The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review
Abstract
1. Introduction
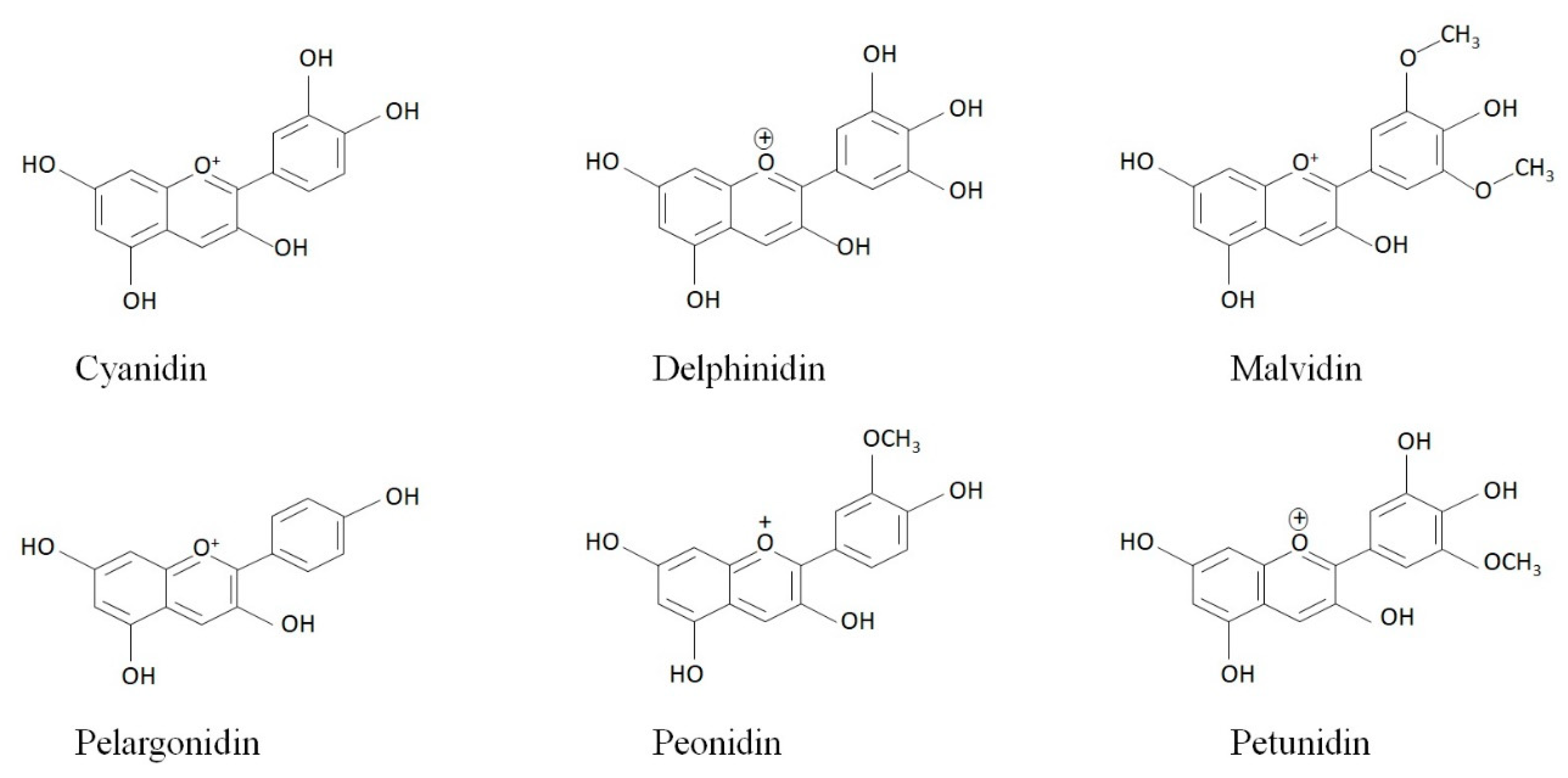
2. Anthocyanins and Obesity
2.1. Outcomes of Human Clinical Studies
2.2. Results of In Vivo Studies
2.3. Results of in vitro Studies
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980e2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 March 2020).
