Effects of Sugars and Sugar Alcohols on the Gelatinization Temperatures of Wheat, Potato, and Corn Starches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sweetener Solutions
2.2.2. Gelatinization Temperature (Tgel)
2.2.3. Data Analysis
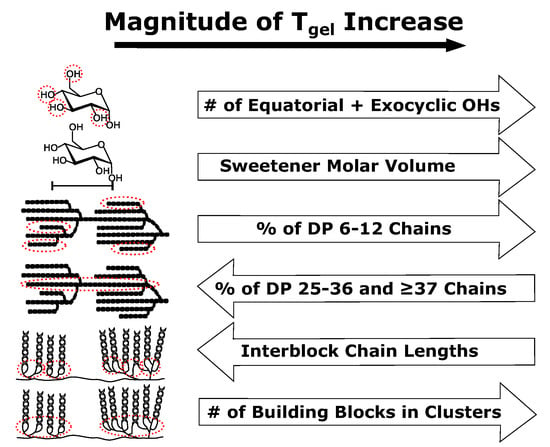
3. Results and Discussion
3.1. Effects of Sweetener Properties on the Gelatinization Temperatures of Starches
3.1.1. Wheat Starch
3.1.2. Waxy, Dent, and High Amylose Corn Starches
3.1.3. Potato Starch
3.2. Effects of Starch Properties
3.2.1. Amylose Content
3.2.2. Amylopectin Architecture
3.2.3. Amylopectin Crystalline Structure
3.2.4. Percent Crystallinity
3.2.5. Potato Starch—The Anomaly
- (1)
- Size Exclusion within the Granule
- (2)
- Phosphorous Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huber, K.C.; BeMiller, J.N. Carbohydrates. In Fennema’s Food Chemistry, 5th ed.; Damodaran, S., Parkin, K.L., Eds.; CRC Press: New York, NY, USA, 2017; pp. 91–169. [Google Scholar]
- Jane, J. Structural features of starch granules II. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R.L., Eds.; Academic Press: New York, NY, USA, 2009; pp. 193–236. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Perez, S.; Baldwin, P.M.; Gallant, D.J. Structural features of starch granules I. In Starch: Chemistry and Technology, 3rd ed.; BeMiller, J.N., Whistler, R.L., Eds.; Academic Press: New York, NY, USA, 2009; pp. 149–192. [Google Scholar]
- Jenkins, P.J.; Cameron, R.E.; Donald, A.M. A universal feature in the structure of starch granules from different botanical sources. Starch-Stärke 1993, 45, 417–420. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of starch: Evidence of a new level of granule organization. Carbohydr. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Zeleznak, K.; Hoseney, R. The glass transition in starch. Cereal Chem. 1987, 64, 121–124. [Google Scholar]
- Ratnayake, W.S.; Jackson, D.S. Starch gelatinization. Adv. Food Nutr. Res. 2008, 55, 221–268. [Google Scholar]
- Perry, P.; Donald, A. The role of plasticization in starch granule assembly. Biomacromolecules 2000, 1, 424–432. [Google Scholar] [CrossRef]
- Donovan, J.W. Phase transitions of the starch–water system. Biopolymers 1979, 18, 263–275. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E.; Seetharaman, K. On the importance of organization of glucan chains on thermal properties of starch. Carbohydr. Polym. 2013, 92, 1653–1659. [Google Scholar] [CrossRef]
- Pfannemüller, B. Influence of chain length of short monodisperse amyloses on the formation of A-and B-type X-ray diffraction patterns. Int. J. Biol. Macromol. 1987, 9, 105–108. [Google Scholar] [CrossRef]
- Genkina, N.K.; Wikman, J.; Bertoft, E.; Yuryev, V.P. Effects of Structural Imperfection on Gelatinization Characteristics of Amylopectin Starches with A- and B-Type Crystallinity. Biomacromolecules 2007, 8, 2329–2335. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Seib, P.A. The structure of four waxy starches related to gelatinization and retrogradation. Carb. Res. 1992, 227, 131–145. [Google Scholar] [CrossRef]
