Evaluation of the Physicochemical and Sensory Characteristics of Different Fig Cultivars for the Fresh Fruit Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Weight and Size
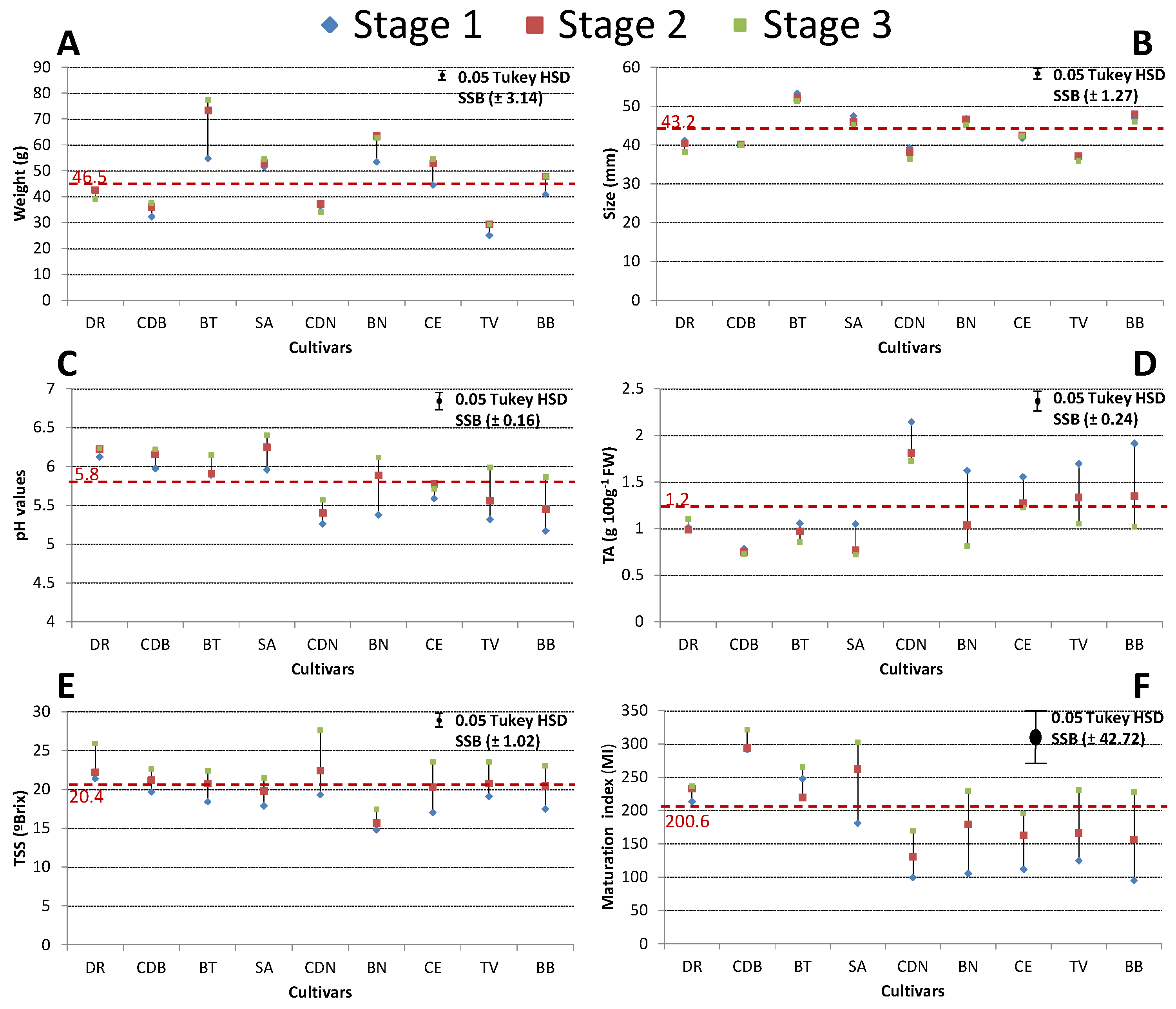
2.3. Total Soluble Solids (TSS), Titratable Acidity (TA), pH, and Maturation Index (MI)
