Hydrocolloid-Based Coatings with Nanoparticles and Transglutaminase Crosslinker as Innovative Strategy to Produce Healthier Fried Kobbah
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticles Preparation
2.3. Kobbah Formulation and Manufacturing
2.4. Preparation of the Coating Solutions
2.5. Dipping and Frying Method
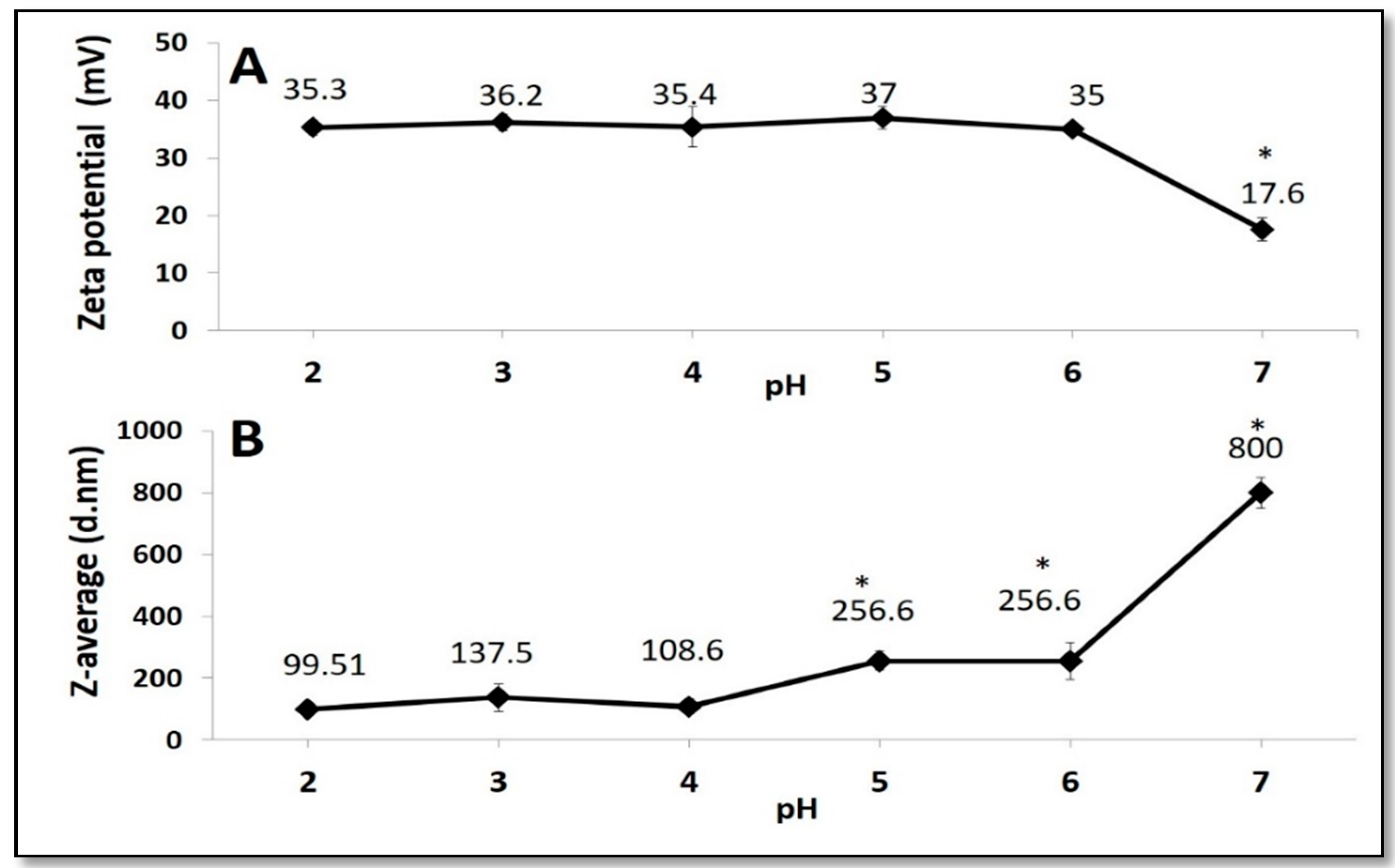
2.6. Zeta Potential and Z-Average of Coating Solutions
2.7. Oil and Water Content
2.8. Acrylamide Detection
2.8.1. Preparation of Acrylamide Standard
2.8.2. Acrylamide Extraction
2.8.3. HPLC-UV Analysis
2.9. Color Analysis
2.10. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and In Vitro Digestion
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chitosan Nanoparticles, Mesoporous Silica Nanoparticles and Film Forming Solutions Characterization
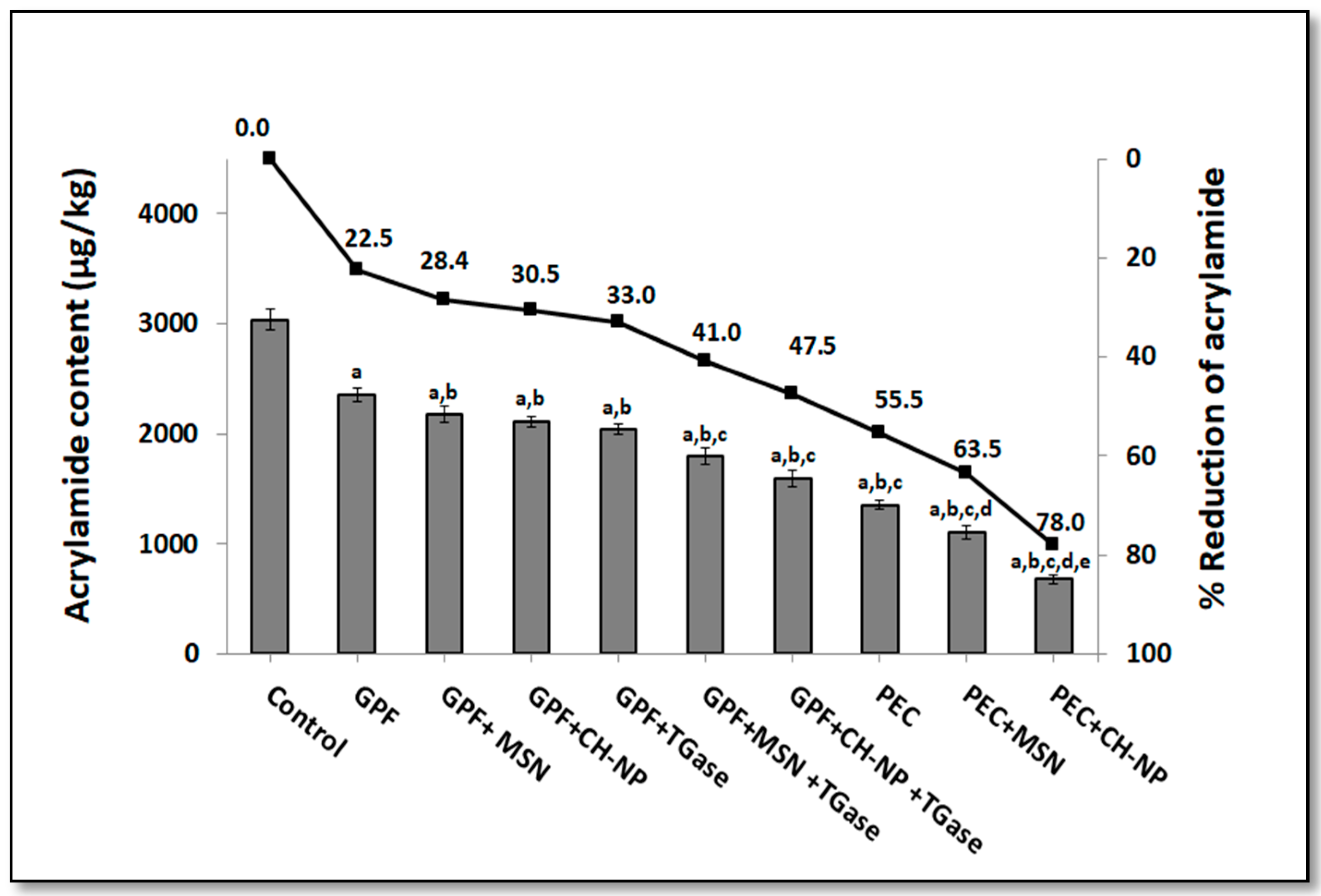
3.2. Influence on Nanoreinforced and TGase-Crosslinked Hydrocolloid Coating Solutions on Acrylamide Content
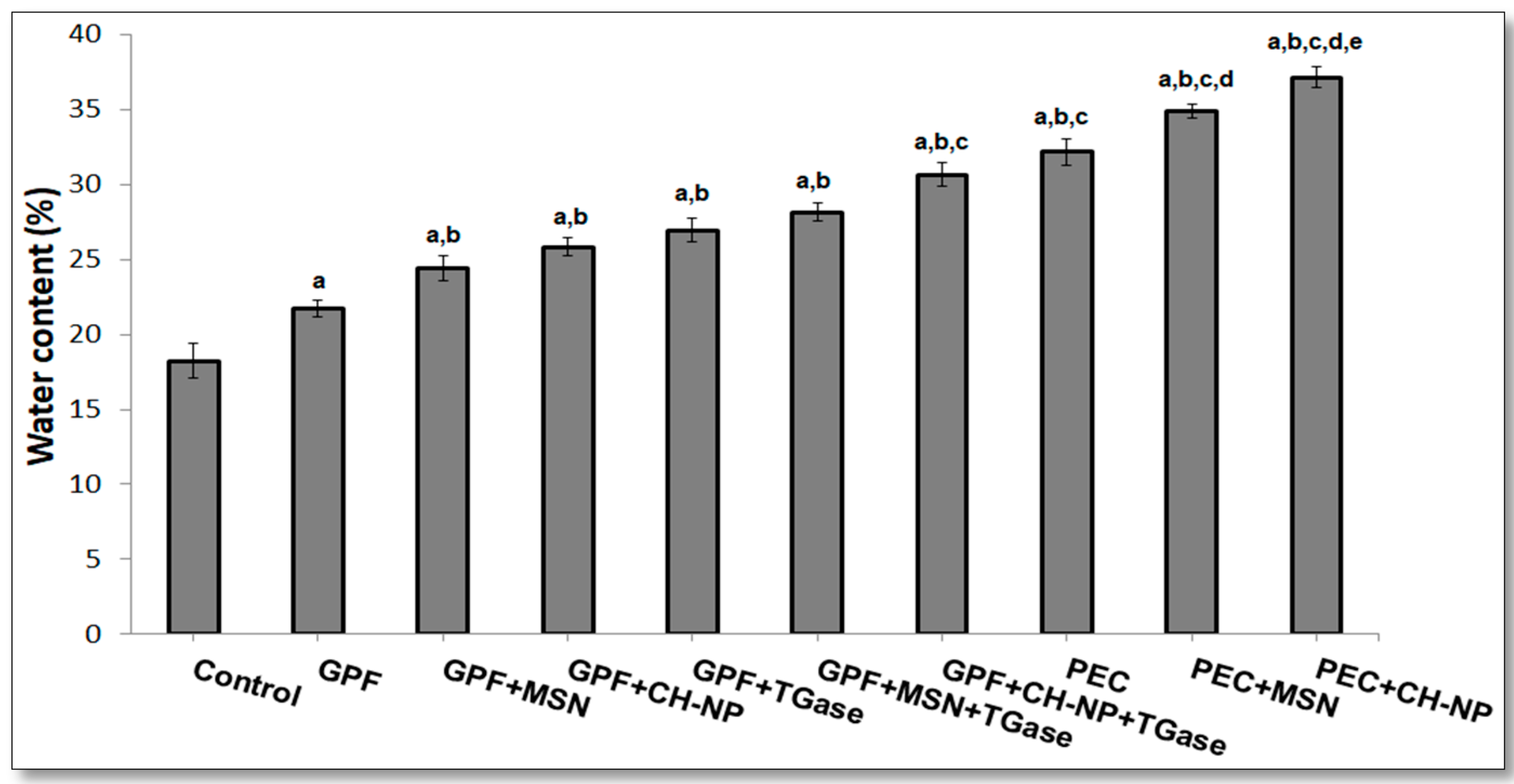
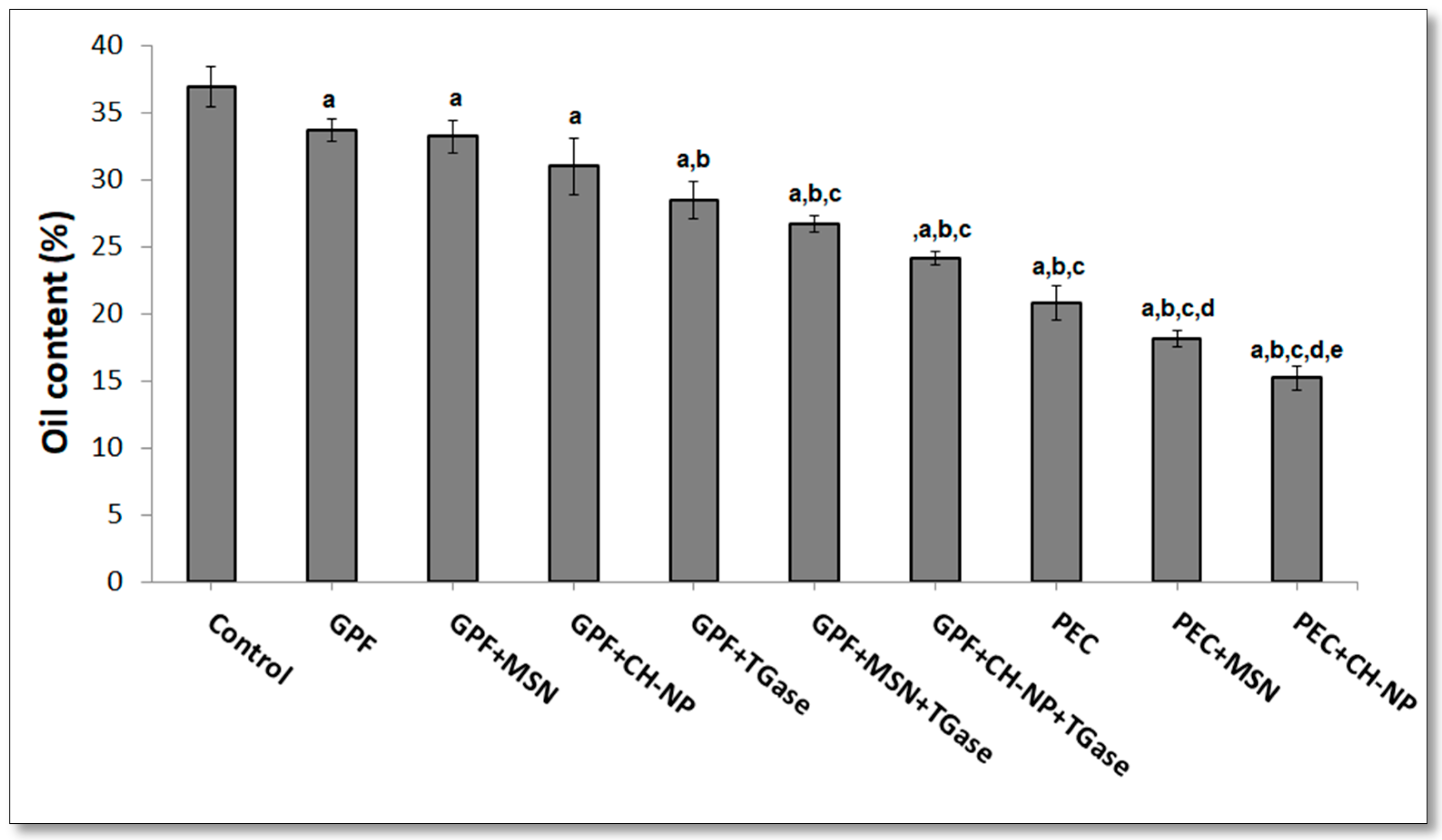
3.3. Influence of Nanoreinforced and Crosslinked Hydrocolloid Coating Solutions on Water and Oil Content
3.4. Influence of Nanoreinforced and Crosslinked Hydrocolloid Coating Solutions on the Kobbah Color
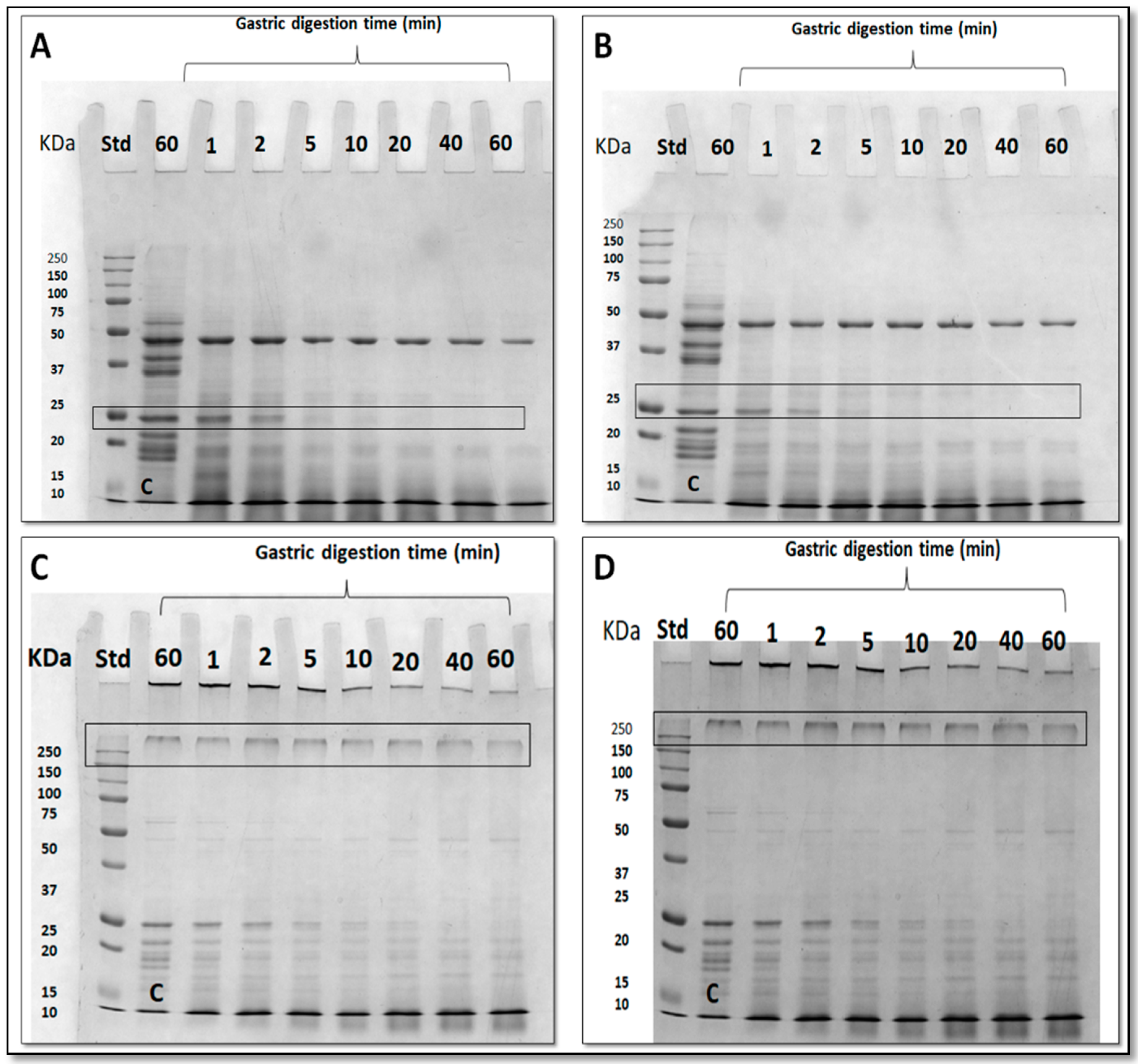
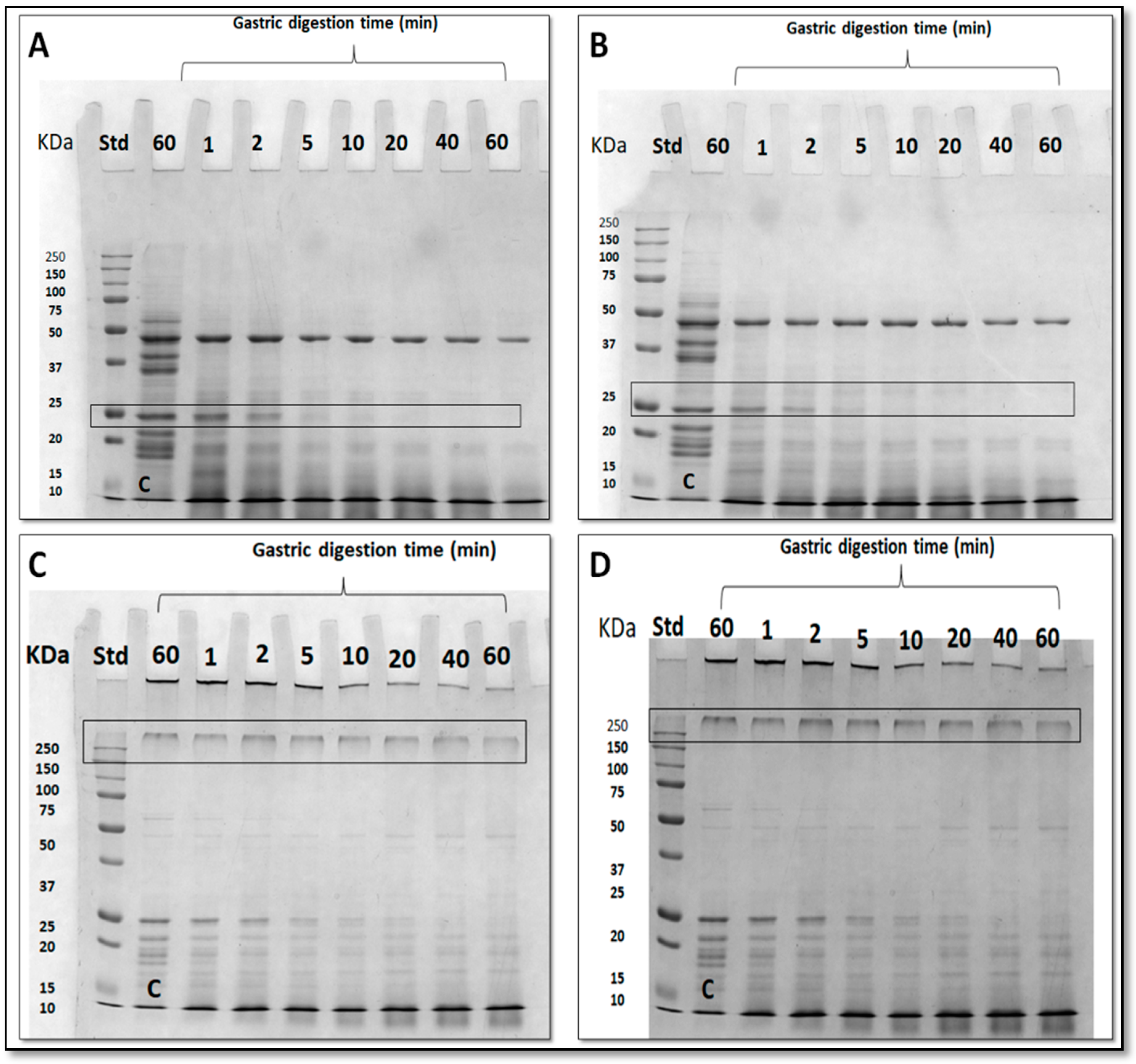