- Noda, T.; Isono, N.; Krivandin, A.V.; Shatalova, O.V.; Błaszczak, W.; Yuryev, V.P. Origin of defects in assembled supramolecular structures of sweet potato starches with different amylopectin chain-length distribution. Carbohydr. Polym. 2009, 76, 400–409. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of cornstarch with different amylose/amylopectin content. Carbohydr. Polym. 2006, 65, 357–363. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. I. Structural aspects provide insight into crystallinity characteristics and gelatinisation behaviour of granular starch. J. Cereal Sci. 2003, 38, 43–52. [Google Scholar] [CrossRef]
- Gomand, S.; Lamberts, L.; Derde, L.; Goesaert, H.; Vandeputte, G.; Goderis, B.; Visser, R.; Delcour, J. Structural properties and gelatinisation characteristics of potato and cassava starches and mutants thereof. Food Hydrocolloids 2010, 24, 307–317. [Google Scholar] [CrossRef]
- Evans, I.; Haisman, D. The effect of solutes on the gelatinization temperature range of potato starch. Starch-Stärke 1982, 34, 224–231. [Google Scholar] [CrossRef]
- Spies, R.D.; Hoseney, R.C. Effect of sugars on starch gelatinization. Cereal Chem. 1982, 59, 128–131. [Google Scholar]
- Beleia, A.; Miller, R.A.; Hoseney, R.C. Starch gelatinization in sugar solutions. Starch-Stärke 1996, 48, 259–262. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Non-equilibrium melting of native granular starch: Part I. Temperature location of the glass transition associated with gelatinization of A-type cereal starches. Carbohydr. Polym. 1988, 8, 183–208. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Non-equilibrium behavior of small carbohydrate-water systems. Pure Appl. Chem. 1988, 60, 1841–1864. [Google Scholar] [CrossRef]
- Hansen, L.; Setser, C.; Paukstelis, J. Investigations of sugar-starch interactions using carbon-13 nuclear magnetic resonance. I. sucrose. Cereal Chem. 1989, 66, 411–415. [Google Scholar]
- Hoover, R.; Senanayake, N. Effect of sugars on the thermal and retrogradation properties of oat starches. J. Food Biochem. 1996, 20, 65–83. [Google Scholar] [CrossRef]
- Perry, P.; Donald, A. The effect of sugars on the gelatinisation of starch. Carbohydr. Polym. 2002, 49, 155–165. [Google Scholar] [CrossRef]
- Allan, M.C.; Rajwa, B.; Mauer, L.J. Effects of sugars and sugar alcohols on the gelatinization temperature of wheat starch. Food Hydrocolloids 2018, 84, 593–607. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Mauer, L.J. Starch gelatinization temperature in sugar and polyol solutions explained by hydrogen bond density. Food Hydrocolloids 2019, 94, 371–380. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Bertoft, E.; Koch, K.; Åman, P. Building block organisation of clusters in amylopectin from different structural types. Int. J. Biol. Macromol. 2012, 50, 1212–1223. [Google Scholar] [CrossRef]
