Bioactive Compounds and Antioxidant Capacity of Small Berries
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Sample Preparation
2.3. Total Anthocyanins Content
2.4. Total Flavonoids Content
2.5. Trolox Equivalent Antioxidant Capacity (TEAC)
2.6. Ferric-Reducing Antioxidant Power (FRAP)
2.7. Scavenging of the DPPH• Radical
2.8. HPLC-DAD-UV-ESI HRMS Analytical Method
2.9. Fatty Acid Composition by Gas Chromatography
2.10. Statistical Analysis
3. Results and Discussion
3.1. Total Anthocyanins and Flavonoids Contents
3.2. Antioxidant Capacity Assays
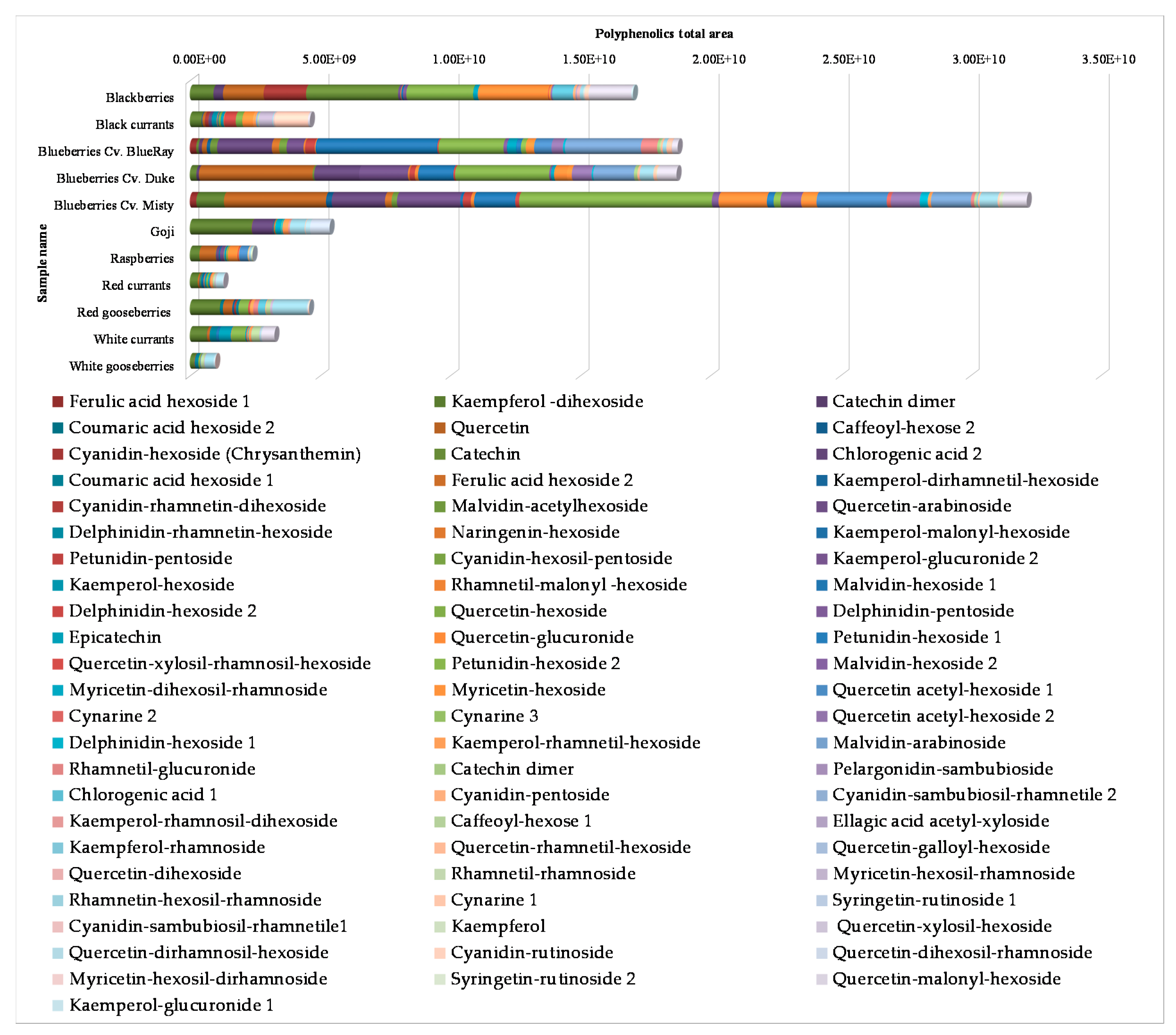
3.3. HPLC-DAD-ESI HRMS
3.4. Fatty Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manganaris:, G.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Hegedűs, A.; Stefanovits-Bányaia, E. Application of and correlation among antioxidant and antiradical assays for characterizing antioxidant capacity of berries. Sci. Hortic. 2010, 125, 332–336. [Google Scholar] [CrossRef]
- Namiesnik, J.; Vearasilp, K.; Kupska, M.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Antioxidant activities and bioactive components in some berries. Eur. Food Res. Technol. 2013, 237, 819–829. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- AGRION. Technical Notes for Vegetables, Strawberries and Small Fruits. Foundation for Research, Innovation and Technological Development of Piedmontese Agriculture. Available online: http://www.agrion.it/gesnew-sche.asp?GesNewId=6849&nomenu=0 (accessed on 30 April 2020). (In Italian).
