13 ± 0.09 | 5.32 ± 0.20 | 9.43 ± 0.06 | 8.47 ± 0.13 | 4.01 ± 0.05 | |
| Raw | 1.55 ± 0.09 | 1.73 ± 0.07 | 0.85 ± 0.03 | 5.95 ± 0.35 | 13.61 ± 0.13 | 12.51 ± 0.14 | 6.16 ± 0.12 | |
| LSD at p < 0.05 | 0.23 | 0.35 | 0.39 | 0.36 | 1.69 | 1.53 | 0.81 | |
| Cooking Method | Solution | Free Phenolic Acids | Bound Phenolic Acids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| p-Hydroxybenzoic | p-Coumaric | Caffeic | Ferulic | p-Hydroxybenzoic | p-Coumaric | Ferulic | Sinapic | ||
| Boiling | Water | 1.14 ± 0.05 | 1.37 ± 0.06 | 1.01 ± 0.05 | 1.95 ± 0.09 | 1.88 ± 0.12 | 3.25 ± 0.02 | 11.16 ± 0.13 | 3.44 ± 0.14 |
| Salt | 1.24 ± 0.06 | 1.36 ± 0.04 | 1.05 ± 0.03 | 1.96 ± 0.07 | 1.97 ± 0.11 | 3.33 ± 0.05 | 11.07 ± 0.14 | 3.34 ± 0.16 | |
| Sugar | 1.14 ± 0.07 | 1.08 ± 0.18 | 1.04 ± 0.04 | 1.88 ± 0.04 | 1.84 ± 0.09 | 3.17 ± 0.02 | 10.87 ± 0.26 | 3.26 ± 0.12 | |
| Pressure | Water | 1.18 ± 0.07 | 1.40 ± 0.04 | 1.13 ± 0.04 | 1.86 ± 0.05 | 1.89 ± 0.11 | 3.29 ± 0.04 | 11.86 ± 0.40 | 3.25 ± 0.15 |
| Salt | 1.35 ± 0.06 | 1.37 ± 0.11 | 1.11 ± 0.02 | 1.89 ± 0.10 | 1.92 ± 0.07 | 3.35 ± 0.05 | 11.79 ± 0.12 | 3.31 ± 0.15 | |
| Sugar | 1.24 ± 0.09 | 1.11 ± 0.07 | 1.09 ± 0.04 | 1.77 ± 0.06 | 1.79 ± 0.01 | 3.21 ± 0.03 | 11.77 ± 0.33 | 3.27 ± 0.17 | |
| Microwave | Water | 1.11 ± 0.09 | 1.30 ± 0.05 | 1.24 ± 0.03 | 1.89 ± 0.10 | 1.95 ± 0.14 | 3.26 ± 0.03 | 11.89 ± 0.36 | 3.42 ± 0.09 |
| Salt | 1.15 ± 0.09 | 1.30 ± 0.02 | 1.16 ± 0.07 | 1.91 ± 0.11 | 1.99 ± 0.13 | 3.33 ± 0.03 | 11.84 ± 0.48 | 3.37 ± 0.23 | |
| Sugar | 1.03 ± 0.07 | 1.13 ± 0.03 | 1.03 ± 0.01 | 1.79 ± 0.08 | 1.80 ± 0.18 | 3.19 ± 0.06 | 11.66 ± 0.11 | 3.28 ± 0.14 | |
| Slow | Water | 1.16 ± 0.10 | 1.33 ± 0.04 | 1.04 ± 0.03 | 1.92 ± 0.05 | 1.92 ± 0.11 | 3.32 ± 0.05 | 11.69 ± 0.85 | 3.37 ± 0.15 |
| Salt | 1.21 ± 0.08 | 1.31 ± 0.06 | 1.07 ± 0.05 | 1.94 ± 0.07 | 1.94 ± 0.10 | 3.33 ± 0.01 | 11.74 ± 0.02 | 3.29 ± 0.18 | |
| Sugar | 1.06 ± 0.07 | 1.04 ± 0.07 | 1.01 ± 0.08 | 1.84 ± 0.07 | 1.83 ± 0.09 | 3.21 ± 0.04 | 11.77 ± 0.40 | 3.25 ± 0.11 | |
| Raw | 1.02 ± 0.05 | 1.51 ± 0.06 | 1.11 ± 0.02 | 1.36 ± 0.02 | 1.98 ± 0.11 | 4.29 ± 0.11 | 15.39 ± 0.29 | 5.63 ± 0.16 | |
| LSD at p < 0.05 | 0.15 | 0.23 | 0.13 | 0.25 | 0.12 | 0.44 | 1.71 | 0.93 | |
| Cooking Method | Solution | Methanol Extract (ME) | Alkaline-Liberated Phenolic Extract (ALPE) | % Increase (+) or Decrease (−) | |
|---|---|---|---|---|---|
| ME | ALPE | ||||
| Faba Bean | |||||
| Boiling | Water | 23.88 ± 2.10 | 2.84 ± 0.18 | −37.7 | −39.2 |
| Salt | 24.73 ± 0.53 | 3.06 ± 0.09 | −35.5 | −34.5 | |
| Sugar | 22.48 ± 0.37 | 2.90 ± 0.15 | −41.4 | −37.9 | |
| Pressure | Water | 17.64 ± 0.99 | 3.81 ± 0.16 | −54.0 | −18.4 |
| Salt | 19.45 ± 1.18 | 3.40 ± 0.14 | −49.3 | −27.2 | |
| Sugar | 19.49 ± 0.28 | 2.31 ± 0.14 | −49.2 | −50.5 | |
