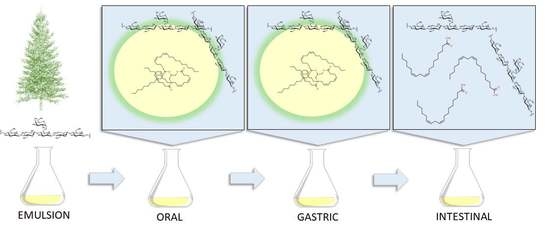
Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. In Vitro Digestion
2.4. Emulsion Morphology
2.5. Analysis of Triacylglycerols by HPLC-ELSD
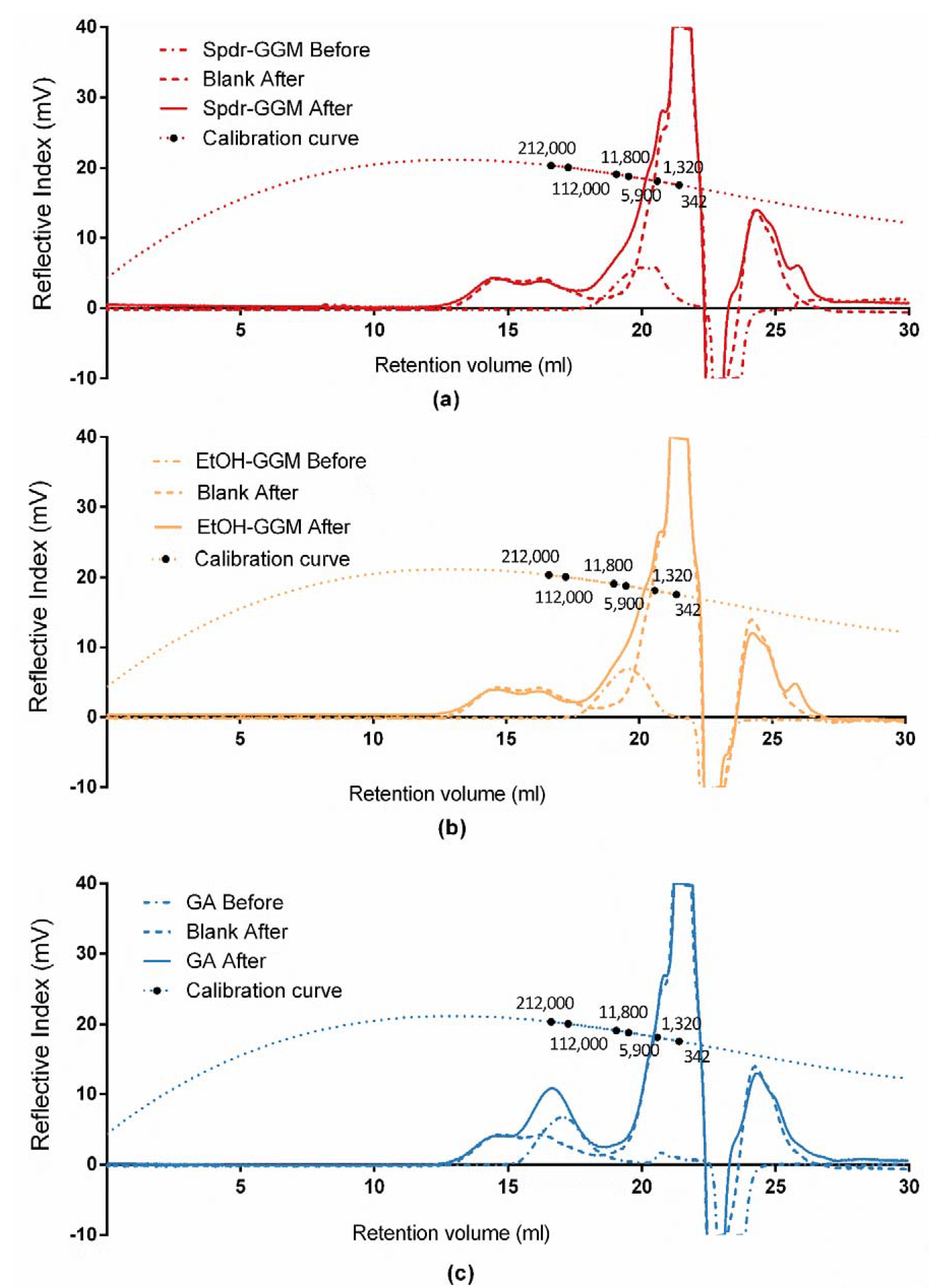
2.6. Determination of Molar Mass by HPSEC-MALLS-RI
2.7. Analysis of Monosaccharides by HPAEC-PAD
2.8. Analysis of Phenolic Compounds by UHPLC-DAD-FLD
2.9. Statistical Analyses
3. Results
3.1. Lipid Release from GGM-Stabilized Emulsions
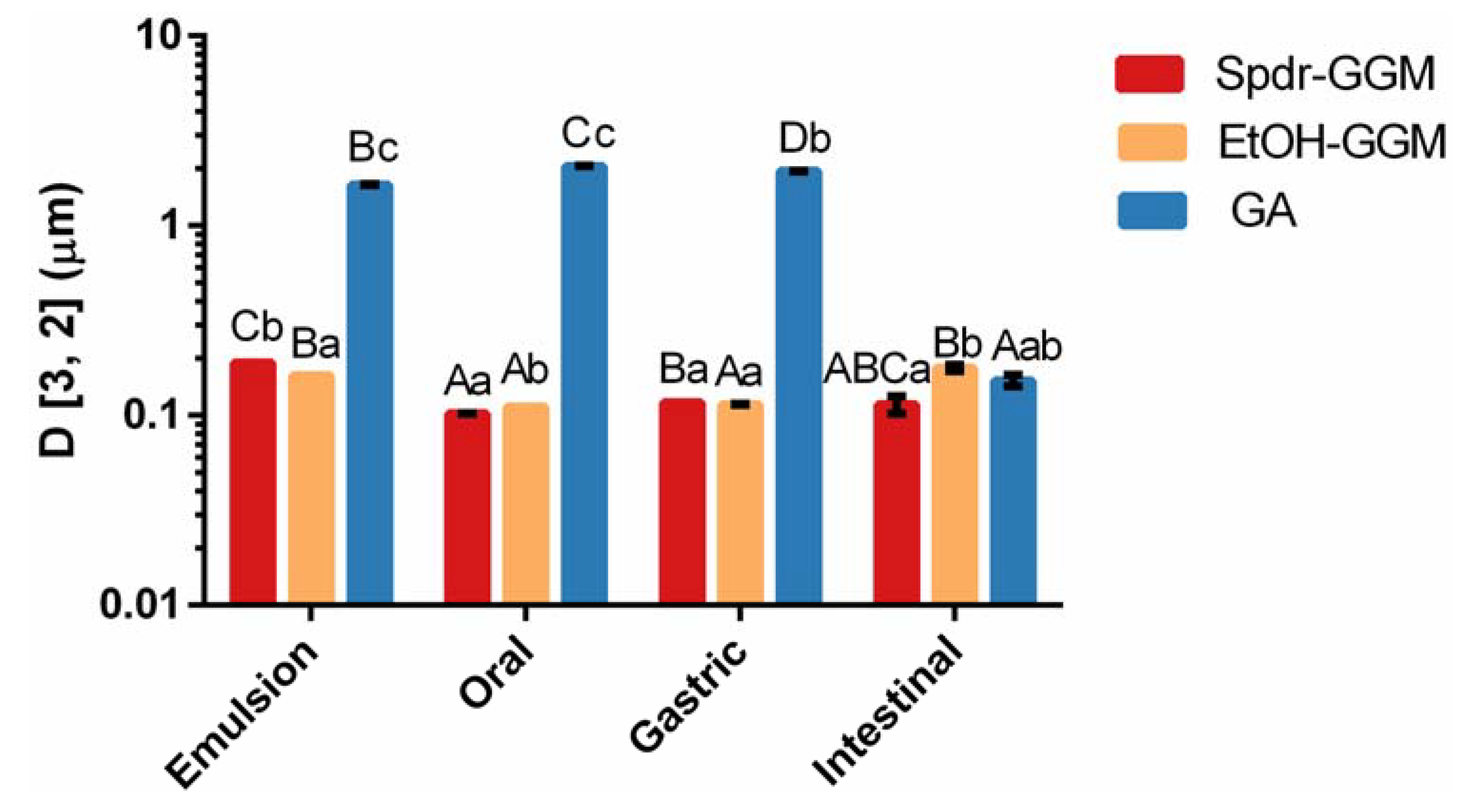
3.2. Physical Stability of GGM Stabilized Emulsions
3.3. Stability of GGM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sjöström, E. Wood Chemistry Fundamentals and Applications; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Lee, K.N.; Kritchevsky, D.; Parizaa, M.W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 1994, 108, 19–25. [Google Scholar] [CrossRef]
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; Di Gregorio, D.; Di Mascio, R.; Grazia Franzosi, M.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef]