3.5. Effect of Nanoreinforced and Crosslinked Hydrocolloid Coating Solutions on the Digestibility of Fried Kobbah
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Dmoor, H.M.; Humeid, M.A.; Alawi, M.A. Investigation of acrylamide levels in selected fried and baked foods in Jordan. Food Agric. Environ. 2004, 2, 157–165. [Google Scholar]
- Sabbah, M.; Giosafatto, C.V.L.; Esposito, M.; Di Pierro, P.; Mariniello, L.; Porta, R. Transglutaminase cross-linked edible films and coatings for food applications. In Enzymes in Food Biotechnology, 1st ed.; Kuddus, M., Ed.; Academic Press: New York, NY, USA, 2019; pp. 369–388. [Google Scholar]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Villalonga Santana, R.; Mariniello, L. Effect of mesoporous silica nanoparticles on the physicochemical properties of pectin packaging material for strawberry wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, N.; Bosco, S.J.D. Preservation of jujube fruits by edible Aloe vera gel coating to maintain quality and safety. Indian J. Sci. Res. Technol. 2014, 3, 79–88. [Google Scholar]
- Yossef, M.A. Comparison of different edible coatings materials for improvement of quality and shelf life of perishable fruits. Middle East J. Applied Sci. 2014, 4, 416–424. [Google Scholar]
- Campbell, C.G. Grass Pea, Lathyrus sativus L.; Promoting the Conservation and Use of Underutilized and Neglected Crops; IPGRI: Rome, Italy, 1997; Volume 18, pp. 1–91. [Google Scholar]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef]