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A review on role of microbiome in obesity and antiobesity properties of probiotic supplements. Biomed Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef] [PubMed]
- Mandviwala, T.; Khalid, U.; Deswal, A. Obesity and cardiovascular disease: A risk factor or a risk marker? Curr. Atheroscler. Rep. 2016, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Genser, L.; Mariolo, J.R.C.; Castagneto-Gissey, L.; Panagiotopoulos, S.; Rubino, F. Obesity, type 2 diabetes, and the metabolic syndrome: Pathophysiologic relationships and guidelines for surgical intervention. Surg. Clin. N. Am. 2016, 96, 681–701. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Lysaght, J.; O’Sullivan, J.; Reynolds, J.V. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol. Metab. 2017, 28, 46–62. [Google Scholar] [CrossRef]
- Bonfrate, L.; Wang, D.Q.; Garruti, G.; Portincasa, P. Obesity and the risk and prognosis of gallstone disease and pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 623–635. [Google Scholar] [CrossRef]
- Martin-Jiménez, C.A.; Gaitán-Vaca, D.M.; Echeverria, V.; González, J.; Barreto, G.E. Relationship between obesity, Alzheimer’s disease, and Parkinson’s disease: An astrocentric view. Mol. Neurobiol. 2017, 54, 7096–7115. [Google Scholar] [CrossRef]
- Wirth, A.; Wabitsch, M.; Hauner, H. The prevention and treatment of obesity. Dtsch. Arztebl. Int. 2014, 111, 705–713. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Ghobadi, S.; Mohammad Hosseini, M.; Gholami, Z.; Mohammadian, F. Flavanols are potential anti-obesity agents, a systematic review and meta-analysis of controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Giacometti, J.; Russo, G.L. Antiobesity effects of anthocyanins in preclinical and clinical studies. Oxid. Med. Cell. Longev. 2017, 2017, 2740364. [Google Scholar] [CrossRef] [PubMed]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Anthocyanins in Thai rice varieties: Distribution and pharmacological significance. Int. Food Res. J. 2018, 25, 2024–2032. [Google Scholar]
- Najafabadi, N.S.; Sahari, M.A.; Barzegar, M.; Esfahani, Z.H. Effects of concentration method and storage time on some bioactive compounds and color of jujube (Ziziphus jujuba var vulgaris) concentrate. J. Food Sci. Technol. 2017, 54, 2947–2955. [Google Scholar] [CrossRef]
- Alighourchi, H.; Barzegar, M.; Abbasi, S. Anthocyanins characterization of 15 Iranian pomegranate (Punica granatum L.) varieties and their variation after cold storage and pasteurization. Eur. Food Res. Technol. 2008, 227, 881–887. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Jbir, R.; Mars, M.; Trifi, M.; Kamal-Eldin, A.; Melgarejo, P.; Hernandez, F. Organic acids, sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. Int. J. Food Prop. 2011, 14, 741–757. [Google Scholar] [CrossRef]
- Batista, A.G.; da Silva, J.K.; Cazarin, C.B.B.; Biasoto, A.C.T.; Sawaya, A.C.H.F.; Prado, M.A.; Júnior, M.R.M. Red-jambo (Syzygium malaccense): Bioactive compounds in fruits and leaves. LWT Food Sci. Technol. 2017, 76, 284–291. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Zhang, J.; Celli, G.B.; Brooks, M.S. Natural sources of anthocyanins. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health, 1st ed.; Brooks, M.S.-L., Celli, G.B., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2019; pp. 154–196. [Google Scholar]
- Sivamaruthi, B.S.; Kesika, P.; Subasankari, K.; Chaiyasut, C. Beneficial effects of anthocyanins against diabetes mellitus associated consequences-A mini review. Asian Pac. J. Trop. Biomed. 2018, 8, 471–477. [Google Scholar]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124 086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Mondello, L.; Morabito, D.; Dugo, G. Characterization of the anthocyanin fraction of sicilian blood orange juice by micro-HPLC-ESI/MS. J. Agric. Food Chem. 2003, 51, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.Q.; Dourado, G.K.Z.S.; Cesar, T.B. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int. J. Food Sci. Nutr. 2015, 66, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Venneria, E.; Ciarapica, D.; Foddai, M.S.; Intorre, F.; Zaccaria, M.; Maiani, F.; Palomba, L.; Barnaba, L.; Tubili, C.; et al. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: A pilot study. Oxid. Med. Cell. Longev. 2017, 2017, 1672567. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Sabatino, L.; Lazzaro, F.; Borzì, M.A.; Gargano, M.; Traulo, P.; Gagliano, G. Blood orange anthocyanins in fruit beverages: How the commercial shelf life reflects the quality parameter. Beverages 2015, 1, 82–94. [Google Scholar] [CrossRef]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an anthocyanin and prebiotic blend on intestinal environment in obese male and female subjects. J. Nutr. Metab. 2018, 2018, 7497260. [Google Scholar] [CrossRef]