- Rowland, L.J.; Hancock, J.F.; Bassil, N.V. Blueberry. In Genetics, Genomics and Breeding of Berries; Folta, K.M., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–32. [Google Scholar]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Troszyńska, A. Antioxidant activity of extract of adzuki bean and its fractions. J. Food Lipid. 2008, 15, 119–136. [Google Scholar] [CrossRef]
- Wang, X.; Tong, H.; Chen, F.; Gangemi, J.D. Chemical characterization and antioxidant evaluation of muscadine grape pomace extract. Food Chem. 2010, 123, 1156–1162. [Google Scholar] [CrossRef]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F.; Tassone, S. Fatty acid profile and nutritive value of quinoa (Chenopodium quinoa Willd.) seeds and plants at different growth stages. Anim. Feed Sci. Technol. 2013, 183, 56–61. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Mondello, L.; Errante, G.; Zappia, G.; Dugo, G. Identification of anthocyanins in berries by narrow-bore high-performance liquid chromatography with electrospray ionization detection. J. Agric. Food Chem. 2001, 49, 3987–3992. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Singh Badwal, T.; Xu, B. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G.J.; Mezzetti, B. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009, 67, S145–S150. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Savić, Z.; Brala, A.; Levaj, B.; Bursać Kovačević, D.; Biško, A. Evaluation of phenolic content and antioxidant capacity of blueberry cultivars (Vaccinium corymbosum L.) grown in the Northwest Croatia. Food Technol. Biotechnol. 2010, 48, 214–221. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R. Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinone, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Lee, S.G.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Contribution of anthocyanin composition to total antioxidant capacity of berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef]
- Manríquez-Torres, J.J.; Sánchez-Franco, J.A.; Ramírez-Moreno, E.; Cruz-Cansino, N.D.; Ariza-Ortega, J.A.; Torres-Valencia, J.M. Effect of thermoultrasound on the antioxidant compounds and fatty acid profile of blackberry (Rubus fruticosus spp.) juice. Molecules 2016, 21, 1624. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef] [PubMed]
- Radočaj, O.; Vujasinović, V.; Dimić, E.; Basić, Z. Blackberry (Rubus fruticosus L.) and raspberry (Rubus idaeus L.) seed oils extracted from dried press pomace after longterm frozen storage of berries can be used as functional food ingredients. Eur. J. Lipid Sci. Technol. 2014, 116, 1015–1024. [Google Scholar] [CrossRef]
- Van Hoed, V.; Barbouche, I.; De Clercq, N.; Dewettinck, K.; Slah, M.; Leber, E.; Verhé, R. Influence of filtering of cold pressed berry seed oils on their antioxidant profile and quality characteristics. Food Chem. 2011, 127, 1848–1855. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Bushman, B.S.; Phillips, B.; Isbell, T.; Ou, B.; Crane, J.M.; Knapp, S.J. Chemical composition of caneberry (Rubus spp.) seeds and oils and their antioxidant potential. J. Agric. Food Chem. 2004, 52, 7982–7987. [Google Scholar] [CrossRef]
- Parry, J.; Yu, L. Fatty acid content and antioxidant properties of cold-pressed black raspberry seed oil and meal. J. Food Sci. 2004, 69, 189–193. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, M.P.; Whittaker, P.; Yu, L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- FAO. Fats and Fatty Acids in Human Nutrition—Report. 2010. Available online: http://www.fao.org/3/a-i1953e.pdf (accessed on 19 November 2019).