- Romano, A.; Giosafatto, C.V.L.; Al-Asmar, A.; Aponte, M.; Masi, P.; Mariniello, L. Grass pea (Lathyrus sativus) flour: Microstructure, physico-chemical properties and in vitro digestion. Eur. Food Res. Technol. 2019, 245, 191–198. [Google Scholar] [CrossRef]
- Romano, A.; Giosafatto, C.V.L.; Al-Asmar, A.; Masi, P.; Romano, R.; Mariniello, L. Structure and in vitro digestibility of grass pea (Lathyrus sativus L.) flour following transglutaminase treatment. Eur. Food Res. Technol. 2019, 245, 1899–1905. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Al-Asmar, A.; Mariniello, L. Transglutaminase protein substrates of food interest. In Enzymes in Food Technology: Improvement and Innovation; Kuddus, M., Ed.; Springer Nature; Singapore Pte Ltd.: Singapore, 2018; pp. 293–317. [Google Scholar]
- Sabbah, M.; Altamimi, M.; Di Pierro, P.; Schiraldi, C.; Cammarota, M.; Porta, R. Black edible films from protein-containing defatted cake of Nigella sativa seeds. Int. J. Mol. Sci. 2020, 21, 832. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Liu, J.; He, D.; Liu, Q.; He, X.; Wang, K.; Yang, X.; Shangguan, J.; Tang, J.; Mao, Y. Vertically ordered mesoporous silica film-assisted label-free and universal electrochemiluminescence aptasensor platform. Anal. Chem. 2016, 88, 11707–11713. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef]