- Istek, N.; Gurbuz, O. Investigation of the impact of blueberries on metabolic factors influencing health. J. Funct. Foods 2017, 38, 298–307. [Google Scholar] [CrossRef]
- Bhaswant, M.; Brown, L.; Mathai, M.L. Queen Garnet plum juice and raspberry cordial in mildly hypertensive obese or overweight subjects: A randomized, double-blind study. J. Funct. Foods 2019, 56, 119–126. [Google Scholar] [CrossRef]
- Bicudo, M.O.P.; Ribani, R.H.; Beta, T. Anthocyanins, phenolic acids and antioxidant properties of juçara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Foods Hum. Nutr. 2014, 69, 142–147. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; Cesar, H.C.; Vasconcelos, J.R.; Oyama, L.M.; de Rosso, V.V.; Pisani, L.P. Obesity-related inflammatory modulation by juçara berry (Euterpe edulis Mart.) supplementation in Brazilian adults: A double-blind randomized controlled trial. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef]
- Vugic, L.; Colson, N.; Nikbakht, E.; Gaiz, A.; Olivia, J. Holland, Avinash Reddy Kundur, Indu Singh. Anthocyanin supplementation inhibits secretion of pro-inflammatory cytokines in overweight and obese individuals. J. Funct. Foods 2020, 64, 103596. [Google Scholar] [CrossRef]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qi, X.; Liu, Y.; Guo, J.; Zhu, R.; Chen, W.; Zheng, X.; Yu, T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013, 141, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, D.; Yang, Y.; Xia, M.; Li, D.; Li, G.; Zhu, Y.; Xiao, Y.; Ling, W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011, 91, 1006–1013. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.H.; Jung, K.O.; Jung, K.H.; Kwon, K.I.; Jeong, T.C.; Chung, Y.C.; et al. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Tomay, F.; Marinelli, A.; Leoni, V.; Caccia, C.; Matros, A.; Mock, H.P.; Tonelli, C.; Petroni, K. Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice. J. Transl. Med. 2019, 17, 237. [Google Scholar] [CrossRef]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Badshah, H.; Ullah, I.; Kim, S.E.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rat’s hypothalamus. Neuropeptides 2013, 47, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Seifert, S.; Jaudszus, A.; Bub, A.; Watzl, B. Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in Fischer rats. PLoS ONE 2013, 8, e66690. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z.; Matusevicius, P.; Kołodziejczyk, K. Polyphenol-rich extract from blackcurrant pomace attenuates the intestinal tract and serum lipid changes induced by a high-fat diet in rabbits. Eur. J. Nutr. 2014, 53, 1603–1613. [Google Scholar] [CrossRef]
- Kim, S.Y.; Wi, H.R.; Choi, S.; Ha, T.J.; Lee, B.W.; Lee, M. Inhibitory effect of anthocyanin-rich black soybean testa (Glycine max (L.) Merr.) on the inflammation-induced adipogenesis in a DIO mouse model. J. Funct. Foods 2015, 14, 623–633. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich phytochemicals from aronia fruits inhibit visceral fat accumulation and hyperglycemia in high-fat diet-induced dietary obese rats. J. Oleo Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef]
- van der Heijden, R.A.; Morrison, M.C.; Sheedfar, F.; Mulder, P.; Schreurs, M.; Hommelberg, P.P.; Hofker, M.H.; Schalkwijk, C.; Kleemann, R.; Tietge, U.J.; et al. Effects of anthocyanin and flavanol compounds on lipid metabolism and adipose tissue associated systemic inflammation in diet-induced obesity. Mediat. Inflamm. 2016, 2016, 2042107. [Google Scholar] [CrossRef]
- Peixoto, T.C.; Moura, E.G.; de Oliveira, E.; Soares, P.N.; Guarda, D.S.; Bernardino, D.N.; Ai, X.X.; Rodrigues, V.D.S.T.; de Souza, G.R.; da Silva, A.J.R.; et al. Cranberry (Vaccinium macrocarpon) extract treatment improves triglyceridemia, liver cholesterol, liver steatosis, oxidative damage and corticosteronemia in rats rendered obese by high fat diet. Eur. J. Nutr. 2018, 57, 1829–1844. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high fat diet-treated mice. Int. J. Food Sci. Nutr. 2016, 67, 257–264. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishida, M.; Saito, M.; Tanabe, A.; Eitsuka, T.; Yuan, S.H.; Ikekawa, N.; Nishida, H. The fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms improves insulin resistance and hepatic lipid accumulation by modulation of liver adenosine monophosphate-activated protein kinase activity and lipogenic gene expression in high-fat diet-fed obese mice. Nutr. Res. 2016, 36, 1090–1097. [Google Scholar] [PubMed]