- Peymanpour, G.; Marcone, M.; Ragaee, S.; Tetlow, I.; Lane, C.C.; Seetharaman, K.; Bertoft, E. On the molecular structure of the amylopectin fraction isolated from “high-amylose” ae maize starches. Int. J. Biol. Macromol. 2016, 91, 768–777. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolym. Orig. Rese. Biomol. 2008, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Bertoft, E. Composition of building blocks in clusters from potato amylopectin. Carbohydr. Polym. 2007, 70, 123–136. [Google Scholar] [CrossRef]
- Kalinga, D.N.; Bertoft, E.; Tetlow, I.; Seetharaman, K. Structure of clusters and building blocks in amylopectin from developing wheat endosperm. Carbohydr. Polym. 2014, 112, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C. Effect of water content on the gelatinization of wheat starch. Starch-Stärke 1980, 32, 270–272. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists., 3rd ed.; Elsevier: Cambridge, MA, USA, 2018. [Google Scholar]
- Shahidi, F.; Farrell, P.G.; Edward, J.T. Partial molar volumes of organic compounds in water. III. Carbohydrates. J. Solut. Chem. 1976, 5, 807–816. [Google Scholar] [CrossRef]
- Buttersack, C. Hydrophobicity of carbohydrates and related hydroxy compounds. Carb. Res. 2017, 446, 101–112. [Google Scholar] [CrossRef]
- Høiland, H.; Holvik, H. Partial molal volumes and compressibilities of carbohydrates in water. J. Solut. Chem. 1978, 7, 587–596. [Google Scholar] [CrossRef]
- Shamil, S.; Birch, G.; Mathlouthi, M.; Clifford, M. Apparent molar volumes and tastes of molecules with more than one sapophore. Chem. Senses 1987, 12, 397–409. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, C.; Ma, Y. Density and viscosity of sorbitol/maltitol in L-ascorbic acid aqueous solutions at T=(293.15 to 323.15)K. J. Mol. Liq. 2013, 188, 67–73. [Google Scholar] [CrossRef]
- Jang, J.; Pyun, Y. Effect of moisture content on the melting of wheat starch. Starch-Stärke 1996, 48, 48–51. [Google Scholar] [CrossRef]
- Stoddart, J.F. Stereochemistry of Carbohydrates; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1971. [Google Scholar]
- Uedaira, H.; Ishimura, M.; Tsuda, S.; Uedaira, H. Hydration of oligosaccharides. Bull. Chem. Soc. Jpn. 1990, 63, 3376–3379. [Google Scholar] [CrossRef]
- Miljković, M. Relative reactivity of hydroxyl groups in monosaccharides. In Carbohydrates: Synthesis, Mechanisms, and Stereoelectronic Effects; Miljković, M., Ed.; Springer: New York, NY, USA, 2010; pp. 113–142. [Google Scholar]
- Hsieh, C.-F.; BeMiller, J.N.; Huber, K.C. Impact of granule hydration on maize and wheat starch chemical reactivity at the granular and molecular levels. Food Hydrocoll. 2019, 105374. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Predictions of glass transition temperature for hydrogen bonding biomaterials. J. Phys. Chem. B 2013, 117, 16303–16313. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.H.; Yoo, B.; Lim, S.T. Effects of sugars and sugar alcohols on thermal transition and cold stability of corn starch gel. Food Hydrocoll. 2004, 18, 133–142. [Google Scholar] [CrossRef]