2.4. Colour
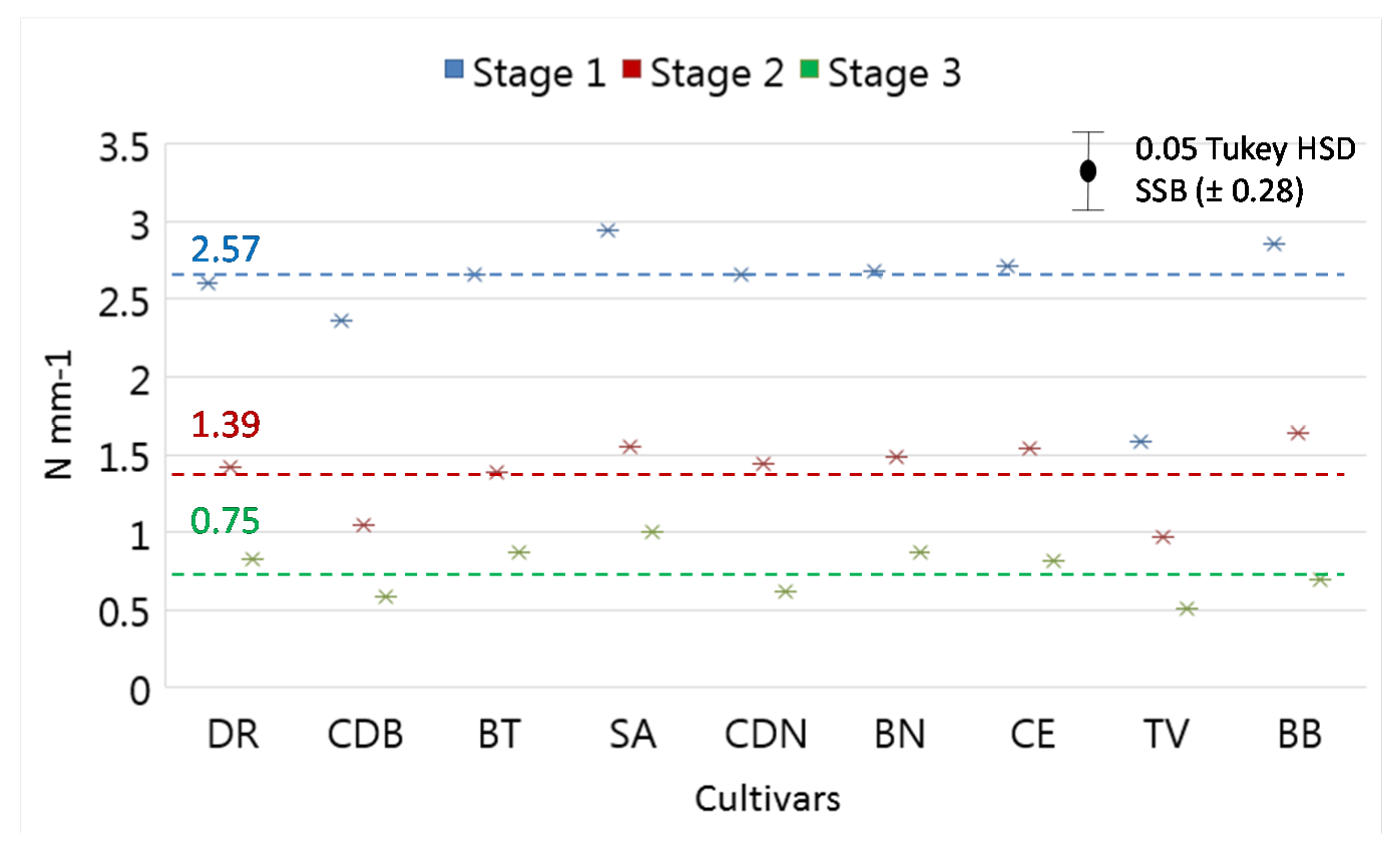
2.5. Firmness
2.6. Determination of Volatile Compounds
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Colour and Firmness
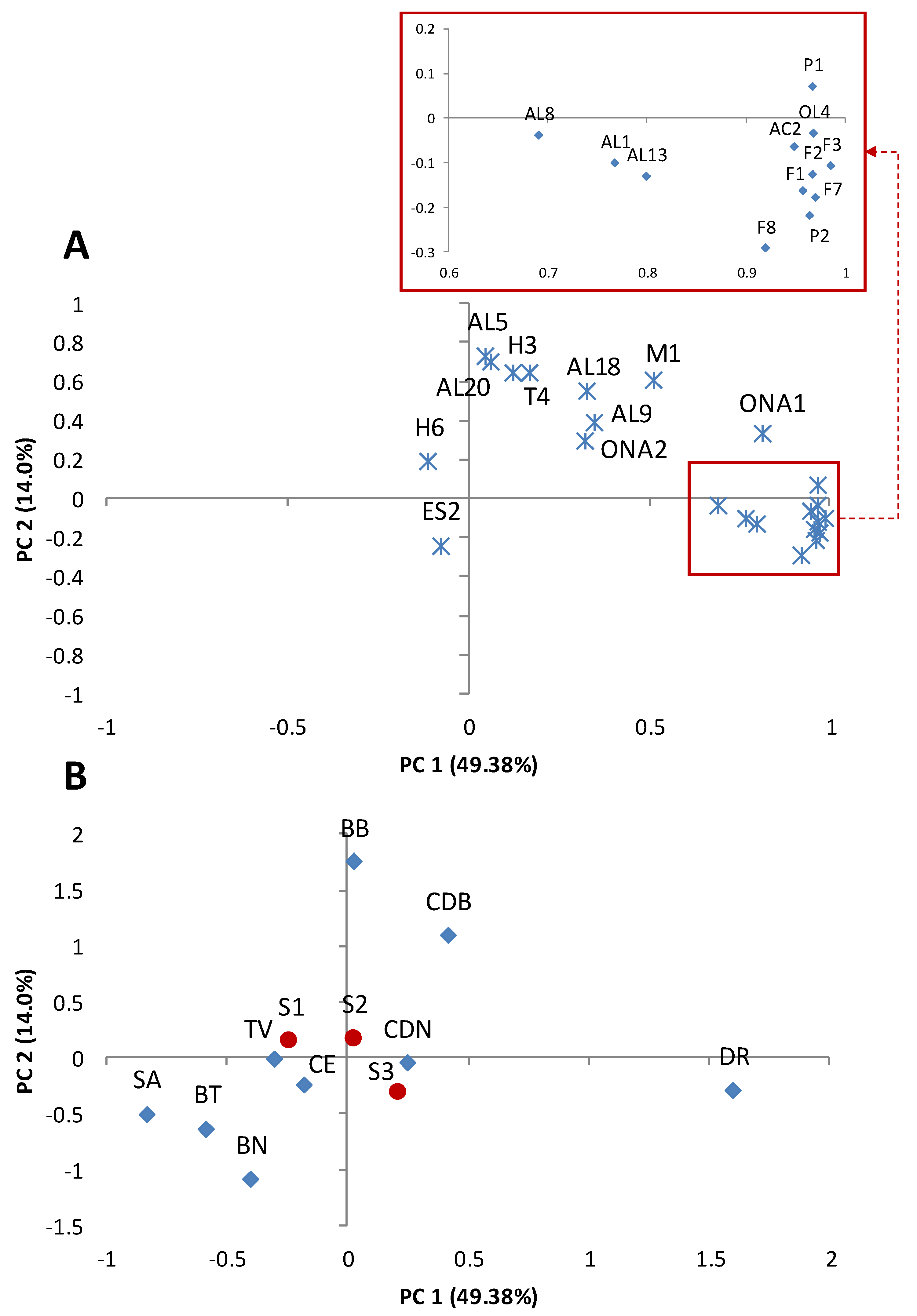