- Tyagi, V.; Sharma, A.; Gupta, R.K. Design and properties of polysaccharide based: Silica hybrid packaging material. Int. J. Food Sci. Nutr. 2017, 2, 140–150. [Google Scholar]
- Fernandez-Bats, I.; Di Pierro, P.; Villalonga-Santana, R.; Garcia-Almendarez, B.; Porta, R. Bioactive mesoporous silica nanocomposite films obtained from native and transglutaminase-crosslinked bitter vetch proteins. Food Hydrocolloid. 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga-Santana, R.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of mesoporous silica nanoparticles on glycerol plasticized anionic and cationic polysaccharide edible films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef]
- Dekkers, S.; Krystek, P.; Peters, R.J.B.; Lankveld, D.P.K.; Bokkers, B.G.H.; van Hoeven-Arentzen, P.H.; Bouwmeester, H.; Oomen, A.G. Presence and risks of nanosilica in food products. Nanotoxicology 2011, 5, 393–405. [Google Scholar] [CrossRef]
- McCarthy, J.; Inkielewicz-Stępniak, I.; Corbalan, J.J.; Radomski, M.W. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: Protective Effects of Fisetin. Chem. Res. Toxicol. 2012, 25, 2227–2235. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Jahanian, M.A.G.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Otoni, C.G.; de Moura, M.R.; Mattoso, L.H.C. Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high-and low-methyl pectin films. Food Hydrocoll. 2016, 52, 732–740. [Google Scholar] [CrossRef]