- Morrison, W.R.; Tester, R.F.; Snape, C.E.; Law, R.; Gidley, M. Swelling and gelatinization of cereal starches. IV. Some effects of lipid-complexed amylose and free amylose in waxy and normal barley starches. Cereal Chem. 1993, 70, 385. [Google Scholar]
- Matveev, Y.I.; Van Soest, J.; Nieman, C.; Wasserman, L.; Protserov, V.; Ezernitskaja, M.; Yuryev, V. The relationship between thermodynamic and structural properties of low and high amylose maize starches. Carbohydr. Polym. 2001, 44, 151–160. [Google Scholar] [CrossRef]
- Biliaderis, C.G.; Page, C.M.; Maurice, T.J.; Juliano, B.O. Thermal characterization of rice starches: A polymeric approach to phase transitions of granular starch. J. Agric. Food Chem. 1986, 34, 6–14. [Google Scholar] [CrossRef]
- Juliano, B.; Perez, C. Crystallinity of raw rice starch granules as indexed by corrosion with hydrochloric acid and amylase. Starch-Stärke 1990, 42, 49–52. [Google Scholar] [CrossRef]
- Noda, T.; Takahata, Y.; Sato, T.; Suda, I.; Morishita, T.; Ishiguro, K.; Yamakawa, O. Relationships between chain length distribution of amylopectin and gelatinization properties within the same botanical origin for sweet potato and buckwheat. Carbohydr. Polym. 1998, 37, 153–158. [Google Scholar] [CrossRef]
- Karim, A.; Toon, L.; Lee, V.; Ong, W.; Fazilah, A.; Noda, T. Effects of phosphorus contents on the gelatinization and retrogradation of potato starch. J. Food Sci. 2007, 72, C132–C138. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Yang, H.; Zhao, S.; Liu, Y.; Liu, R. Effects of salts on the gelatinization and retrogradation properties of maize starch and waxy maize starch. Food Chem. 2017, 214, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-C.; Seib, P.A. Fine structure of maize starches from four wx-containing genotypes of the W64A inbred line in relation to gelatinization and retrogradation. Carbohydr. Polym. 1995, 26, 141–147. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Amylopectin molecular structure in relation to physicochemical properties of quinoa starch. Carbohydr. Polym. 2017, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bertoft, E.; Bao, J.; Corke, H. Molecular structure of amylopectin from amaranth starch and its effect on physicochemical properties. Int. J. Biol. Macromol. 2008, 43, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Hanashiro, I.; Abe, J.-I.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carb. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Hizukuri, S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carb. Res. 1986, 147, 342–347. [Google Scholar] [CrossRef]
- Donald, A.M.; Perry, P.A.; Waigh, T.A. The impact of internal granule structure on processing and properties. In Starch: Advances in Structure and Function; Barsby, T.L., Donald, A.M., Frazier, P.J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2001; Volume 271, pp. 45–52. [Google Scholar]