| Microwave | Water | 22.45 ± 0.49 | 2.61 ± 0.05 | −41.4 | −44.1 |
| Salt | 23.26 ± 0.27 | 2.55 ± 0.11 | −39.3 | −45.4 | |
| Sugar | 23.82 ± 0.26 | 2.51 ± 0.16 | −37.9 | −46.3 | |
| Slow | Water | 19.15 ± 0.86 | 2.81 ± 0.09 | −50.1 | −39.8 |
| Salt | 17.25 ± 0.46 | 2.95 ± 0.07 | −55.0 | −36.8 | |
| Sugar | 17.08 ± 0.56 | 2.62 ± 0.01 | −55.4 | −43.9 | |
| Raw | 38.34 ± 3.23 | 4.67 ± 0.15 | - | - | |
| LSD at p < 0.05 | 7.71 | 0.90 | - | - | |
| Lentil | |||||
| Boiling | Water | 8.67 ± 0.14 | 4.14 ± 0.09 | −38.6 | −40.1 |
| Salt | 9.10 ± 0.11 | 4.51 ± 0.49 | −35.6 | −34.7 | |
| Sugar | 9.56 ± 0.19 | 3.81 ± 0.19 | −32.3 | −44.9 | |
| Pressure | Water | 14.48 ± 0.69 | 6.06 ± 0.54 | 2.5 | −12.3 |
| Salt | 16.49 ± 0.27 | 5.89 ± 0.70 | 16.7 | −14.8 | |
| Sugar | 16.94 ± 2.47 | 3.56 ± 0.04 | 19.9 | −48.5 | |
| Microwave | Water | 22.45 ± 0.49 | 7.23 ± 0.80 | 58.9 | 4.6 |
| Salt | 23.26 ± 0.07 | 8.22 ± 0.36 | 64.6 | 19.0 | |
| Sugar | 23.82 ± 0.26 | 7.05 ± 0.84 | 68.6 | 2.0 | |
| Slow | Water | 7.89 ± 0.58 | 4.33 ± 0.81 | −44.2 | −37.3 |
| Salt | 8.38 ± 0.01 | 4.78 ± 0.30 | −40.7 | −30.8 | |
| Sugar | 8.44 ± 0.01 | 6.60 ± 0.07 | −40.3 | −4.5 | |
| Raw | 14.13 ± 0.90 | 6.91 ± 0.23 | - | - | |
| LSD at p < 0.05 | 8.45 | 2.13 | - | - | |
| Pea | |||||
| Boiling | Water | 13.24 ± 0.69 | 4.79 ± 0.22 | 28.5 | 146.9 |
| Salt | 11.23 ± 0.17 | 3.97 ± 0.01 | 9.0 | 104.6 | |
| Sugar | 14.01 ± 0.03 | 2.69 ± 0.11 | 36.0 | 38.7 | |
| Pressure | Water | 13.26 ± 0.50 | 3.83 ± 0.06 | 28.7 | 97.4 |
| Salt | 11.27 ± 0.41 | 2.15 ± 0.09 | 9.4 | 10.8 | |
| Sugar | 11.16 ± 0.03 | 3.03 ± 0.15 | 8.3 | 56.2 | |
| Microwave | Water | 14.23 ± 0.76 | 2.02 ± 0.01 | 38.2 | 4.1 |
| Salt | 13.28 ± 0.53 | 2.77 ± 0.05 | 28.9 | 42.8 | |
| Sugar | 11.76 ± 0.19 | 2.81 ± 0.07 | 14.2 | 44.8 | |
| Slow | Water | 11.84 ± 0.10 | 2.68 ± 0.25 | 15.0 | 38.1 |
| Salt | 15.29 ± 1.65 | 4.09 ± 0.04 | 48.4 | 110.8 | |
| Sugar | 14.36 ± 3.76 | 3.81 ± 0.16 | 39.4 | 96.4 | |
| Raw | 10.30 ± 0.06 | 1.94 ± 0.02 | - | - | |
| LSD at p < 0.05 | 2.17 | 1.25 | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.-S.M. Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions. Foods 2020, 9, 908. https://doi.org/10.3390/foods9070908
Liu Y, Ragaee S, Marcone MF, Abdel-Aal E-SM. Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions. Foods. 2020; 9(7):908. https://doi.org/10.3390/foods9070908
Chicago/Turabian StyleLiu, Yihan, Sanaa Ragaee, Massimo F. Marcone, and El-Sayed M. Abdel-Aal. 2020. "Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions" Foods 9, no. 7: 908. https://doi.org/10.3390/foods9070908
APA StyleLiu, Y., Ragaee, S., Marcone, M. F., & Abdel-Aal, E.-S. M. (2020). Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions. Foods, 9(7), 908. https://doi.org/10.3390/foods9070908






