Quantitative Analysis of Spectinomycin and Lincomycin in Poultry Eggs by Accelerated Solvent Extraction Coupled with Gas Chromatography Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. GC-MS/MS Analysis
2.3. Preparation of the Samples
2.3.1. Liquid–Liquid Extraction
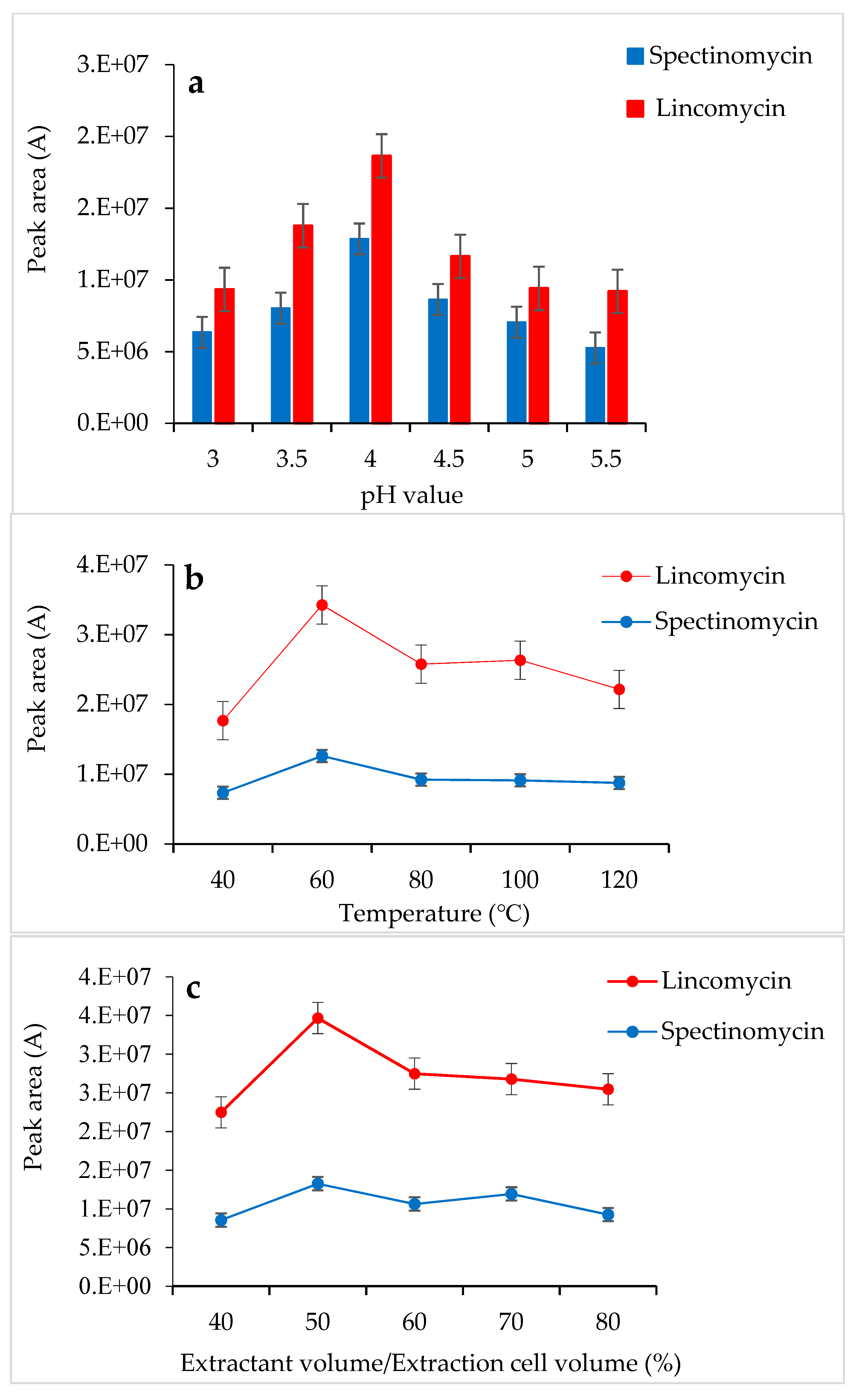
2.3.2. Accelerated Solvent Extraction
2.3.3. Solid-Phase Extraction
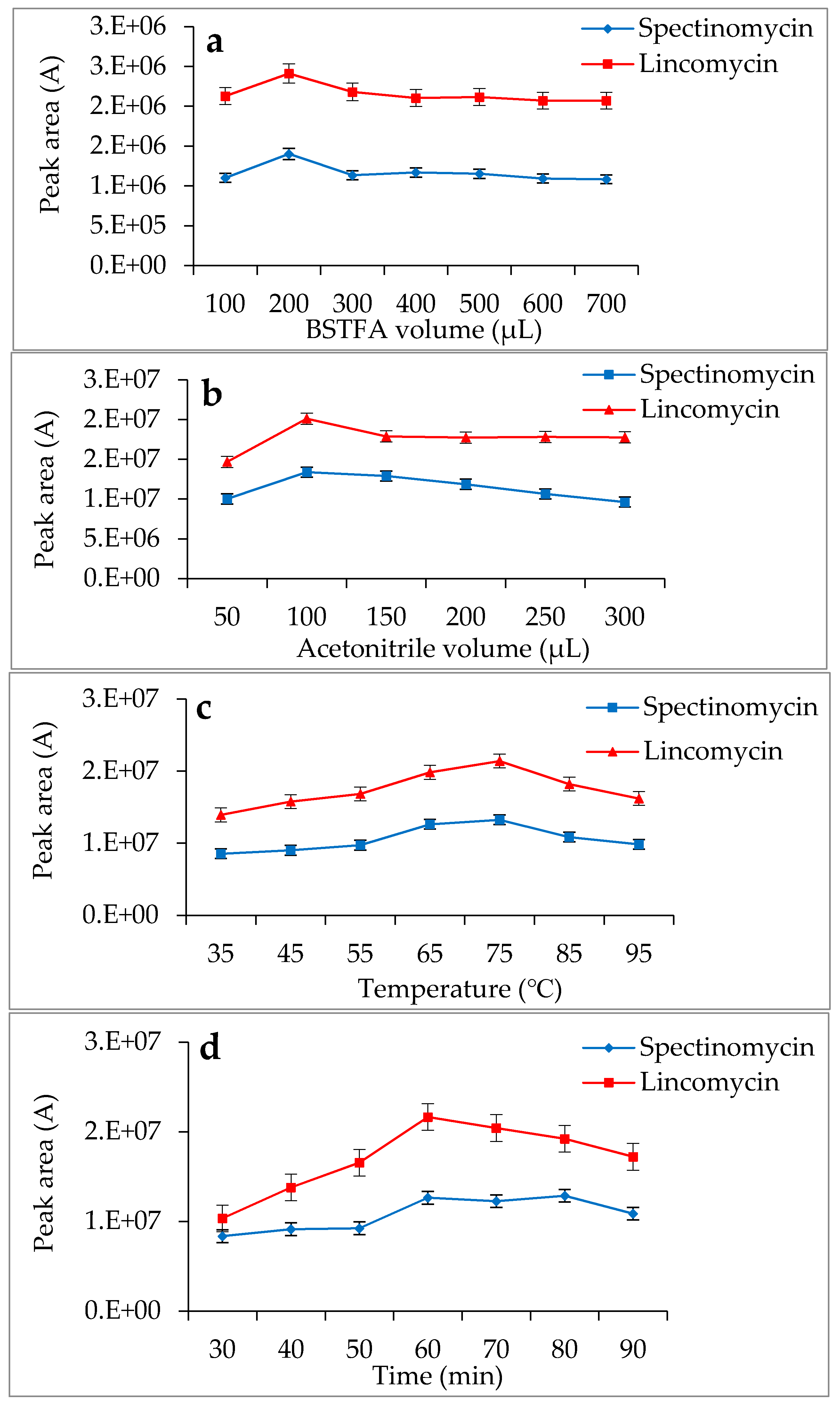
2.4. Derivatization Reaction
2.5. Quality Parameters
3. Results and Discussion
3.1. Optimization of the ASE Conditions
3.2. Optimization of the SPE Conditions
3.3. Optimization of the GC-MS/MS Analysis
3.4. Bioanalytical Method Validation
3.5. Comparison of Different Detection Methods
3.6. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Butler, M.M.; Waidyarachchi, S.L.; Connolly, K.L.; Jerse, A.E.; Chai, W.; Lee, R.E.; Kohlhoff, S.A.; Shinabarger, D.L.; Bowlin, T.L. Aminomethyl spectinomycins as therapeutics for drug-resistant gonorrhea and chlamydia coinfections. Antimicrob. Agents Chemother. 2018, 62, e00325-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.H.; Murray, R.W.; Vidmar, T.J.; Marotti, K.R. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 1997, 41, 2127–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Hou, Q.; Kwok, L.-Y.; Huo, D.; Feng, S.; Zhang, H. Effect of feeding Lactobacillus plantarum P-8 on the faecal microbiota of broiler chickens exposed to lincomycin. Sci. Bull. 2017, 62, 105–113. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, J.-T.; Zheng, M.; Zhang, Q.; Walsh, T.R.; Liao, X.-P.; Sun, J.; Liu, Y.-H. Combination therapy strategies against multiple-resistant Streptococcus suis. Front. Pharmacol. 2018, 9, 489. [Google Scholar] [CrossRef]