- Ai, Y.; Jane, J.-l. Understanding starch structure and functionality. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–178. [Google Scholar]
- Semeijn, C.; Buwalda, P.L. Potato Starch. In Starch in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 353–372. [Google Scholar]
- Jane, J.; Chen, Y.; Lee, L.; McPherson, A.; Wong, K.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Blennow, A.; Bay-Smidt, A.M.; Olsen, C.E.; Møller, B.L. The distribution of covalently bound phosphate in the starch granule in relation to starch crystallinity. Int. J. Biol. Macromol. 2000, 27, 211–218. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics; 1st student ed.; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Marsh, R.; Waight, S. The effect of pH on the zeta potential of wheat and potato starch. Starch-Stärke 1982, 34, 149–152. [Google Scholar] [CrossRef]
- Huber, K.C.; BeMiller, J.N. Location of sites of reaction within starch granules. Cereal Chem. 2001, 78, 173–180. [Google Scholar] [CrossRef]
- Lim, S.; Seib, P. Location of Phosphate Esters in a Wheat Starch Phosphate by 31P-Nuclear Magnetic Resonance Spectroscopy. Cereal Chem. 1993, 70, 145. [Google Scholar]
- Takeda, Y.; Hizukuri, S. Location of phosphate groups in potato amylopectin. Carb. Res. 1982, 102, 321–327. [Google Scholar] [CrossRef]
- McPherson, A.E.; Jane, J. Comparison of waxy potato with other root and tuber starches. Carbohydr. Polym. 1999, 40, 57–70. [Google Scholar] [CrossRef]
- Mali, S.; Ferrero, C.; Redigonda, V.; Beleia, A.P.; Grossmann, M.V.E.; Zaritzky, N.E. Influence of pH and hydrocolloids addition on yam (Dioscorea alata) starch pastes stability. LWT Food Sci. Technol. 2003, 36, 475–481. [Google Scholar] [CrossRef]





| Amylose Content [2] | Average | % Distribution [2] | Granule | Phosp- | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starch | Tgel Onset (°C) † | Percent Crystallinity ‡ | Crystal Type [2] | Apparent | Absolute | Chain Length (DP) [2] | DP 6–12 | DP 13–24 | DP 25–36 | DP ≥ 37 | NBbl | IB-CL | Size (μm) [1] | Horus (%DS) [1] |
| Waxy corn | 65.84 ± 0.25 B | 41.8 [31] | A | <1% | <1% | 23.5 | 17.0 | 49.4 | 17.1 | 16.5 | 5.2 [32] | 6.2 [32] | 2–30 | 0.00 |
| Dent corn | 66.19 ± 0.65 B | 30.3 [31] | A | 29.4 | 22.5 | 24.4 | 17.9 | 47.9 | 14.9 | 19.3 | 6.2 [33] | 6.8 [33] | 2–30 | 0.00 |
| HACS55 | 71.81 ± 0.25 C | 19.5 [31] | B | 52 | 27.3 | 28.9 | 9.7 | 43.9 | 20.3 | 26.1 | 5.4 [33] | 9.1 [33] | 2–24 | 0.00 |
| HACS70 | 71.27 ± 0.37 C | 20.7 [34] | B | 68 | 40.2 | 30.7 | 8.5 | 40.7 | 21.3 | 29.5 | 5.6 [33] | 8.9 [33] | 2–24 | 0.00 |
| Potato | 61.32 ± 0.19 A | 45.5 [34] | B | 36 | 16.9 | 29.4 | 12.3 | 43.3 | 15.5 | 28.9 | 3–5 [35] | 7–8 [35] | 5–100 | 0.08 |
| Wheat | 60.78 ± 0.09 A | 22.8 [34] | A | 28.8 | 25.8 | 22.7 | 19.0 | 41.7 | 16.2 | 13.0 | 6.2–6.3 [36] | 6.4–6.5 [36] | 2–55 | 0.00 |