- Basu, A.; Imrhan, V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: Conclusions from clinical trials. Eur. J. Clin. Nutr. 2007, 61, 295–303. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, 109. [Google Scholar] [CrossRef]
- McClements, D.J. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J. Food Sci. 2010, 75, 30–42. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, D.G. Food emulsions: Their structures and properties. In Food Emulsions, 4th ed.; Friberg, S.E., Larsson, K., Sjoblom, J., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 17–44. [Google Scholar]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S.; Tenkanen, M. Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food Sci. Technol. 2012, 28, 90–102. [Google Scholar] [CrossRef]
- Willför, S.; Sjöholm, R.; Laine, C.; Roslund, M.; Hemming, J.; Holmbom, B. Characterisation of water-soluble galactoglucomannans from Norway spruce wood and thermomechanical pulp. Carbohydr. Polym. 2003, 52, 175–187. [Google Scholar] [CrossRef]
- Willför, S.; Rehn, P.; Sundberg, A.; Sundberg, K.; Holmbom, B. Recovery of water-soluble acetyl-galactoglucomannans from mechanical pulp of spruce. Tappi J. 2003, 2, 27–32. [Google Scholar]
- Willför, S.; Sundberg, K.; Tenkanen, M.; Holmbom, B. Spruce-derived mannans: A potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr. Polym. 2008, 72, 197–210. [Google Scholar] [CrossRef]
- Kilpeläinen, P.O.; Hautala, S.S.; Byman, O.O.; Tanner, L.J.; Korpinen, R.I.; Lillandt, M.K.-J.; Pranovich, A.V.; Kitunen, V.H.; Willför, S.M.; Ilvesniemi, H.S.; et al. Pressurized hot water flow-through extraction system scale up from the laboratory to the pilot scale. Green Chem. 2014, 16, 3186–3194. [Google Scholar] [CrossRef]
- Von Schoultz, S. US20150167234: Method for Extracting Biomass. U.S. Available online: https://patents.google.com/patent/US20150167234 (accessed on 18 June 2015).