- Pokrant, E.; Maddaleno, A.; Lobos, R.; Trincado, L.; Lapierre, L.; San Martín, B.; Cornejo, J. Assessing the depletion of lincomycin in feathers from treated broiler chickens: A comparison with the concentration of its residues in edible tissues. Food Addit. Contam. 2019, 36, 1647–1653. [Google Scholar] [CrossRef]
- Verrette, L.; Fairbrother, J.M.; Boulianne, M. Effect of cessation of ceftiofur and substitution with lincomycin-spectinomycin on extended-spectrum-β-lactamase/AmpC genes and multidrug resistance in escherichia coli from a canadian broiler production pyramid. Appl. Environ. Microbiol. 2019, 85, e00037-19. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Agriculture of the People’s Republic of China. Maxium Residue Level of Veterinary Drugs in Food of Animal Origin; Notice no. 235 (Appendix 4); Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2002.
- The European Medicines Agency. Pharmacologically Active Substances and their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. In Commission Regulation (EU); No. 37/2010 of 22 December 2009; The European Medicines Agency: Amsterdam, The Netherlands, 2010. [Google Scholar]
- U.S. Food and Drug Administration. CFR-Code of Federal Regulations Title 21 Part 556 Tolerances for Residue of New Animal Drugs in Food; U.S. Food and Drug Administration: Rockville, MD, USA, 2014.