3.3. Volatile Compounds
3.4. Sensory Analysis
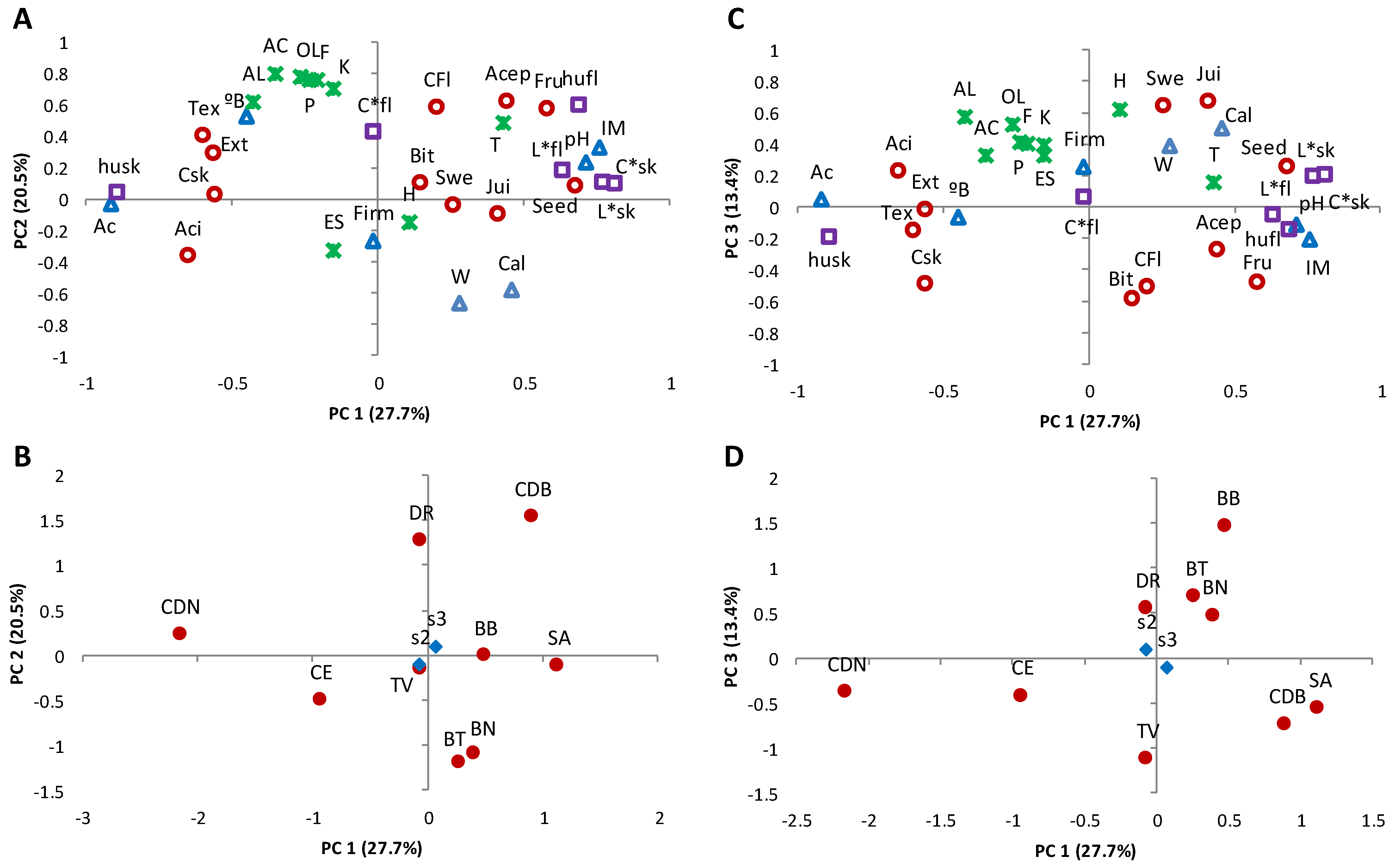
3.5. Interactions between the Analytical Parameters and Sensory Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ercisli, S.; Tosun, M.; Karlidag, H.; Dzubur, A.; Hadziabulic, S.; Aliman, Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from northeastern Turkey. Plant Food Hum. Nutr. 2012, 67, 271–276. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT, FAO Database. 2017. Available online: http://faostat.fao.org (accessed on 15 January 2020).