| Sample | Scientific Name | Latitude | Longitude | DM | Yield 1 |
|---|---|---|---|---|---|
| Blackberry Cv. Loch Tay | Rubus fruticosus | 45°35′ N | 8°04′ E | 284.5 | 378.1 |
| Black currants | Ribes nigrum | 45°35′ N | 8°04′ E | 753.5 | 241.9 |
| Blueberries Cv. Blue Ray | Vaccinium corymbosum | 45°35′ N | 8°04′ E | 498.8 | 264.5 |
| Blueberries Cv. Duke | Vaccinium corymbosum | 45°03′ N | 7°30′ E | 589.0 | 197.7 |
| Blueberries Cv. Misty | Vaccinium corymbosum | 45°35′ N | 8°04′ E | 473.2 | 366.5 |
| Goji | Lycium barbarum L. | 45°04′ N | 7°43′ E | 266.3 | 769.9 |
| Raspberries | Rubus idaeus | 45°03′ N | 7°30′ E | 249.2 | 451.6 |
| Red currants | Ribes rubrum | 44°32′ N | 7°65′ E | 655.3 | 162.6 |
| Red gooseberries | Ribes grossularia L. | 45°03′ N | 7°30′ E | 691.1 | 186.9 |
| White currants | Ribes pallidum | 44°82′ N | 7°97′ E | 655.7 | 178.1 |
| White gooseberries | Ribes grossularia L. | 44°79′ N | 7°37′ E | 866.4 | 120.2 |
| Sample | TAC | TFC | ||
|---|---|---|---|---|
| g Extract | g FM | g Extract | g FM | |
| Blackberry | 2.10 b | 0.57 b | 24.00 a | 6.54 cd |
| Black currants | 7.59 a | 5.92 a | 24.78 a | 19.34 a |
| Blueberries Cv. Blue Ray | 0.03 d | 0.02 d | 24.73 a | 12.24 b |
| Blueberries Cv. Duke | 0.07 d | 0.03 d | 18.84 a | 10.10 bc |
| Blueberries Cv. Misty | 0.66 c | 0.30 c | 24.67 a | 11.11 b |
| Raspberries | 0.20 d | 0.05 d | 6.48 b | 1.72 e |
| Goji | nd 1 | nd | 2.78 b | 0.75 e |
| Red currants | nd | nd | 4.03 b | 2.78 de |
| Red gooseberries | nd | nd | 8.32 b | 5.74 d |
| White currants | nd | nd | 4.54 b | 3.12 de |
| White gooseberries | nd | nd | 7.06 b | 6.19 d |
| Sample | TEAC | FRAP | DPPH | ||
|---|---|---|---|---|---|
| g Extract | g FM | g Extract | g FM | mg/mL | |
| Blackberry | 10.25 ab | 2.94 de | 7.02 b | 2.01 cd | 0.28 e |
| Black currants | 12.09 a | 9.00 a | 10.29 a | 7.72 a | 0.20 e |
| Blueberries Cv. Blue Ray | 7.58 bcd | 3.78 cd | 4.61 cd | 2.57 c | 0.48 cd |
| Blueberries Cv. Duke | 8.79 abc | 5.21 bc | 4.78 cd | 2.61 c | 0.50 cd |
| Blueberries Cv. Misty | 6.09 cde | 2.87 de | 4.18 de | 1.98 cd | 0.57 c |
| Goji | 2.79 e | 0.74 e | 1.46 g | 0.39 f | 1.18 a |
| Raspberries | 4.38 de | 1.08 e | 3.08 ef | 0.76 ef | 0.60 c |
| Red currants | 9.39 abc | 6.17 b | 3.95 de | 2.56 c | 0.35 de |
| Red gooseberries | 3.47 e | 2.40 de | 2.02 fg | 1.40 de | 0.96 b |
| White currants | 9.08 abc | 5.96 bc | 5.87 bc | 3.85 b | 0.26 e |
| White gooseberries | 8.23 bc | 7.12 ab | 4.53 cd | 3.92 b | 0.49 cd |
| TEAC 1 | FRAP 2 | DPPH 3 | |
|---|---|---|---|
| TAC | 0.580 ** | 0.729 ** | −0.338 * |
| TFC | 0.424 ** | 0.567 ** | −0.333 * |