- The Japan Food Chemical Research Foundation. Maximum Residue Limits (MRLs) List of Agricultural Chemicals in Foods; The Japan Food Chemical Research Foundation: Tokyo, Japan, 2015. [Google Scholar]
- Medina, M.B. Development of a fluorescent latex immunoassay for detection of a spectinomycin antibiotic. J. Agric. Food Chem. 2004, 52, 3231–3236. [Google Scholar] [CrossRef]
- Kowalski, P.; Konieczna, L.; Olędzka, I.; Plenis, A.; Bączek, T. Development and validation of electromigration technique for the determination of lincomycin and clindamycin residues in poultry tissues. Food Anal. Methods 2014, 7, 276–282. [Google Scholar] [CrossRef]
- Cao, S.; Song, S.; Liu, L.; Kong, N.; Kuang, H.; Xu, C. Comparison of an enzyme-linked immunosorbent assay with an immunochromatographic assay for detection of lincomycin in milk and honey. Immunol. Investig. 2015, 44, 438–450. [Google Scholar] [CrossRef]
- Schermerhorn, P.G.; Chu, P.-S.; Kijak, P.J. Determination of spectinomycin residues in bovine milk using liquid chromatography with electrochemical detection. J. Agric. Food Chem. 1995, 43, 2122–2125. [Google Scholar] [CrossRef]
- Szúnyog, J.; Adams, E.; Liekens, K.; Roets, E.; Hoogmartens, J. Analysis of a formulation containing lincomycin and spectinomycin by liquid chromatography with pulsed electrochemical detection. J. Pharm. Biomed. Anal. 2002, 29, 213–220. [Google Scholar] [CrossRef]
- Haagsna, N.; Scherpenisse, P.; Simmonds, R.J.; Wood, S.A.; Rees, S.A. High-performance liquid chromatographic determination of spectinomycin in swine, calf and chicken plasma using post-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1995, 672, 165–171. [Google Scholar] [CrossRef]
- Hamamoto, K.; Mizuno, Y.; Koike, R.; Yamaoka, R.; Takahashi, T.; Takahashi, Y. Residue analysis of spectinomycin in tissues of chicken and swine by HPLC. J. Food Hyg. Soc. Jpn. 2003, 44, 114–118. [Google Scholar] [CrossRef]
- Negarian, M.; Mohammadinejad, A.; Mohajeri, S.A. Preparation, evaluation and application of core–shell molecularly imprinted particles as the sorbent in solid-phase extraction and analysis of lincomycin residue in pasteurized milk. Food Chem. 2019, 288, 29–38. [Google Scholar] [CrossRef]
- Wang, M.J.; Hu, C.Q. Analysis of spectinomycin by HPLC with evaporative light-scattering detection. Chromatographia 2006, 63, 255–260. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Wang, Y.; Yan, C. HPLC-ELSD analysis of spectinomycin dihydrochloride and its impurities. J. Sep. Sci. 2011, 34, 1811–1819. [Google Scholar] [CrossRef]
- Peru, K.M.; Kuchta, S.L.; Headley, J.V.; Cessna, A.J. Development of a hydrophilic interaction chromatography–mass spectrometry assay for spectinomycin and lincomycin in liquid hog manure supernatant and run-off from cropland. J. Chromatogr. A 2006, 1107, 152–158. [Google Scholar] [CrossRef]
- Molognoni, L.; de Souza, N.C.; de Sá Ploêncio, L.A.; Micke, G.A.; Daguer, H. Simultaneous analysis of spectinomycin, halquinol, zilpaterol, and melamine in feedingstuffs by ion-pair liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2018, 1569, 110–117. [Google Scholar] [CrossRef]
- Thompson, T.S.; Noot, D.K.; Calvert, J.; Pernal, S.F. Determination of lincomycin and tylosin residues in honey using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 1020, 241–250. [Google Scholar] [CrossRef]
- Sin, D.W.-M.; Wong, Y.-C.; Ip, A.C.-B. Quantitative analysis of lincomycin in animal tissues and bovine milk by liquid chromatography electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2004, 34, 651–659. [Google Scholar] [CrossRef]
- Sin, D.W.M.; Ho, C.; Wong, Y.C.; Ho, S.K.; Ip, A.C.B. Simultaneous determination of lincomycin and virginiamycin M1 in swine muscle, liver and kidney by liquid chromatography–electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2004, 517, 39–45. [Google Scholar] [CrossRef]
- van Holthoon, F.L.; Essers, M.L.; Mulder, P.J.; Stead, S.L.; Caldow, M.; Ashwin, H.M.; Sharman, M. A generic method for the quantitative analysis of aminoglycosides (and spectinomycin) in animal tissue using methylated internal standards and liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2009, 637, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Moltó, J.C.; Mañes, J.; Font, G. Determination of macrolide and lincosamide antibiotics by pressurised liquid extraction and liquid chromatography-tandem mass spectrometry in meat and milk. Food Control 2010, 21, 1703–1709. [Google Scholar] [CrossRef]
- Maddaleno, A.; Pokrant, E.; Yanten, F.; San Martin, B.; Cornejo, J. Implementation and validation of an analytical method for lincomycin determination in feathers and edible tissues of broiler chickens by liquid chromatography tandem mass spectrometry. J. Anal. Methods Chem. 2019, 2019, 4569707. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Yin, B.; Ang, C.Y.W.; Rushing, L.; Thompson, H.C. Determination of lincomycin residues in salmon tissues by gas chromatography with nitrogen-phosphorus detection. J. Chromatogr. B Biomed. Sci. Appl. 1996, 687, 405–411. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, D.; Yu, G.; Yu, H.; Pan, Y.; Wang, Y.; Huang, L.; Yuan, Z. Simultaneous determination of lincomycin and spectinomycin residues in animal tissues by gas chromatography-nitrogen phosphorus detection and gas chromatography-mass spectrometry with accelerated solvent extraction. Food Addit. Contam. A 2011, 28, 145–154. [Google Scholar] [CrossRef]
- The European Communities. Commission decision 2002/657/EC of 12 August 2002 implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, 221, 8–36. [Google Scholar]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research; Center for Veterinary Medicine. Guidance for Industry: Bioanalytical Method Validation; US Department of Health and Human Services: Washington, DC, USA, 2001.