- Song, H.; Wu, T.; Xu, D.; Chu, Q.; Lin, D.; Zheng, X. Dietary sweet cherry anthocyanins attenuates diet-induced hepatic steatosis by improving hepatic lipid metabolism in mice. Nutrition 2016, 32, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lai, J.; Tang, Q.; Zheng, X. Mulberry ethanol extract attenuates hepatic steatosis and insulin resistance in high-fat diet-fed mice. Nutr. Res. 2016, 36, 710–718. [Google Scholar] [CrossRef]
- Ayoub, H.M.; McDonald, M.R.; Sullivan, J.A.; Tsao, R.; Platt, M.; Simpson, J.; Meckling, K.A. The effect of anthocyanin-rich purple vegetable diets on metabolic syndrome in obese Zucker rats. J. Med. Food 2017, 20, 1240–1249. [Google Scholar] [CrossRef]
- da Costa, G.F.; Santos, I.B.; de Bem, G.F.; Cordeiro, V.S.C.; da Costa, C.A.; de Carvalho, L.C.R.M.; Ognibene, D.T.; Resende, A.C.; de Moura, R.S. The beneficial effect of anthocyanidin-rich Vitis vinifera L. grape skin extract on metabolic changes induced by high-fat diet in mice involves antiinflammatory and antioxidant actions. Phytother. Res. 2017, 31, 1621–1632. [Google Scholar] [CrossRef]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.F.; Zheng, G.H.; Wang, A.M.; Sun, C.H.; Qin, S.P.; Zhuang, J.; Lu, J.; Ma, D.F.; Zheng, Y.L. The inhibitory effects of purple sweet potato color on hepatic inflammation is associated with restoration of NAD+ levels and attenuation of NLRP3 inflammasome activation in high-fat-diet-treated mice. Molecules 2017, 22, 1315. [Google Scholar] [CrossRef]
- You, Y.; Yuan, X.; Liu, X.; Liang, C.; Meng, M.; Huang, Y.; Han, X.; Guo, J.; Guo, Y.; Ren, C.; et al. Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- You, Y.; Han, X.; Guo, J.; Guo, Y.; Yin, M.; Liu, G.; Huang, W.; Zhan, J. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J. Funct. Foods 2018, 41, 62–71. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and blueberry anthocyanin supplementation counteract high-fat-diet-induced obesity by alleviating oxidative stress and inflammation and accelerating energy expenditure. Oxid. Med. Cell. Longev. 2018, 2018, 4051232. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Mathai, M.L.; Xu, G.; McAinch, A.J.; Su, X.Q. The effects of supplementation with blueberry, cyanidin-3-O-β-glucoside, yoghurt and its peptides on obesity and related comorbidities in a dietinduced obese mouse model. J. Funct. Foods 2019, 56, 92–101. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.H.C.; Cunha, M.G.; Alezandro, M.R.; Genovese, M.I. Phenolic-rich jaboticaba (Plinia jaboticaba (Vell.) Berg) extracts prevent high-fat-sucrose diet-induced obesity in C57BL/6 mice. Food Res. Int. 2018, 107, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef]
- John, O.D.; Mouatt, P.; Prasadam, I.; Xiao, Y.; Panchal, S.K.; Brown, L. The edible native Australian fruit, Davidson’s plum (Davidsonia pruriens), reduces symptoms in rats with diet-induced metabolic syndrome. J. Funct. Foods 2019, 56, 204–215. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.S.; Kim, E.J. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 2019, 11, 1190. [Google Scholar] [CrossRef]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of anthocyanin-rich tart cherry extract on inflammatory mediators and adipokines involved in type 2 diabetes in a high fat diet induced obesity mouse model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef]
- Sandoval, V.; Femenias, A.; Martínez-Garza, Ú.; Sanz-Lamora, H.; Castagnini, J.M.; Quifer-Rada, P.; Lamuela-Raventós, R.M.; Marrero, P.F.; Haro, D.; Relat, J. Lyophilized Maqui (Aristotelia chilensis) berry induces browning in the subcutaneous white adipose tissue and ameliorates the insulin resistance in high fat diet-induced obese mice. Antioxidants 2019, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.R.; Kang, J.H.; Yoo, H.; Choe, S.Y.; Yang, C.H.; Kim, M.O.; Yu, R. Cyanidin and cyanidin-3-O-β-D-glucoside suppress the inflammatory responses of obese adipose tissue by inhibiting the release of chemokines MCP-1 and MRP-2. J. Food Sci. Nutr. 2007, 12, 148–153. [Google Scholar] [CrossRef][Green Version]
- Guo, H.; Guo, J.; Jiang, X.; Li, Z.; Ling, W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012, 50, 3040–3047. [Google Scholar] [CrossRef]
- Lee, B.; Lee, M.; Lefevre, M.; Kim, H.R. Anthocyanins inhibit lipogenesis during adipocyte differentiation of 3T3-L1 preadipocytes. Plant Foods Hum. Nutr. 2014, 69, 137–141. [Google Scholar] [CrossRef]
- Han, M.H.; Kim, H.J.; Jeong, J.W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of Vitis coignetiae Pulliat is associated with the activation of AMPK signaling pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef]
- Park, M.; Sharma, A.; Lee, H.J. Anti-adipogenic effects of delphinidin-3-O-β-glucoside in 3T3-L1 preadipocytes and primary white adipocytes. Molecules 2019, 24, 1848. [Google Scholar] [CrossRef]