| Sample | C16:0 | C16:1n9 | C17:0 | C18:0 | C18:1n9 | C18:1n11 | C18:2n6 | C18:3n6 | C18:3n3 | C20:0 | C24:0 | SFA 1 | MUFA 2 | PUFA 3 | n−6/n−3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blackberries | 26.40 ab | 1.10 | 2.80 | 16.87 b | 12.02 c | 0.13 c | 23.48 b | Nd 4 | 17.25 bc | nd | nd | 46.05 e | 13.23 c | 40.72 c | 1.36 cd |
| Black currants | 28.65 a | 0.55 | 5.90 | 38.23 a | 1.63 f | 0.32 c | 14.58 cd | nd | 8.68 ef | 1.47 | nd | 74.25 a | 2.50 e | 23.25 f | 1.67 bc |
| Blueberries Cv. Blue Ray | 20.40 c | nd | 8.28 | 18.20 b | 3.68 ef | 1.60 abc | 28.75 b | nd | 19.18 ab | nd | nd | 54.75 cd | 4.82 de | 40.37 c | 2.08 b |
| Blueberries Cv. Duke | 22.35 bc | nd | 9.95 | 22.48 b | 3.70 ef | 1.15 bc | 27.05 b | nd | 13.33 cde | nd | nd | 46.80 e | 5.25 de | 47.92 b | 1.50 cd |
| Blueberries Cv. Misty | 22.43 bc | nd | 6.38 | 24.55 b | 5.23 ef | 1.95 ab | 26.25 b | nd | 13.15 cde | nd | nd | 53.42 de | 7.17 d | 39.40 c | 2.07 b |
| Goji | 12.50 d | 0.55 | 1.63 | 3.68 c | 16.55 b | 1.13 bc | 54.05 a | 1.50 | 6.83 f | 0.63 | 0.23 | 18.70 f | 18.25 b | 62.37 a | 8.26 a |
| Raspberries | 22.13 bc | 1.08 | 6.00 | 17.85 b | 21.57 a | 0.07 c | 9.15 d | nd | 22.17 a | nd | nd | 46.00 e | 22.72 a | 31.30 de | 0.41 e |
| Red currants | 23.20 bc | 0.67 | 10.65 | 26.65 ab | 7.17 de | 3.12 a | 16.07 c | nd | 11.83 de | 0.65 | nd | 61.15 bc | 10.92 c | 27.90 def | 1.36 cd |
| Red gooseberries | 23.65 abc | nd | 13.85 | 23.53 b | 5.85 e | 0.35 bc | 17.40 c | nd | 13.43 cde | 1.93 | nd | 62.92 b | 6.20 d | 30.87 de | 1.30 cd |
| White currants | 26.43 ab | 1.27 | 8.77 | 28.05 ab | 10.40 cd | nd | 12.07 cd | nd | 13.02 cde | nd | nd | 63.23 b | 11.65 c | 25.10 ef | 0.92 de |
| White gooseberries | 24.70 abc | nd | 10.87 | 27.10 ab | 4.03 ef | 1.60 abc | 17.40c | nd | 14.30 cd | nd | nd | 62.67 b | 5.67 de | 31.67 d | 1.22 cd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods 2020, 9, 623. https://doi.org/10.3390/foods9050623
Zorzi M, Gai F, Medana C, Aigotti R, Morello S, Peiretti PG. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods. 2020; 9(5):623. https://doi.org/10.3390/foods9050623
Chicago/Turabian StyleZorzi, Michael, Francesco Gai, Claudio Medana, Riccardo Aigotti, Sara Morello, and Pier Giorgio Peiretti. 2020. "Bioactive Compounds and Antioxidant Capacity of Small Berries" Foods 9, no. 5: 623. https://doi.org/10.3390/foods9050623
APA StyleZorzi, M., Gai, F., Medana, C., Aigotti, R., Morello, S., & Peiretti, P. G. (2020). Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods, 9(5), 623. https://doi.org/10.3390/foods9050623