- Martelli, M.R.; Barros, T.T.; de Moura, M.R.; Mattoso, L.H.C.; Assis, O.B.G. Effect of chitosan nanoparticles and pectin content on mechanical properties and water vapor permeability of banana puree films. J. Food Sci. 2013, 78, N98–N104. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, Y.; Gopal, G.; Lakshmanan, C.C.; Nandakumar, K.S. Chitosan nanoparticles for generating novel systems for better applications: A Review. J. Mol. Genet. Med. 2015, S4-005. [Google Scholar] [CrossRef]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as a food preservative. J. Appl. Microbiol. 2018, 126, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Food chemistry: Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- Mekawi, E.M.; Sharoba, A.M.; Ramadan, M.F. Reduction of acrylamide formation in potato chips during deep-frying in sunflower oil using pomegranate peel nanoparticles extract. J. Food Meas. Characteriz. 2019. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Man, Y.; Liu, Y. Reduction of acrylamide content in bread crust by starch coating. J. Sci. Food Agric. 2017, 98, 336–345. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Ulfah, K.; Prangdimurti, E.; Ishikawa, Y. Effect of blanching and pectin coating as pre-frying treatments to reduce acrylamide formation in banana chips. Int. Food. Res. J. 2015, 22, 936–942. [Google Scholar]