- Chaiittianan, R.; Sutthanut, K.; Rattanathongkom, A. Purple corn silk: A potential anti-obesity agent with inhibition on adipogenesis and induction on lipolysis and apoptosis in adipocytes. J. Ethnopharmacol. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Fotschki, B.; Laparra, J.M.; Sójka, M. Raspberry polyphenolic extract regulates obesogenic signals in hepatocytes. Molecules 2018, 23, 2103. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Shin, T.S.; Kim, M.Y.; Cho, N.J.; Kim, J.D. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS ONE 2018, 13, e0208556. [Google Scholar] [CrossRef]
- Muscarà, C.; Molonia, M.S.; Speciale, A.; Bashllari, R.; Cimino, F.; Occhiuto, C.; Saija, A.; Cristani, M. Anthocyanins ameliorate palmitate-induced inflammation and insulin resistance in 3T3-L1 adipocytes. Phytother. Res. 2019, 33, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, P.; Nakagawa, H.; Yamaki, K. Cyanidin and cyanidin-3-glucoside derived from Vigna unguiculata act as noncompetitive inhibitors of pancreatic lipase. J. Food Biochem. 2019, 43, e12774. [Google Scholar] [CrossRef] [PubMed]

| Subjects | Study Type | Intervention | Dose & Duration | Results | References |
|---|---|---|---|---|---|
| i. Healthy nurses; n = 121 701 females; Age = 30–55 years ii. Health professionals; n = 51 529 males; Age = 40–75 years iii. Healthy nurses (n = 116 686 females); Age = 25–42 years | 3 Cohort study (i. Study began in 1976; ii. Study began in 1986; iii. Study began in 1989) | Dietary flavonoids (the major dietary sources include fruits such as apples, blueberries, grapefruit, orange, strawberries; juices such as orange juices, tomato juices; vegetables such as onions, celery, peppers; beverages such as tea, beer, red wine) | Median daily intake of anthocyanins (i. 11.5 mg; ii. 7.7 mg; iii. 5.7 mg), flavonoid polymers (i. mg; ii. mg; iii. mg), flavan-3-ols (i. 24.2 mg; ii. 23.9 mg; iii. 26.1 mg), flavanones (i. 37.6 mg; ii. 41.7 mg; iii. 21.7 mg), flavanols (i. 12.1 mg; ii. 11.7 mg; iii. 14.2 mg), flavones (i. 2.1 mg; ii. 2.2 mg; iii. 1.2 mg); 24 years | Increased flavonoid consumption helps to maintain body weight and prevent the incidence of obesity. | [23] |
| Overweight/obese individuals; n = 12 (3 females, 9 males)/6 (2 females, 4 males); Age = 23–59 years; Average BMI ≥ 25 kg/m2 Normal weight individuals; n = 17 (11 females, 6 males); Age = 23–59 years; Average BMI = 18.5–24.9 kg/m2 | Randomized clinical trial | 100% ready-to-drink red-fleshed sweet orange juice | 750 mL of orange juice per day; 8 weeks | Increased serum antioxidant activity, reduced levels of C-reactive protein, TC, and LDL-C in both groups; reduced diastolic blood pressure in overweight/obese individuals; reduced systolic blood pressure, fasting insulin levels, insulin resistance in normal weight individuals | [25] |
| Overweight/obese individuals; n = 11 females; Age = 36 ± 7 years; Average BMI = 34.4 ± 4.8 kg/m2 | Pilot Study | Orange juice rich in anthocyanins | 500 mL containing 250 mg of anthocyanins per day; 12 weeks. | No change in body weight. Reduced the TC, LDL-C. | [26] |
| Individuals with uncomplicated obesity; n = 46 (34 females, 12 males); Age = 42.9 ± 10 years; Average BMI = 34.2 ± 3.1 kg/m2 | Open-label study | Anthocyanins and prebiotic powder mix | One sachet of powder containing 618 mg of black rice extract (120 mg of anthocyanins), 202 mg of black currant extract (60 mg of anthocyanins), 144 mg of European blueberry extract (35 mg of anthocyanins), and prebiotic fibers (1.9 g of inulin, 1.1 g of fructooligosaccharides) per day; 8 weeks. | Reduced firmicutes and actinobacteria load. Increased Bacteroidetes content. Improved bowel habits and intestinal ecosystem. | [28] |
| Overweight/obese individuals; Average BMI ≥ 25 kg/m2 Blueberry group, n = 27 (70% females, 30% males); Age = 34.9 ± 9.7 years; Control group, n = 27 (80% females, 20% males); Age = 32.2 ± 9.7 years | Double-blind, placebo-controlled trial | Blueberry | After 1st six weeks of nutrition therapy, 50 g of carbohydrate source was replaced with 50 g of blueberries in the daily diet; 12 weeks of nutrition therapy for both groups | Reduced body weight and body fat. Reduced TC, LDL-C significantly. | [29] |