| Sweetener | Number of Carbons | Sweetener Type | Reducing Sugar | Glycosidic Linkage [38] | Number of OH Groups for Inter-Molecular H-Bonding [30] | Calculated Equatorial and Exo-Cyclic OHs in Solution [29] | Dry Tg (°C) [39] | Molar Volume (cm3/mol) | Capacity Factor (Kc) [40] |
|---|---|---|---|---|---|---|---|---|---|
| Fructose | 6 | Sugar | Yes | NA | 3.98 | 2.8 | 15.16 ± 0.11 | 110.4 ± 0.4 [39] | 0.029 |
| Mannose | 6 | Sugar | Yes | NA | 4.05 | 3.3 | 35.91 ± 0.10 | 111.7 ± 0.5 [39] | 0.026 |
| Galactose | 6 | Sugar | Yes | NA | 3.95 | 3.6 | 31.92 ± 0.47 | 111.9 ± 0.3 [39] | −0.006 |
| Glucose | 6 | Sugar | Yes | NA | 3.98 | 4.6 | 38.30 ± 0.01 | 112.2 ± 0.4 [39] | 0.016 |
| Sorbitol | 6 | Sugar Alcohol | No | NA | 3.21 | 6 | −1.54 ± 0.71 | 119.9 [41] | 0.012 |
| Sucrose | 12 | Sugar | No | αGlcp(1 → 2)βFruf | 4.48 | 6 | 59.36 ± 0.56 | 210.2 ± 0.8 [39] | 0.47 |
| Isomaltulose | 12 | Sugar | Yes | αGlcp(1 → 6)Fru | 4.75 | 5.2 | 60.56 ± 0.61 | 219.5 [42] | 0.177 |
| Isomalt | 12 | Sugar Alcohol | No | αGlcp(1 → 6)Sor & αGlcp(1 → 6)Mtl | 4.69 | 9 | 58.73 ± 1.63 | NA | 0.1260.143 |
| Trehalose | 12 | Sugar | No | αGlcp(1 → 1)αGlcp | 7.72 | 8 | 117.51 ± 2.01 | 206.9 ± 0.5 [39] | 0.128 |
| Maltose | 12 | Sugar | Yes | αGlcp(1 → 4)Glc | 5.74 | 7.4 | 48.99 ± 3.83 | 208.8 ± 0.8 [39] | 0.195 |
| Maltitol | 12 | Sugar Alcohol | No | αGlcp(1 → 4)Sor | 4.33 | 9 | 46.40 ± 0.11 | 215.367 [43] | NA |
| p-Values | |||||||
|---|---|---|---|---|---|---|---|
| Starch | Sweetener Concen-Tration | Size (6 OR 12-C) | Type (Sug./Sug. Alc.) | Reducing Sugar (Red./Nonred.) | Conc.* Size | Conc.* Type | Conc.* Red. |
| Waxy corn | <0.001 | <0.001 | <0.001 | 0.981 | 0.001 | 0.060 | 0.660 |
| Dent corn | <0.001 | <0.001 | <0.001 | 0.428 | <0.001 | 0.198 | 0.948 |
| HACS55 | <0.001 | <0.001 | 0.004 | 0.913 | 0.070 | 0.401 | 0.358 |
| HACS70 | <0.001 | <0.001 | <0.001 | 0.899 | 0.196 | 0.403 | 0.042 |
| Potato | <0.001 | 0.661 | 0.003 | 0.133 | 0.911 | 0.423 | 0.671 |
| Wheat | <0.001 | 0.001 | 0.118 | 0.513 | <0.001 | 0.302 | 0.005 |
| Correlation Coefficients (R) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NOH,s | Equatorial and Exocyclic OHs | Dry Tg (°C) | Molar Volume (cm3/mol) | Capacity Factor (Kc) | ||||||
| Starch | log Tgels | ΔTgel(3M-0) | log Tgels | ΔTgel(3M-0) | log Tgels | ΔTgel(3M-0) | log Tgels | ΔTgel(3M-0) | log Tgels | ΔTgel(3M-0) |
| Waxy | 0.087 | 0.260 | 0.747 ** | 0.771 ** | 0.296 | 0.506 | 0.589 * | 0.767 ** | 0.385 | 0.099 |
| Dent corn | 0.188 | 0.342 | 0.755 ** | 0.780 ** | 0.379 | 0.563 * | 0.703 ** | 0.812 ** | 0.084 | 0.389 |
| HACS55 | 0.094 | 0.294 | 0.558 * | 0.649 ** | 0.321 | 0.543 | 0.444 | 0.605 * | 0.409 | 0.087 |
| HACS70 | 0.075 | 0.145 | 0.662 ** | 0.743 ** | 0.306 | 0.358 | 0.599 * | 0.618 * | 0.522 | 0.310 |
| Potato | 0.361 | 0.039 | 0.024 | 0.159 | 0.164 | 0.197 | 0.340 | 0.077 | 0.066 | 0.321 |
| Wheat | 0.074 | 0.204 | 0.606 ** | 0.676 ** | 0.315 | 0.419 | 0.623 * | 0.770 ** | 0.272 | 0.122 |
| Correlation Coefficients (R) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average Chain Length | % DP 6–12 | % DP 13–24 | % DP 25–36 | % DP ≥ 37 | ||||||