- National Food Safety Standard. GB 29685-2013: Determination of Lincomycin, Clindamycin and Spectinomycin in Animal Derived Food by Gas Chromatography-Mass Spectrometry Method; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2013.

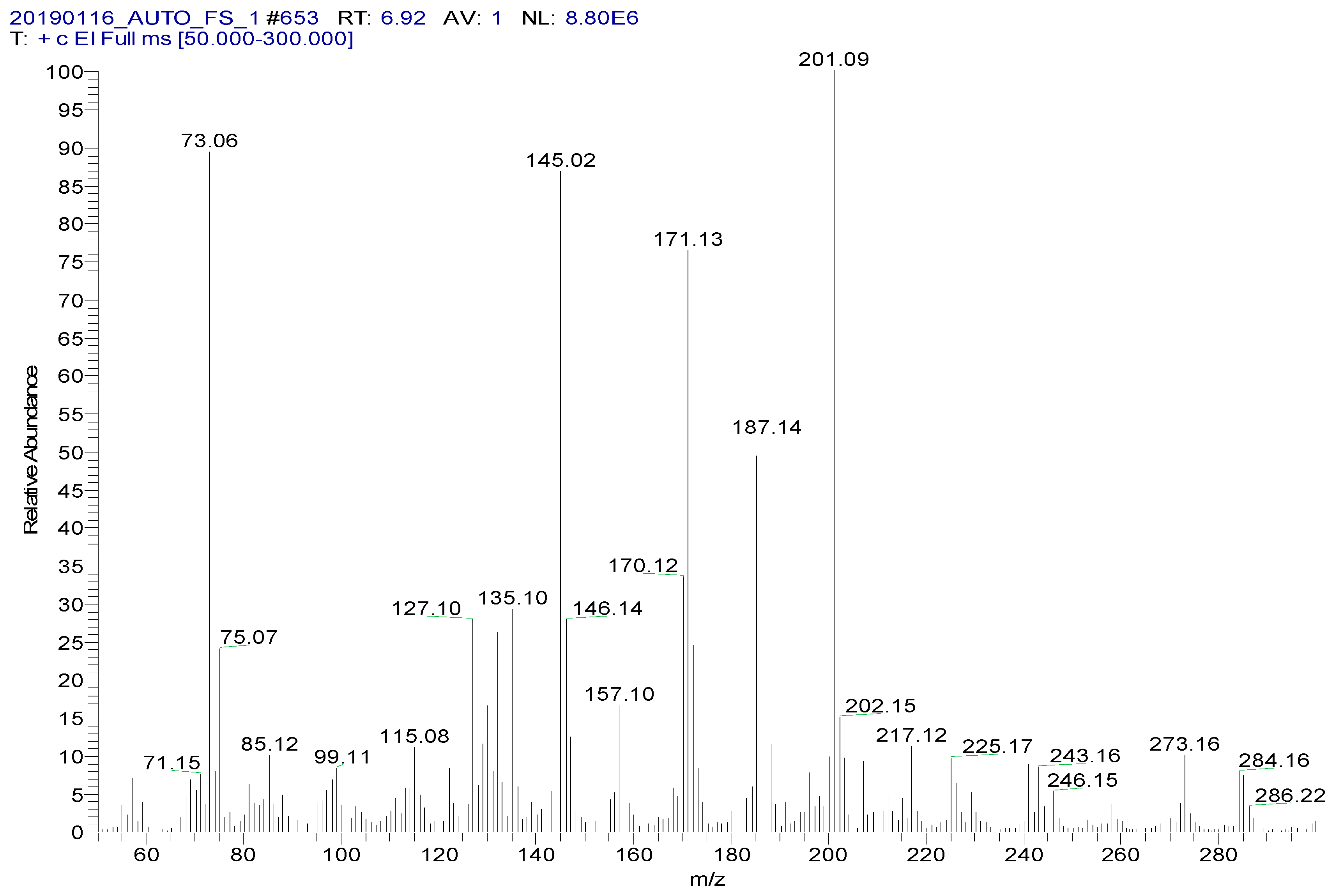


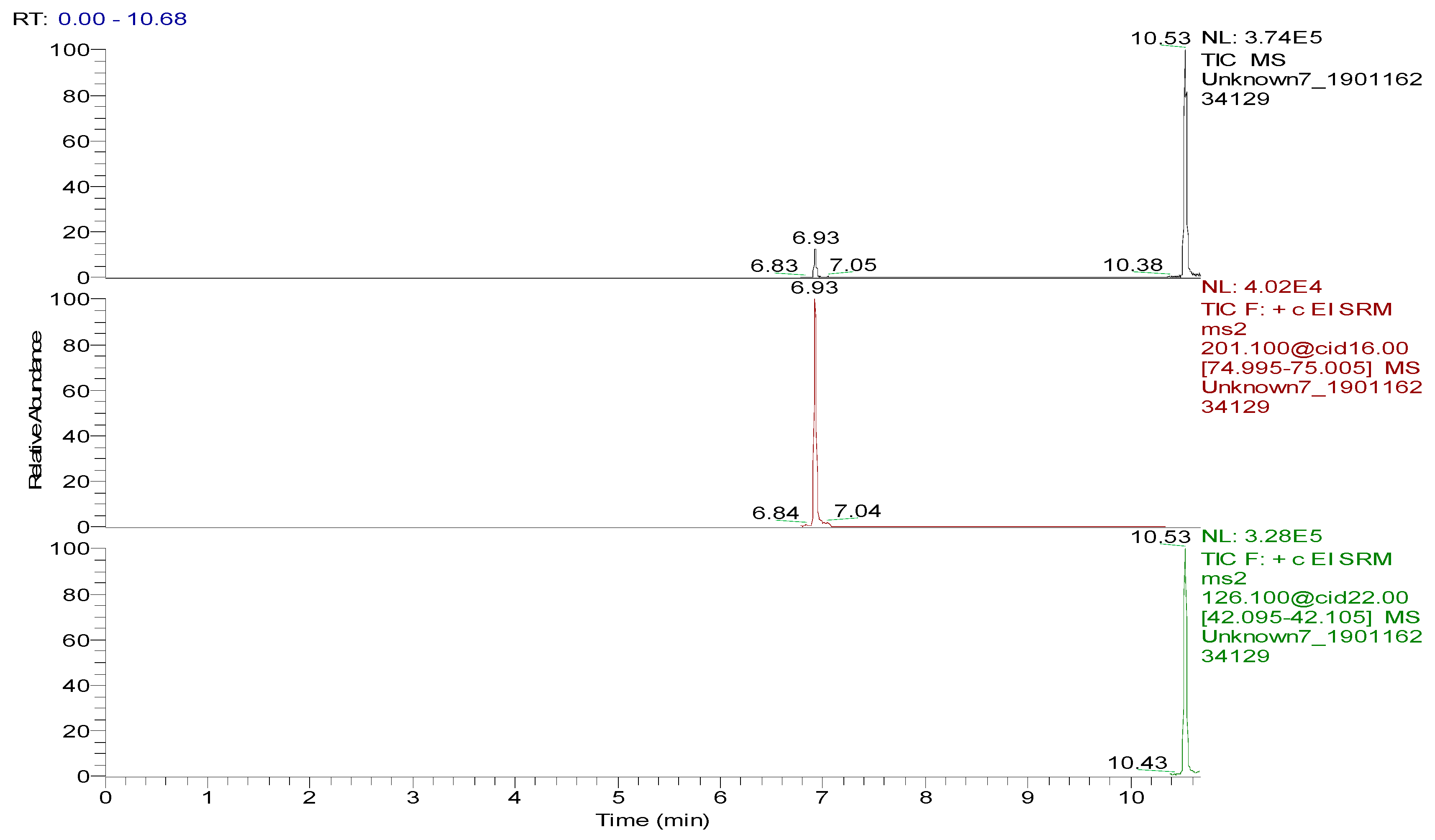
| Analyte | Retention Time (min) | Molecular Weight (m/z) | Mass Transitions (m/z) | Collision Energy (eV) |
|---|---|---|---|---|
| Spectinomycin | 6.93 | 332.15 | 201.1 > 75.0 * 201.1 > 185.1 | 16 8 |
| Lincomycin | 10.53 | 406. 21 | 126.1 > 42.0 * 126.1 > 82.0 | 22 22 |
| Analyte | Matrix | Sample Preparation Method | |
|---|---|---|---|
| LLE-SPE | ASE-SPE | ||
| Spectinomycin | Hen whole egg | 80.5 ± 1.6 | 86.0 ± 1.5 |
| Albumen | 81.4 ± 1.4 | 86.6 ± 2.0 | |
| Yolk | 81.2 ± 1.7 | 86.6 ± 2.1 | |
| Duck whole egg | 80.9 ± 3.4 | 86.1 ± 2.7 | |
| Albumen | 74.4 ± 1.4 | 85.7 ± 2.2 | |
| Yolk | 82.1 ± 3.4 | 87.1 ± 1.3 | |
| Goose whole egg | 79.2 ± 2.2 | 87.7 ± 1.7 | |
| Albumen | 81.1 ± 1.6 | 83.4 ± 1.4 | |
| Yolk | 80.9 ± 3.7 | 85.9 ± 1.2 | |
| Pigeon whole egg | 80.5 ± 2.7 | 87.7 ± 2.2 | |
| Quail whole egg | 79.0 ± 3.2 | 87.2 ± 1.9 | |
| Lincomycin | Hen whole egg | 79.4 ± 1.6 | 84.8 ± 1.6 |
| Albumen | 78.7 ± 2.7 | 85.8 ± 2.2 | |
| Yolk | 81.3 ± 3.3 | 84.8 ± 2.0 | |
| Duck whole egg | 75.4 ± 1.7 | 85.4 ± 2.6 | |
| Albumen | 76.0 ± 2.4 | 93.4 ± 2.3 | |
| Yolk | 83.5 ± 1.6 | 87.9 ± 2.1 | |
| Goose whole egg | 82.3 ± 3.7 | 87.9 ± 1.1 | |
| Albumen | 75.7 ± 2.0 | 89.1 ± 1.1 | |
| Yolk | 76.6 ± 2.2 | 84.6 ± 2.5 | |
| Pigeon whole egg | 83.2 ± 3.6 | 86.2 ± 2.2 | |
| Quail whole egg | 79.3 ± 1.2 | 86.4 ± 1.5 | |
| Analyte | Matrix | Regression Equation | Determination Coefficient (R2) | Linear Range (μg/kg) | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|
| Spectinomycin | Hen whole egg | y = 1251x + 16346 | 0.9993 | 6.0–2000 | 3.1 | 6.0 |
| Albumen | y = 1298.4x + 34049 | 0.9992 | 6.4–2000 | 3.0 | 6.4 | |
| Yolk | y = 1199.1x − 4540.7 | 0.9997 | 5.6–2000 | 2.3 | 5.6 | |