| Mildly hypertensive overweight/obese individuals; QC group, n = 15 out of 16 (8 females, 8 males); Age = 47 ± 11 years; Average BMI = 31 ± 5 kg/m2; SBP = 142 ± 7 mmHg; DBP = 92 ± 4 mmHg; Placebo group, n = 14 out of 16 (8 females, 8 males); Age = 38 ± 14 years; Average BMI = 32 ± 5 kg/m2; SBP = 139 ± 5 mmHg; DBP = 91 ± 3 mmHg | Randomized, double-blind study | Queen Garnet (QC) plum juice; Placebo (raspberry cordial drink) | 250 mL per day; QC juice contains C3G (78 mg C3GE/100 mL), C3R (24 mg C3GE/100 mL), quercetin glycosides (36 mg/100 mL); placebo drink contains C3G (0.1 mg C3GE/100 mL), C3R (0.1 mg C3GE/100 mL), quercetin glycosides (0.4 mg/100 mL);12 weeks | Reduced the LDL level. Reduced plasma glucose, insulin, and C-peptide, leptin and GLP-1 concentrations. Increased adiponectin content. Reduced the levels of IL-2, IL-6, IL-13, and TNF-α. | [30] |
| Obese individuals; Juҫara group, n = 13 (8 females, 5 males); Age = 45.76 ± 2.58 years; Average BMI = 34.63 ± 1.2 kg/m2; Placebo group, n = 14 (8 females, 6 males); Age = 45.07 ± 3.42 years; Average BMI = 33.82 ± 0.71 kg/m2 | Randomized, double-blind controlled study | Juҫara berry (freeze-dried pulp); Placebo (maltodextrin) | 5 g per day; 6 weeks | Reduced the TLR4, IL-6, MYD88, TNF-α, and MCP-1 level. Increased Ob-R protein and IL-10. | [32] |
| Lean individuals, n = 12 (6 females, 6 males); Age = 33 ± 3.2 years; Average BMI = 22.8 ± 0.6 kg/m2; Overweight individuals, n = 9 (4 females, 5 males); Age = 49.9 ± 4.2 years; Average BMI = 27.7 ± 0.4 kg/m2; Obese individuals, n = 8 (4 females, 4 males); Age = 43.3 ± 4.5 years; Average BMI = 34.3 ± 0.9 kg/m2 | Open-label study | MEDOX® capsules (Medapalett Pharmaceuticals, Biolink, Sandnes, Norway) containing purified anthocyanins of blackcurrant and bilberries | 320 mg per 4 capsules per day; 2 capsules consumed twice daily along with usual diet; each capsule contains 3-O-rutinoside of cyanidin and delphinidin (1%), and 3-O-β-galactosides, 3-O-β-glucosides and 3-O-β-arabinosides of cyanidin (33%), delphinidin (58%), malvidin (3%), petunidin (2.5%) and peonidin (2.5%); 28 days | Reduced plasma CCL2, and IL-6 level | [33] |
| Model | Intervention | Dose | Duration | Results | Reference |
|---|---|---|---|---|---|
| High-fat-diet-induced obese mice | Grape pomace extract | 250 mg/kg/day | 12 weeks | Reduced the plasma C-reactive protein level. | [34] |
| High-fat-diet-fed hamsters | Mulberry water extracts | 0.5 or 1.0 or 2.0% in diet | 12 weeks | Reduced body weight and visceral fat. Reduced the TG, TC, LDL/HDL ratio, free fatty acids level, and hepatic lipids. Induced the hepatic carnitine palmitoyltransferase-1, and peroxisome proliferator-activated receptor α expressions. Reduced the HMG-CoA reductase, and fatty acid synthase expressions. | [35] |
| Male Zucker fatty rats | Freeze dried whole highbush blueberry powder | 2.0% (wt/wt) in high fat (45% of kcal) diet | 90 days | Reduced the TG, fasting insulin, HOMA-IR, glucose area under curve values, and abdominal fat mass. Increased PPAR activity and affected the PPAR expression associated with fat and glucose oxidation. | [36] |
| High-fat-diet-fed mice | Blueberry and Mulberry Juice | Average amount of blueberry juice (4.83 ± 0.36 mL per day per mouse) or mulberry juice (4.56 ± 0.57 mL per day per mouse) consumption | 12 weeks | Inhibited body weight gain. Decreased serum cholesterol and leptin secretion. Reduced insulin resistance and lipid accumulation. | [37] |
| High-fat-diet-fed mice | Mulberry anthocyanins | 40 or 200 mg/kg of food | 12 weeks | Inhibited body weight gain. Reduced the resistance to insulin, leptin secretion, adipocytes size, and lipid accumulation. | [38] |
| KK-Ay mice | Cyanidin-3-O-β-D-glucoside (C3G) | 1 g/kg of body weight | 12 weeks | Reduced body weight, liver, and visceral adipose tissue weight. Reduced the plasma and hepatic TG, and steatosis score. Increased the pAMPK in skeletal muscle and visceral adipose. Stimulated the lipoprotein lipase activity. | [39] |
| Obese mice | Purple sweet potato-anthocyanins | 200 mg/kg body weight/day | 4 weeks | Reduced weight gain, TG, TC. Reduced the TBARSs content. Reduced the lipid accumulation. Increased the pAMPK and pACC activity. | [40] |
| Obese mice | C3G-rich purple corn color | 2 g/kg in the diet | 12 weeks | Reduced weight gain, and adipose tissue weight. Normalized the expression of TNF-α. Suppressed fatty acid and triacylglycerol synthesis. | [41] |
| High-fat-diet-fed mice | Purple corn extract | 290 mg of anthocyanin concentration/kg/day | 12 weeks | Reduced inflammatory mediators and attenuated adipose tissue inflammation. | [42] |
| Wistar rats | Anthocyanins-rich chokeberry extract | 100 or 200 mg/kg body weight/day | 6 weeks | Reduced epididymal fat, TC, LDL-C, blood glucose. Suppressed the expression of inflammatory cytokines, plasma TNF-α and IL-6. Increased expressions of Gys1, Irs1, Irs2, Pi3k, Glut1, Glut4, Pparγ, adiponectin, and zinc finger protein. Suppressed the expressions of Fabp4, Fas, and Lpl. | [43] |