- Al-Asmar, A.; Naviglio, D.; Giosafatto, C.V.L.; Mariniello, L. Hydrocolloid-based coatings are effective at reducing acrylamide and oil content of French fries. Coatings 2018, 8, 147. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Panzella, L.; Mariniello, L. The effect of transglutaminase to improve the quality of either traditional or pectin-coated falafel (Fried Middle Eastern Food). Coatings 2019, 9, 331. [Google Scholar] [CrossRef]
- Chang, P.R.; Jian, R.; Yu, J.; Ma, X. Fabrication and characterisation of chitosan nanoparticles/plasticised-starch composites. Food Chem. 2010, 120, 736–740. [Google Scholar] [CrossRef]
- Brasil, T.A.; Capitani, C.D.; Takeuchi, K.P.; de Castro Ferreira, T.A.P. Physical, chemical and sensory properties of gluten-free kibbeh formulated with millet flour (Pennisetum glaucum (L.) R. Br.). Food Sci. Technol. 2015, 35, 361–367. [Google Scholar] [CrossRef][Green Version]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Esposito, M.; Mariniello, L.; Porta, R. Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food Bioprocess. Technol. 2013, 7, 447–455. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC) Official Method 960.39 Fat (Crude) or Ether Extract in Meat First Action 1960 Final Action; AOAC International: Arlington, MA, USA, 2006.