| Duck whole egg | y = 1059.5x − 3709.3 | 0.9999 | 6.3–2000 | 3.5 | 6.3 | |
| Albumen | y = 1113.1x − 4463.4 | 0.9994 | 7.9–2000 | 3.8 | 7.9 | |
| Yolk | y = 1051.5x − 2573.8 | 0.9993 | 8.0–2000 | 2.7 | 8.0 | |
| Goose whole egg | y = 1113.5x − 3245.8 | 0.9996 | 7.1–2000 | 3.5 | 7.1 | |
| Albumen | y = 1179.6x − 3611.7 | 0.9995 | 7.8–2000 | 3.2 | 7.8 | |
| Yolk | y = 1074.7x − 2777.5 | 0.9996 | 6.7–2000 | 3.0 | 6.7 | |
| Pigeon whole egg | y = 1022.9x − 6310.6 | 0.9991 | 8.0–2000 | 4.0 | 8.0 | |
| Quail whole egg | y = 1073.1x − 4316.6 | 0.9998 | 7.6–2000 | 3.8 | 7.6 | |
| Lincomycin | Hen whole egg | y = 10074x − 29019 | 0.9994 | 8.4–200 | 3.1 | 8.4 |
| Albumen | y = 7829.4x + 98669 | 0.9992 | 6.7–200 | 2.6 | 6.7 | |
| Yolk | y = 13453x − 27487 | 0.9994 | 5.9–200 | 2.5 | 5.9 | |
| Duck whole egg | y = 8127.3x − 16359 | 0.9996 | 6.5–200 | 2.8 | 6.5 | |
| Albumen | y = 7772.3x − 312.45 | 0.9992 | 7.3–200 | 3.0 | 7.3 | |
| Yolk | y = 7759.2x + 32016 | 0.9992 | 8.0–200 | 4.3 | 8.0 | |
| Goose whole egg | y = 8825.4x + 2625.7 | 0.9996 | 8.5–200 | 3.5 | 8.5 | |
| Albumen | y = 9240.1x + 6253.1 | 0.9994 | 9.0–200 | 3.8 | 9.0 | |
| Yolk | y = 8381.4x + 27696 | 0.9994 | 9.2–200 | 4.0 | 9.2 | |
| Pigeon whole egg | y = 8578.4x − 37597 | 0.9993 | 9.5–200 | 3.9 | 9.5 | |
| Quail whole egg | y = 8736.2x − 61525 | 0.9994 | 8.2–200 | 4.3 | 8.2 |
| Analyte | Matrix | Spike Level (μg/kg) | Recovery (%) | RSD (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|---|
| Spectinomycin | Hen whole egg | 6.0 | 82.9 ± 1.5 | 1.8 | 3.0 | 4.2 |
| 1000.0 | 84.5 ± 1.0 | 1.2 | 4.1 | 3.6 | ||
| 2000.0 a | 86.0 ± 1.5 | 1.7 | 4.0 | 4.1 | ||
| 4000.0 | 95.7 ± 1.2 | 1.3 | 3.7 | 5.5 | ||
| Duck whole egg | 6.3 | 80.9 ± 1.3 | 1.6 | 4.6 | 5.0 | |
| 1000.0 | 85.5 ± 1.5 | 1.8 | 3.6 | 4.5 | ||
| 2000.0 a | 86.1 ± 2.7 | 3.1 | 2.9 | 3.2 | ||
| 4000.0 | 89.4 ± 1.8 | 2.0 | 2.9 | 3.4 | ||
| Goose whole egg | 7.1 | 82.1 ± 1.7 | 2.1 | 3.2 | 5.0 | |
| 1000.0 | 86.8 ± 1.3 | 1.5 | 2.7 | 4.9 | ||
| 2000.0 a | 87.7 ± 1.7 | 1.9 | 2.5 | 3.0 | ||