| Sprague-Dawley Rat | Anthocyanins | 6 or 24 mg/kg | 40 days | Lowered body weight and food consumption. Reduced adipose tissue volume, and expression of neuropeptide Y. Increased expression of GABA-receptor Suppressed the expressions of PKA, and p-CREB. | [44] |
| Fat diet fed fischer rats | Anthocyanins grape-bilberry rich juice | 1551 mg/L | 10 weeks | Reduced serum cholesterol, TG, leptin, and resistin. Decreased saturated fatty acids and increased polyunsaturated fatty acids level. | [45] |
| High fat diet fed Rabbits | Blackcurrant pomace | 1.5% in the diet | 4 weeks | Reduced the putrefactive metabolites and β-glucuronidase activity. Reduced the TG, TC, LDL-C and free fatty acid content. Increased antioxidant activity. | [46] |
| High fat diet fed mice | Black soybean testa | 1 g/kg bodyweight | 12 weeks | Reduced food consumption, fat accumulation, lipogenesis, and inflammation. Increased lipolysis. | [47] |
| Rats | Aronia fruit-anthocyanins | 0.4% in the diet | 4 weeks | Reduced visceral fat mass, perirenal, mesenteric and epididymal adipose mass. Reduced fasting glucose level, TG, and suppressed the increase in blood glucose level. | [48] |
| High fat diet fed mice | Bilberry extract | 0.1% | 24 weeks | Reduced the expression of Tnf and regulated the neutrophil chemoattractant mKC. | [49] |
| High fat diet fed rat | Cranberry extract | 200 mg/kg | 30 days | Reduced body weight gain, TG, hepatic cholesterol content, fatty acid synthase, hypocorticosteronemia, lipid peroxidation, protein carbonylation, and fat accumulation. | [50] |
| High fat diet induced obese mice | Blueberry anthocyanins | 50 or 100 or 200 mg/kg | 8 weeks | Reduced body weight, serum glucose level, and expression of TNF-α, IL-6, PPARγ, and FAS genes. Decreased epididymal adipocytes and improved the serum and liver lipid profiles. | [51] |
| High fat diet fed mice | Mulberry and cherry anthocyanins | 200 mg/kg | 8 weeks | Reduced body weight, serum glucose and leptin levels, and expression of TNF-α, IL-6, iNOS, and NF-кB genes. Improved lipid profiles, and SOD and GPx activities. Reduced the production of MDA. | [52] |
| High fat diet fed rats | Blueberry | 10% in feed | 8 weeks | Increased Gammaproteobacteria abundance. Retained ileal villus height. Regularized the expression of TNF-α and IL-1β. Improved insulin signaling | [53] |
| High fat diet fed mice | whole berries | 400 μg of anthocyanins equivalent/g | 12 weeks | Improved body composition, energy expenditure, mitochondrial respiration. Reduced metabolic stress. | [54] |
| High fat diet fed mice | Acanthopanax senticosus fruits | 0.5 or 1.0 g/kg | 12 weeks | Improved glucose tolerance and insulin sensitivity. Reduced insulin level and lipid accumulation. Reduced the expression of FAS and induced the expression of cholesterol 7-α-hydroxylase. Increased pAMPK. | [55] |
| High fat diet fed mice | Sweet cherry anthocyanins | 200 mg/kg | 15 weeks | Nullified diet-induced hepatic steatosis | [56] |
| High fat diet fed mice | Mulberry ethanol extract | 100 mg/kg | 14 weeks | Reduced weight gain. Improved hepatic steatosis, adipose hypertrophy, and insulin resistance. Altered lipid and cholesterol metabolism. | [57] |
| Obese Zucker rats | Freeze dried baked purple potatoes | 450 g/kg diet | 8 weeks | Improved glucose tolerance and insulin sensitivity. Controlled blood pressure. | [58] |
| High fat diet fed mice | Vitis vinifera L. grape skin extract | 200 mg/kg per day | 12 weeks | Prohibited weight gain, insulin resistance, and dyslipidemia. Reduced oxidative stress | [59] |
| Obese diabetic Mice | Red raspberry fruit | 5.3% freeze-dried raspberry | 8 weeks | Improved antioxidant activity. Increased GPx activity | [60] |
| High fat diet fed mice | Purple sweet potato color | 700 mg/kg/day | 20 weeks | Prevented obesity and liver damages. Reduced oxidative stress. Suppressed NF-κB, NOD1/2 signaling, NLRP3 inflammasome activation. Restored NAD+ level. | [61] |
| Obese mice | C3G | 1 mg/mL | 16 weeks | Reduced weight gain, increased energy expenditure, retained glucose homeostasis, normalized hepatic steatosis and enhanced cold tolerance. Induced the formation of brown-like adipocytes. Influenced the expression of uncoupling protein 1 (UCP1). | [62] |
| High fructose and high fat diet induced obese mice | C3G | 1 mg/mL | 15 weeks | Improved energy expenditure and thermogenic capacity. Increased the expression of UCP1. | [63] |
| High fat diet fed mice | Blackberry or blueberry anthocyanins | 200 mg/kg | 12 weeks | Reduced lipid level, increased the SOD, GPx activity. Increased fecal SCFs level, suppressed the expression of IL-6, NF-κB, TNF-α. Affected the glycerophospholipid, glutathione metabolism, and the insulin-signaling pathway. | [64] |