- Association of Official Analytical Chemists (AOAC) Official Method 950.46 (39.1.02) Moisture (M); AOAC International: Arlington, MA, USA, 2006.
- Zeng, X.; Cheng, K.-W.; Du, Y.; Kong, R.; Lo, C.; Chu, I.K.; Chen, F.; Wang, M. Activities of hydrocolloids as inhibitors of acrylamide formation in model systems and fried potato strips. Food Chem. 2010, 121, 424–428. [Google Scholar] [CrossRef]
- Wang, H.; Feng, F.; Guo, Y.; Shuang, S.; Choi, M.M.F. HPLC-UV quantitative analysis of acrylamide in baked and deep-fried Chinese foods. J. Food Compos. Anal. 2013, 31, 7–11. [Google Scholar] [CrossRef]
- Michalak, J.; Gujska, E.; Kuncewicz, A. RP-HPLC-DAD studies on acrylamide in cereal-based baby foods. J. Food Compos. Anal. 2013, 32, 68–73. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Papadakis, S.E.; Abdul-Malek, S.; Kamdem, R.E.; Yam, K.L. A versatile and inexpensive technique for measuring colour of foods. Food Technol. 2000, 54, 48–51. [Google Scholar]
- Palou, E.; Lopez-Malo, A.; Barbosa-Canovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and colour of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature 1970, 227, 680–985. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Rigby, N.M.; Wellner, N.; Ridout, M.; Husband, F.; Mackie, A.R. Microbial transglutaminase-mediated modification of ovalbumin. Food Hydrocoll. 2012, 26, 261–267. [Google Scholar] [CrossRef]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of Infant Digestive Conditions: Some Clues for Developing Relevant In Vitro Models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Ali, S.W.; Joshi, M.; Rajendran, S. Synthesis and characterization of chitosan nanoparticles with enhanced antimicrobial activity. Int. J. Nanosci. 2011, 10, 979–984. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Zhong, F. Characterization of tara gum edible films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Food Hydrocoll. 2015, 44, 309–319. [Google Scholar] [CrossRef]
- Ali, S.W.; Joshi, M.; Rajendran, S. Modulation of size, shape and surface charge of chitosan nanoparticles with reference to antimicrobial activity. American Scientific Publishers. Adv. Sci. Lett. 2010, 3, 452–460. [Google Scholar] [CrossRef]
- Osheba, S.A.; Sorour, A.M.; Abdou, E.S. Effect of chitosan nanoparticles as active coating on chemical quality and oil uptake of fish fingers. J. Agri. Environ. Sci. 2013, 2, 1–14. [Google Scholar]
- Melo, N.F.C.B.; de MendonçaSoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.; da Costa Tavares-Filho, J.H.; Galembeck, A.; Stamford, T.L.M.; Stamford-Arnaud, T.M.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of postharvest table grapes. Postharvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Angor, M.K.M.; Ajo, R.; Al-Rousan, W.; Al-Abdullah, B. Effect of starchy coating films on the reduction of fat uptake in deep-fat fried potato pellet chips. Ital. J. Food Sci. 2013, 25, 45–50. [Google Scholar]
- Jackson, L.S.; Al-Taher, F. Effects of Consumer Food Preparation on Acrylamide Formation. In Chemistry and Safety of Acrylamide in Food. Advances in Experimental Medicine and Biology; Friedman, M., Mottram, D., Eds.; Springer: Boston, MA, USA, 2005; Volume 561, pp. 447–465. [Google Scholar]
- FA-1005—Improving Health Properties of Food by Sharing Our Knowledge on the Digestive Process (INFOGEST). Available online: https://www.cost.eu/actions/FA1005/#tabs|Name:overview (accessed on 17 February 2020).