| 4000.0 | 90.6 ± 1.5 | 1.7 | 4.0 | 4.1 | ||
| Pigeon whole egg | 8.0 | 82.3 ± 1.3 | 1.6 | 3.3 | 6.7 | |
| 1000.0 | 83.5 ± 1.1 | 1.3 | 3.1 | 4.6 | ||
| 2000.0 a | 87.7 ± 2.2 | 2.5 | 3.7 | 4.8 | ||
| 4000.0 | 88.7 ± 1.3 | 1.5 | 2.5 | 3.7 | ||
| Quail whole egg | 7.6 | 85.1 ± 1.4 | 1.6 | 3.1 | 6.0 | |
| 1000.0 | 85.3 ± 1.6 | 1.9 | 3.2 | 4.4 | ||
| 2000.0 a | 87.1 ± 1.9 | 2.2 | 3.0 | 3.9 | ||
| 4000.0 | 94.8 ± 1.1 | 1.2 | 2.5 | 3.5 | ||
| Lincomycin | Hen whole egg | 8.4 | 82.7 ± 1.3 | 1.6 | 4.6 | 5.7 |
| 25.0 | 85.4 ± 2.4 | 2.8 | 5.0 | 5.6 | ||
| 50.0 a | 84.8 ± 1.6 | 1.9 | 3.1 | 4.5 | ||
| 100.0 | 87.1 ± 1.9 | 2.2 | 3.2 | 4.9 | ||
| Duck whole egg | 6.5 | 81.8 ± 1.1 | 1.3 | 4.3 | 4.7 | |
| 25.0 | 86.7 ± 2.5 | 2.9 | 3.7 | 5.4 | ||
| 50.0 a | 85.4 ± 2.6 | 3.0 | 2.7 | 3.7 | ||
| 100.0 | 86.1 ± 2.7 | 3.1 | 4.6 | 5.0 | ||
| Goose whole egg | 8.5 | 80.1 ± 1.5 | 1.9 | 2.2 | 4.1 | |
| 25.0 | 84.0 ± 2.6 | 3.1 | 3.4 | 4.1 | ||
| 50.0 a | 87.9 ± 1.1 | 1.3 | 2.3 | 3.7 | ||
| 100.0 | 93.5 ± 1.8 | 1.9 | 3.5 | 3.9 | ||
| Pigeon whole egg | 9.5 | 81.4 ± 1.6 | 2.0 | 3.9 | 6.5 | |
| 25.0 | 82.2 ± 2.1 | 2.6 | 2.3 | 3.1 | ||
| 50.0 a | 86.2 ± 2.2 | 2.6 | 5.6 | 6.7 | ||
| 100.0 | 91.3 ± 1.6 | 1.8 | 3.0 | 3.9 | ||
| Quail whole egg | 8.2 | 80.0 ± 1.3 | 1.6 | 2.6 | 3.3 | |
| 25.0 | 86.7 ± 2.1 | 2.4 | 3.9 | 3.7 | ||
| 50.0 a | 86.4 ± 1.5 | 1.7 | 2.6 | 4.4 | ||
| 100.0 | 87.3 ± 1.1 | 1.3 | 3.5 | 5.5 |
| Analyte | Matrix | Spike Level (μg/kg) | Recovery (%) | RSD (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|---|
| Spectinomycin | Hen egg albumen | 6.4 | 83.8 ± 2.4 | 2.9 | 3.0 | 4.6 |
| 1000.0 | 84.2 ± 2.3 | 2.7 | 2.9 | 3.7 | ||
| 2000.0 a | 86.6 ± 2.0 | 2.3 | 2.8 | 3.6 | ||
| 4000.0 | 93.0 ± 1.9 | 2.0 | 2.2 | 4.3 | ||
| Yolk | 5.6 | 83.9 ± 2.5 | 3.0 | 3.5 | 3.7 | |
| 1000.0 | 84.1 ± 1.7 | 2.0 | 3.0 | 3.4 | ||
| 2000.0 a | 86.6 ± 2.1 | 2.4 | 3.7 | 5.5 | ||
| 4000.0 | 94.5 ± 1.4 | 1.5 | 2.6 | 3.8 | ||
| Duck egg albumen | 7.9 | 83.6 ± 1.3 | 1.6 | 2.7 | 4.7 | |
| 1000.0 | 84.7 ± 1.5 | 1.8 | 2.0 | 2.2 | ||
| 2000.0 a | 85.7 ± 2.2 | 2.6 | 3.0 | 3.9 | ||