- Mikkonen, K.S.; Xu, C.; Berton-Carabin, C.; Schroën, K. Spruce galactoglucomannans in rapeseed oil-in-water emulsions: Efficient stabilization performance and structural partitioning. Food Hydrocoll. 2016, 52, 615–624. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Merger, D.; Kilpeläinen, P.; Murtomäki, L.; Schmidt, U.S.; Wilhelm, M. Determination of physical emulsion stabilization mechanisms of wood hemicelluloses via rheological and interfacial characterization. Soft Matter 2016, 12, 8690–8700. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S. Strategies for structuring diverse emulsion systems by using wood lignocellulose-derived stabilizers. Green Chem. 2020. [Google Scholar] [CrossRef]
- Lehtonen, M.I.; Teräslahti, S.; Xu, C.; Yadav, M.P.; Lampi, A.-M.; Mikkonen, K.S. Spruce galactoglucomannans inhibit lipid oxidation in rapeseed oil-in-water emulsions. Food Hydrocoll. 2016, 58, 255–266. [Google Scholar] [CrossRef]
- Lehtonen, M.I.; Merinen, M.; Kilpeläinen, P.O.; Xu, C.; Willför, S.M.; Mikkonen, K.S. Phenolic residues in spruce galactoglucomannans improve stabilization of oil-in-water emulsions. J. Colloid Interface Sci. 2018, 512, 536–547. [Google Scholar] [CrossRef]
- Valoppi, F.; Maina, N.; Allen, M.; Miglioli, R.; Kilpelainen, P.O.; Mikkonen, K. Spruce galactoglucomannan-stabilized emulsions as essential fatty acid delivery systems for functionalized drinkable yogurt and oat-based beverage. Eur. Food Res. Technol. 2019, 245, 1387–1398. [Google Scholar] [CrossRef]
- Valoppi, F.; Lahtinen, M.; Bhattarai, M.; Kirjoranta, S.; Juntti, V.; Peltonen, L.; Mikkonen, K.S. Centrifugal fractionation of softwood extracts improves the biorefinery workflow and yields functional emulsifiers. Green Chem. 2019, 21, 4691–4705. [Google Scholar] [CrossRef]
- Lahtinen, M.; Valoppi, F.; Juntti, V.; Heikkinen, S.; Kilpeläinen, P.; Maina, N.; Mikkonen, K.S. Lignin-rich PHWE hemicellulose extracts responsible for extended emulsion stabilization. Front. Chem. 2019, 7, 871. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; Park, Y.; Weiss, J.; McClements, D.J. Influence of interfacial composition on in vitro digestibility of emulsified lipids: Potential mechanism for Chitosan’s ability to inhibit fat digestion. Food Biophys. 2006, 1, 21–29. [Google Scholar] [CrossRef]
- Klinkesorn, U.; McClements, D.J. Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chem. 2019, 114, 1308–1315. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z.; Hříbalová, V.; Xu, C.; Holmbom, B.; Sundberg, A.; Willför, S. Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities. Int. J. Biol. Macromol. 2008, 42, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Lu, J.; Luo, J.P.; Zha, X.Q.; Wang, J.H. Preventive effect of a galactoglucomannan (GGM) from Dendrobium huoshanense on selenium-induced liver injury and fibrosis in rats. Exp. Toxicol. Pathol. 2020, 64, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Polari, L.; Ojansivu, P.; Mäkelä, S.; Eckerman, C.; Holmbom, B.; Salminen, S. Galactoglucomannan extracted from spruce (Picea abies) as a carbohydrate source for probiotic bacteria. J. Agric. Food Chem. 2012, 60, 11037–11043. [Google Scholar] [CrossRef]
- Deloule, V.; Boisset, C.; Hannani, D.; Suau, A.; Le Gouellec, A.; Chroboczek, J.; Botté, C.; Yamaryo-Botté, Y.; Chirat, C.; Toussaint, B.; et al. Prebiotic role of softwood hemicellulose in healthy mice model. J. Funct. Foods 2020, 64, 103688. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food: An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, H. Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva. Food Hydrocoll. 2009, 23, 1270–1278. [Google Scholar] [CrossRef]