| Sugar | log Tgel | ΔTgel(3M-0) | log Tgel | ΔTgel(3M-0) | log Tgel | ΔTgel(3M-0) | log Tgel | ΔTgel(3M-0) | log Tgel | ΔTgel(3M-0) |
| Fructose | −0.145 | 0.261 | 0.35 | −0.043 | −0.049 | −0.143 | −0.764 * | −0.527 | −0.078 | 0.341 |
| Galactose | −0.734 * | NA | 0.747 * | NA | −0.174 | NA | −0.633 | NA | −0.813 * | NA |
| Glucose | −0.454 | −0.385 | 0.649 | 0.547 | 0.048 | 0.107 | −0.900 ** | −0.751 * | −0.394 | −0.321 |
| Isomalt | −0.774 * | −0.773 * | 0.914 ** | 0.820 ** | 0.365 | 0.551 | −0.933 ** | −0.633 | −0.702 | −0.708 |
| Isomaltulose | −0.746 * | −0.778 * | 0.842 ** | 0.808 * | 0.007 | −0.016 | −0.741 * | −0.541 | −0.748 * | −0.810 ** |
| Maltitol | −0.857 ** | −0.886 ** | 0.938 ** | 0.904 ** | 0.220 | 0.311 | −0.761 * | −0.589 | −0.838 ** | −0.877 ** |
| Maltose | −0.758 * | −0.677 | 0.881 ** | 0.674 | 0.266 | 0.114 | −0.820 ** | −0.343 | −0.707 | −0.695 |
| Mannose | −0.025 | 0.498 | 0.255 | −0.302 | 0.057 | 0.066 | −0.761 * | −0.318 | 0.076 | 0.616 |
| Sorbitol | −0.674 | −0.111 | 0.822 ** | 0.137 | 0.075 | −0.631 | −0.873 ** | 0.104 | −0.640 | −0.207 |
| Sucrose | −0.628 | −0.649 | 0.728 | 0.623 | −0.146 | −0.110 | −0.567 | −0.210 | −0.646 | −0.711 |
| Trehalose | −0.787 * | −0.687 | 0.894 ** | 0.718 | 0.188 | 0.345 | −0.840 * | −0.521 | −0.756 * | −0.655 |
| Correlation Coefficients (R) | ||||
|---|---|---|---|---|
| NBbl | IB-CL | |||
| Sugar | log Tgel | ΔTgel(3M-0) | log Tgel | ΔTgel(3M-0) |
| Fructose | −0.451 | −0.65 | −0.405 | −0.026 |
| Galactose | 0.717 | NA | −0.613 | NA |
| Glucose | −0.116 | 0.247 | −0.65 | −0.369 |
| Isomalt | 0.302 | 0.674 | −0.856 ** | −0.675 |
| Isomaltulose | 0.447 | 0.709 | −0.717 | −0.619 |
| Maltitol | 0.504 | 0.742 * | −0.863 ** | −0.769 * |
| Maltose | 0.509 | 0.918 ** | −0.765 * | −0.434 |
| Mannose | −0.572 | −0.715 | −0.356 | 0.207 |
| Sorbitol | 0.163 | 0.651 | −0.792 * | 0.011 |
| Sucrose | 0.587 | 0.897 ** | −0.609 | −0.427 |
| Trehalose | 0.413 | 0.786 * | −0.774 * | −0.482 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allan, M.C.; Chamberlain, M.; Mauer, L.J. Effects of Sugars and Sugar Alcohols on the Gelatinization Temperatures of Wheat, Potato, and Corn Starches. Foods 2020, 9, 757. https://doi.org/10.3390/foods9060757
Allan MC, Chamberlain M, Mauer LJ. Effects of Sugars and Sugar Alcohols on the Gelatinization Temperatures of Wheat, Potato, and Corn Starches. Foods. 2020; 9(6):757. https://doi.org/10.3390/foods9060757
Chicago/Turabian StyleAllan, Matthew C., MaryClaire Chamberlain, and Lisa J. Mauer. 2020. "Effects of Sugars and Sugar Alcohols on the Gelatinization Temperatures of Wheat, Potato, and Corn Starches" Foods 9, no. 6: 757. https://doi.org/10.3390/foods9060757
APA StyleAllan, M. C., Chamberlain, M., & Mauer, L. J. (2020). Effects of Sugars and Sugar Alcohols on the Gelatinization Temperatures of Wheat, Potato, and Corn Starches. Foods, 9(6), 757. https://doi.org/10.3390/foods9060757