| High fructose and high fat diet induced obese mice | Blueberry or C3G | 6.4 g/kg/dayor 0.02 g/kg/day | 8 weeks | Reduced body weight, body fat, and blood pressure. Improved glucose tolerance. | [65] |
| High fat diet fed mice | Anthocyanins mix | 2 or 20 or 40 mg/kg | 14 weeks | Diminished diet-induced obesity, dyslipidemia, insulin resistance, inflammation, and liver lipid deposition. Suppressed oxidative stress, NF-κB, and JNK activation, and PTP1B overexpression. | [66] |
| High fat diet fed rat | blackberry anthocyanin-rich extract | 25 mg/kg | 17 weeks | Improved the gut microbiota composition. Exhibited neuroprotective activity and improved tryptophan metabolism. | [67] |
| High sucrose and high fat diet induced obese mice | Plinia jaboticaba (Vell.) Berg extract | 50 mg GAE/kg | 8 weeks | Prohibited body weight gain and increase in FBG. Diminished hyperinsulinemia and high TC. | [68] |
| High fat diet induced obese mice | Anthocyanins | 40 mg/kg | 14 weeks | Improved intestinal permeability and endotoxemia. Normalized NADPH oxidase expression and NOS2 level, redox signaling, NF-κB, and ERK activation, and reduced oxidative stress. Reestablished microbial composition and restored MUC2 level. | [69] |
| High carbohydrate and high fat diet fed rat | Davidsonia pruriens | 8 mg of anthocyanins equivalent/kg | 8 weeks | Reduced fat mass, fat accumulation, retroperitoneal dipocytes level, TG, collagen deposition, and inflammation | [70] |
| High fat diet induced obese mice | Aronia melanocarpa extract | 50 or 100 or 200 mg/kg | 8 weeks | Suppressed weight gain and fat mass. Reduced TG, TC, LDL-C, leptin, insulin level. Suppressed adipogenesis. | [71] |
| High fat diet induced obese mice | Tart cherry extract | 60 mg/kg in drinking water | 6 weeks | Reduced IL-6 and leptin levels and improved the antioxidant capacity of the host. | [72] |
| High fat diet induced obese mice | Aristotelia chilensis | 20 mg/mL in water | 16 weeks | Suppressed weight gain. Improved the expression of genes associated with lipogenesis, fatty acid oxidation, thermogenesis. | [73] |
| Model | Intervention | Dose | Results | Reference |
|---|---|---|---|---|
| Rat adipocytes | Cyanidin or Cyanidin 3-O-β-D-glucose (C3G) glucoside | 100 µM | Increased the secretion of adiponectin and leptin, and expression of adipocyte-specific genes. | [74] |
| 3T3-L1 adipocytes | Cyanidin or C3G | 10–100 µM | Suppressed the MCP-1 and MRP-2 activation and release. | [75] |
| 3T3-L1 adipocytes | C3G | 20–100 µM | Suppressed the FFAs and glycerol release. Increased AMP-activated protein kinase activity. Decreased glutamine: fructose 6-phosphate aminotransferase activity. Decreased the adipose triglyceride lipase expression. | [76] |
| 3T3-L1 adipocytes | Grape anthocyanins | 1–40 µg/mL | Reduced the TG accumulation. Altered the lipogenic transcription factors. | [77] |
| 3T3-L1 pre-adipocytes | Anthocyanins from Vitis coignetiae Pulliat | 25–400 μg/mL | Attenuated the pre-adipocytes differentiation. Activated AMPK and suppressed the expression of adipogenic transcription factors. | [78] |
| 3T3-L1 adipocytes | Delphinidin-3-O-β-glucoside | 25, 50, and 100 µM | Reduced lipid accumulation. Suppressed adipogenic and lipogenic genes. Promoted lipid metabolism. | [79] |
| 3T3-L1 pre-adipocytes | Purple corn silk extract | 250–1000 μg/mL | Inhibits adipocyte proliferation. Reduced lipid accumulation | [80] |
| Hepatocytes (HB-8965®) | 5% (v/v) of Raspberry polyphenolic extract | 0.5 nmol/L | Modulated hepatic immune-metabolic mechanisms. Altered the endosome/lysosome activity. | [81] |
| HUVEC and 3T3-L1 cells | Anthocyanins from Cornus kousa | 5–200 μg/mL | Suppressed angiogenesis and lipid accumulation. | [82] |
| 3T3-L1 adipocytes | Anthocyanins | 10 and 20 μg/mL | Reduced lipid accumulation and PPAR- γ level. Nullified the palmitic acid-induced NF-κB activation and adipocyte dysfunction related hypertrophy. | [83] |
| Fluorometric method | Cyanidin and C3G | - | Inhibits pancreatic lipase activity | [84] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review. Foods 2020, 9, 687. https://doi.org/10.3390/foods9060687
Sivamaruthi BS, Kesika P, Chaiyasut C. The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review. Foods. 2020; 9(6):687. https://doi.org/10.3390/foods9060687
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Periyanaina Kesika, and Chaiyavat Chaiyasut. 2020. "The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review" Foods 9, no. 6: 687. https://doi.org/10.3390/foods9060687
APA StyleSivamaruthi, B. S., Kesika, P., & Chaiyasut, C. (2020). The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review. Foods, 9(6), 687. https://doi.org/10.3390/foods9060687