| 4000.0 | 87.3 ± 3.0 | 3.4 | 5.2 | 6.5 | ||
| Yolk | 8.0 | 82.9 ± 1.9 | 2.3 | 2.8 | 3.5 | |
| 1000.0 | 85.3 ± 2.1 | 2.5 | 3.9 | 4.2 | ||
| 2000.0 a | 87.1 ± 1.3 | 1.5 | 2.8 | 3.3 | ||
| 4000.0 | 90.3 ± 2.4 | 2.7 | 4.3 | 6.2 | ||
| Goose egg albumen | 7.8 | 80.4 ± 1.5 | 1.9 | 2.0 | 3.7 | |
| 1000.0 | 84.0 ± 2.5 | 3.0 | 3.3 | 6.3 | ||
| 2000.0 a | 83.4 ± 1.4 | 1.7 | 2.5 | 3.1 | ||
| 4000.0 | 88.1 ± 1.8 | 2.0 | 3.9 | 4.1 | ||
| Yolk | 6.7 | 81.6 ± 2.4 | 2.9 | 3.1 | 3.4 | |
| 1000.0 | 82.4 ± 2.0 | 2.4 | 2.4 | 3.2 | ||
| 2000.0 a | 85.9 ± 1.2 | 1.4 | 2.0 | 3.5 | ||
| 4000.0 | 89.4 ± 1.9 | 2.1 | 3.1 | 4.9 | ||
| Lincomycin | Hen egg albumen | 6.7 | 82.2 ± 1.2 | 1.5 | 2.8 | 3.4 |
| 25.0 | 85.2 ± 1.6 | 1.9 | 3.2 | 4.1 | ||
| 50.0 a | 85.8 ± 2.2 | 2.6 | 3.4 | 4.5 | ||
| 100.0 | 86.5 ± 2.5 | 2.9 | 3.5 | 5.7 | ||
| Yolk | 5.9 | 82.7 ± 1.4 | 1.7 | 3.9 | 4.8 | |
| 25.0 | 85.4 ± 2.0 | 2.3 | 3.0 | 3.6 | ||
| 50.0 a | 84.8 ± 2.0 | 2.4 | 2.6 | 3.8 | ||
| 100.0 | 91.2 ± 2.1 | 2.3 | 4.1 | 5.5 | ||
| Duck egg albumen | 7.3 | 84.9 ± 1.5 | 1.8 | 1.9 | 3.5 | |
| 25.0 | 91.2 ± 2.4 | 2.6 | 6.0 | 5.5 | ||
| 50.0 a | 93.4 ± 2.3 | 2.5 | 3.7 | 4.3 | ||
| 100.0 | 95.1 ± 2.2 | 2.3 | 3.2 | 4.9 | ||
| Yolk | 8.0 | 85.2 ± 1.3 | 1.5 | 3.2 | 5.3 | |
| 25.0 | 86.5 ± 0.9 | 1.0 | 2.5 | 3.6 | ||
| 50.0 a | 87.9 ± 2.1 | 2.4 | 3.8 | 4.1 | ||
| 100.0 | 89.1 ± 2.3 | 2.6 | 3.5 | 3.7 | ||
| Goose egg albumen | 9.0 | 80.8 ± 1.6 | 2.0 | 2.8 | 5.3 | |
| 25.0 | 85.4 ± 1.2 | 1.4 | 2.6 | 4.2 | ||
| 50.0 a | 89.1 ± 1.1 | 1.2 | 2.7 | 3.5 | ||
| 100.0 | 92.1 ± 1.2 | 1.3 | 3.9 | 3.6 | ||
| Yolk | 9.2 | 80.2 ± 1.9 | 2.4 | 4.8 | 4.4 | |
| 25.0 | 85.6 ± 2.1 | 2.5 | 4.3 | 3.9 | ||
| 50.0 a | 84.6 ± 2.5 | 3.0 | 4.0 | 4.1 | ||
| 100.0 | 87.9 ± 1.6 | 1.8 | 2.7 | 4.1 |
| Detection Method | Sample Preparation Method | Analyte | Animal-Derived Food | Analysis Time (min) | LOD (μg/kg) | LOQ (μg/kg) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| HPLC-FLD [16] | SPE | Spectinomycin | Swine, calf and chicken plasma | 12.0 | - | - | 91.0–104 |