- Lampi, A.-N.; Damerau, A.; Li, J.; Moisio, T.; Partanen, R.; Forssell, P.; Piironen, V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J. Cereal Sci. 2015, 62, 102–109. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In situ synthesis of exopolysaccharides by Leuconostoc spp. and Weissella spp. and their rheological impacts in fava bean flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, M.; Pitkänen, L.M.; Kitunen, V.; Korpinen, R.; Ilvesniemi, H.; Kilpeläinen, P.O.; Mikkonen, K.S. Functionality of spruce galactoglucomannans in oil-in-water emulsions. Food Hydrocoll. 2019, 86, 154–161. [Google Scholar] [CrossRef]
- Moore, P.B.; Langley, K.; Wilde, P.J.; Fillery-Travis, A.; Mela, D.J. Effect of emulsifier type on sensory properties of oil-in-water emulsions. J. Sci. Food Agric. 1998, 76, 469–476. [Google Scholar] [CrossRef]
- Malone, M.E.; Appleqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions—Part 1: Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- Chung, C.; Smith, G.; Degner, B.; McClements, D.J. Reduced fat food emulsions: Physicochemical, sensory, and biological aspects. Crit. Rev. Food Sci. Nutr. 2016, 56, 650–685. [Google Scholar] [CrossRef]
- Xu, C.; Pranovich, A.; Vähäsalo, L.; Hemming, J.; Holmbom, B.; Schols, H.A.; Willför, S. Kinetics of acid hydrolysis of water-soluble spruce O-acetyl galactoglucomannans. J. Agric. Food Chem. 2008, 56, 2429–2435. [Google Scholar] [CrossRef]
- Bhattarai, M.; Valoppi, F.; Hirvonen, S.-P.; Hietala, S.; Kilpelainen, P.; Aseyev, V.; Mikkonen, K.S. Time-dependent self-association of spruce galactoglucomannans depends on pH and mechanical shearing. Food Hydrocoll. 2020, 102, 105607. [Google Scholar] [CrossRef]
- Marciani, L.; Faulks, R.; Wickham, M.S.J.; Bush, D.; Pick, B.; Wright, J.; Cox, E.F.; Fillery-Travis, A.; Gowland, P.A.; Spiller, R.C.; et al. Effect of intragastric acid stability of fat emulsions on gastric emptying, plasma lipid profile and postprandial satiety. Br. J. Nutr. 2009, 101, 919–928. [Google Scholar] [CrossRef]
- Atgié, M.; Masbernat, O.; Roger, K. Emulsions stabilized by gum arabic: Composition and packing within interfacial films. Langmuir 2019, 35, 962–972. [Google Scholar] [CrossRef]
- Chu, B.S.; Rich, G.T.; Ridout, M.J.; Faulks, R.M.; Wickham, M.S.J.; Wilde, P.J. Modulating pancreatic lipase activity with galactolipids: Effects of emulsion interfacial composition. Langmuir 2009, 25, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Torcello-Gómez, A.; Foster, T.J. Influence of interfacial and bulk properties of cellulose ethers on lipolysis of oil-in-water emulsions. Carbohydr. Polym. 2016, 144, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of dietary fibers [methyl cellulose, chitosan, and pectin] on digestion of lipids under simulated gastrointestinal conditions. Food Funct. 2014, 5, 3083–3095. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The human gut firmicute roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef]
- Pitkänen, L.; Heinonen, M.; Mikkonen, K.S. Safety considerations of plant polysaccharides for food use: A case study on phenolic-rich softwood galactoglucomannan extract. Food Funct. 2018, 9, 1931–1943. [Google Scholar] [CrossRef]


| TAG % | Spdr-GGM | EtOH-GGM | GA |
|---|---|---|---|
| Initial emulsion | 100 Aa | 100 Aa | 100 Aa |
| Oral phase | 88 Ab ± 2 | 92 Aa ± 7 | 96 Aa ± 1 |
| Gastric phase | 87 Aab ± 5 | 94 Aa ± 7 | 85 Aa ± 8 |
| Intestinal phase | nd | nd | 7 c ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Mikkonen, K.S.; Kilpeläinen, P.O.; Lehtonen, M.I. Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds. Foods 2020, 9, 672. https://doi.org/10.3390/foods9050672
Zhao H, Mikkonen KS, Kilpeläinen PO, Lehtonen MI. Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds. Foods. 2020; 9(5):672. https://doi.org/10.3390/foods9050672
Chicago/Turabian StyleZhao, Hongbo, Kirsi S. Mikkonen, Petri O. Kilpeläinen, and Mari I. Lehtonen. 2020. "Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds" Foods 9, no. 5: 672. https://doi.org/10.3390/foods9050672
APA StyleZhao, H., Mikkonen, K. S., Kilpeläinen, P. O., & Lehtonen, M. I. (2020). Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds. Foods, 9(5), 672. https://doi.org/10.3390/foods9050672