| FFSs | Zeta Potential (mV) | Z-Average (d.nm) |
|---|---|---|
| GPF | −13.7 ± 0.6 | 201.3 ± 11.1 |
| GPF + MSN | −16.8 ± 0.9 a | 191.4 ± 14.2 |
| GPF + CH–NP | −14.1 ± 0.8 b | 385.6 ± 28.2 a,b |
| GPF + TGase | −19.8 ± 1.2 a,b | 240.9 ± 14.4 a,b |
| GPF + MSN + TGase | −18.4 ± 0.5 a | 333.0 ± 22.3 a,b,c |
| GPF + CH–NP + TGase | −18.2 ± 0.9 a | 507.7 ± 18.9 a,b,c,d |
| PEC | −33.7 ± 2.1 | 3198 ± 79 |
| PEC + MSN | −31.8 ± 2.9 | 3110 ± 77 |
| PEC + CH–NP | −32.4 ± 3.2 | 3421 ± 63 * |
| Kobbah Types | ACR Content in Spiked Sample (µg/Kg) | Recovery (%) |
|---|---|---|
| Control | 3186 ± 61 | 98 |
| Dipped in GPF | 2511 ± 135 a | 103 |
| Dipped in GPF + MSN | 2329 ± 103 a,b | 101 |
| Dipped in GPF + CH–NP | 2255 ± 51 a,b | 96 |
| Dipped in GPF + TGase | 2186 ± 48 a,b | 98 |
| Dipped in GPF + MSN + TGase | 1934 ± 70 a,b,c | 93 |
| Dipped in GPF + CH–NP + TGase | 1744 ± 49 a,b,c | 99 |
| Dipped in PEC | 1495 ± 39 a,b,c | 95 |
| Dipped in PEC + MSN | 1250 ± 50 a,b,c,d | 95 |
| Dipped in PEC + CH–NP | 841 ± 37 a,b,c,d,e | 108 |
| Kobbah Types | L* | a* | b* | ΔE | BI |
|---|---|---|---|---|---|
| Control | 49.25 ± 0.68 | 8.28 ± 0.19 | 31.96 ± 0.76 | 0.0 ± 0.00 | 110.09 ± 3.54 |
| Dipped in GPF | 51.04 ± 0.34 * | 7.77 ± 0.12 | 31.26 ± 0.57 | 2.02 ± 0.46 * | 100.80 ± 2.99 * |
| Dipped in GPF + MSN | 52.08 ± 1.01 * | 6.69 ± 0.33 *,a | 33.14 ± 0.18 | 3.48 ± 0.93 * | 104.20 ± 3.36 |
| Dipped in GPF CH–NP | 53.23 ± 0.97 *,a | 5.96 ± 0.16 *,a | 31.88 ± 1.62 | 4.82 ± 0.76 *,a | 95.00 ± 8.61 * |
| Dipped in GPF + TGase | 54.93 ± 1.11 *,a | 6.10 ± 0.82 *,a | 33.01 ± 0.54 | 6.20 ± 1.30 *,a | 95.16 ± 4.36 *,a |
| Dipped in GPF + MSN + TGase | 55.35 ± 1.08 *,a | 5.97 ± 0.31 *,a | 31.55 ± 1.80 | 6.70 ± 1.10 *,a | 88.68 ± 8.02 *,a |
| Dipped in GPF + CH–NP + TGase | 55.70 ± 1.00 *,a | 5.81 ± 0.42 *,a | 32.06 ± 1.29 | 7.01 ± 0.84 *,a | 89.44 ± 6.35 *,a |
| Dipped in PEC | 56.56 ± 0.69 *,a,b | 5.44 ± 0.31 *,a | 32.58 ± 0.36 | 7.87 ± 0.75 *,a,b | 88.75 ± 2.02 *,a |
| Dipped in PEC + MSN | 58.03 ± 0.77 *,a,b | 5.01 ± 0.25 *,a | 32.99 ± 0.38 | 9.36 ± 0.77 *,a,b,c | 86.78 ± 3.18 *,a |
| Dipped in PEC + CH–NP | 60.78 ± 1.02 *,a,b,c | 3.32 ± 1.35 *,a,c | 33.34 ± 1.00 a | 12.70 ± 0.98 *,a,b,c | 79.91 ± 1.72 *,a,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Mariniello, L. Hydrocolloid-Based Coatings with Nanoparticles and Transglutaminase Crosslinker as Innovative Strategy to Produce Healthier Fried Kobbah. Foods 2020, 9, 698. https://doi.org/10.3390/foods9060698
Al-Asmar A, Giosafatto CVL, Sabbah M, Mariniello L. Hydrocolloid-Based Coatings with Nanoparticles and Transglutaminase Crosslinker as Innovative Strategy to Produce Healthier Fried Kobbah. Foods. 2020; 9(6):698. https://doi.org/10.3390/foods9060698
Chicago/Turabian StyleAl-Asmar, Asmaa, Concetta Valeria L. Giosafatto, Mohammed Sabbah, and Loredana Mariniello. 2020. "Hydrocolloid-Based Coatings with Nanoparticles and Transglutaminase Crosslinker as Innovative Strategy to Produce Healthier Fried Kobbah" Foods 9, no. 6: 698. https://doi.org/10.3390/foods9060698
APA StyleAl-Asmar, A., Giosafatto, C. V. L., Sabbah, M., & Mariniello, L. (2020). Hydrocolloid-Based Coatings with Nanoparticles and Transglutaminase Crosslinker as Innovative Strategy to Produce Healthier Fried Kobbah. Foods, 9(6), 698. https://doi.org/10.3390/foods9060698