| HPLC-UVD [18] | CSMISPE | Lincomycin | Milk | 9.0 | 20.0 | 80.0 | 80.0–89.0 |
| HILIC-MS/MS [22] | LLE | Spectinomycin | Feedstuffs | 10.0 | - | - | 80.0–92.0 |
| HPLC-MS [23] | SPE | Lincomycin | Honey | 10.0 | 7.0 | 10.0 | 102–105 |
| HPLC-MS/MS [24] | LLE | Lincomycin | Animal tissues and milk | 10.0 | 1.5–8.8 | 25.0–50.0 | 93.9–107 |
| HPLC-MS/MS [27] | ASE | Lincomycin | Meat and milk | 40.0 | 5.0–10.0 | 10.0–15.0 | 86.0–91.0 |
| GC-NPD [30] | ASE | Spectinomycin Lincomycin | Animal tissues | 13.0 | 8.1–9.4 | 16.4–21.4 | 73.0–97.0 |
| GC-MS [30] | ASE | Spectinomycin Lincomycin | Animal tissues | 15.0 | 1.9–3.1 | 4.7–5.7 | 70.0–93.0 |
| GC-MS/MS | ASE | Spectinomycin Lincomycin | Poultry eggs | 10.8 | 2.3–4.3 | 5.6–9.5 | 80.0–95.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Wang, Y.; Xie, X.; Diao, Z.; Xie, K.; Zhang, G.; Zhang, T.; Dai, G. Quantitative Analysis of Spectinomycin and Lincomycin in Poultry Eggs by Accelerated Solvent Extraction Coupled with Gas Chromatography Tandem Mass Spectrometry. Foods 2020, 9, 651. https://doi.org/10.3390/foods9050651
Wang B, Wang Y, Xie X, Diao Z, Xie K, Zhang G, Zhang T, Dai G. Quantitative Analysis of Spectinomycin and Lincomycin in Poultry Eggs by Accelerated Solvent Extraction Coupled with Gas Chromatography Tandem Mass Spectrometry. Foods. 2020; 9(5):651. https://doi.org/10.3390/foods9050651
Chicago/Turabian StyleWang, Bo, Yajuan Wang, Xing Xie, Zhixiang Diao, Kaizhou Xie, Genxi Zhang, Tao Zhang, and Guojun Dai. 2020. "Quantitative Analysis of Spectinomycin and Lincomycin in Poultry Eggs by Accelerated Solvent Extraction Coupled with Gas Chromatography Tandem Mass Spectrometry" Foods 9, no. 5: 651. https://doi.org/10.3390/foods9050651
APA StyleWang, B., Wang, Y., Xie, X., Diao, Z., Xie, K., Zhang, G., Zhang, T., & Dai, G. (2020). Quantitative Analysis of Spectinomycin and Lincomycin in Poultry Eggs by Accelerated Solvent Extraction Coupled with Gas Chromatography Tandem Mass Spectrometry. Foods, 9(5), 651. https://doi.org/10.3390/foods9050651