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Contador, L.; Shinya, P.; Infante, R. Texture phenotyping in fresh fleshy fruit. Sci. Hortic. 2015, 193, 40–46. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, L.R.; de Pinho, P.G.; Gil-Izquierdo, A.; Valentão, P.; Silva, B.M.; Pereira, J.A.; Andrade, P.B. Volatile profiling of Ficus carica varieties by HS-SPME and GC–IT-MS. Food Chem. 2010, 123, 548–557. [Google Scholar] [CrossRef]
- Pereira, J.; Pereira, J.; Câmara, J.S. Effectiveness of different solid-phase microextraction fibres for differentiation of selected Madeira island fruits based on their volatile metabolite profile—Identification of novel compounds. Talanta 2011, 83, 899–906. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A. The composition of strawberry aroma is influenced by cultivar, maturity, and storage. HortScience 2000, 35, 1022–1026. [Google Scholar] [CrossRef]
- Gibernau, M.; Buser, H.R.; Frey, J.E.; Hossaert-McKey, M. Volatile compounds from extracts of figs of Ficus carica. Phytochem 1997, 46, 241–244. [Google Scholar] [CrossRef]
- Grison, L.; Edwards, A.A.; Hossaert-McKey, M. Interspecies variation in floral fragrances emitted by tropical Ficus species. Phytochem 1999, 52, 1293–1299. [Google Scholar] [CrossRef]
- Grison-Pigé, L.; Hossaert-McKey, M.; Greeff, J.M.; Bessière, J.M. Fig volatile compounds a first comparative study. Phytochem 2002, 61, 61–71. [Google Scholar] [CrossRef]
- Gozlekci, S.; Kafkas, E.; Ercisli, S. Volatile compounds determined by HS/GC-MS technique in peel and pulp of fig (Ficus carica L.) cultivars grown in Mediterranean region of Turkey. Not. Bot. Horti Agrobot Cluj-Napoca 2011, 39, 105–108. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Aranda, E.; López-Corrales, M.; Córdoba, M.G. Influence of modified atmosphere packaging (MAP) on aroma quality of figs (Ficus carica L.). Postharvest Biol. Technol. 2018, 136, 145–151. [Google Scholar] [CrossRef]
- Wang, M.Y.; MacRae, E.; Wohlers, M.; Marsh, K. Changes in volatile production and sensory quality of kiwifruit during fruit maturation in Actinidia deliciosa ‘Hayward’ and A. chinensis ‘Hort16A’. Postharvest Biol. Technol. 2011, 59, 16–24. [Google Scholar] [CrossRef]
- Polat, A.A.; Çalişkan, O. Fruit characteristics of table fig (Ficus carica L) cultivars in subtropical climate conditions of the Mediterranean region. New Zeal J. Crop. Hort. 2008, 36, 107–115. [Google Scholar] [CrossRef]
- Çalişkan, O.; Polat, A.A. Effects of genotype and harvest year on phytochemical and fruit quality properties of Turkish fig genotypes. Span. J. Agric Res. 2012, 10, 1048–1058. [Google Scholar] [CrossRef]
- Gozlekci, S. Pomological traits of fig (Ficus carica L.) genotypes collected in the west Mediterranean region in Turkey. J. Anim. Plant Sci. 2011, 21, 646–652. [Google Scholar]
- Crisosto, C.H.; Bremer, V.; Ferguson, L.; Crisosto, G.M. Fresh fig (Ficus carica L.) cultivars harvested at two maturity stages. HortScience 2010, 45, 707–710. [Google Scholar] [CrossRef]
- King, E.S.; Hopfer, H.; Haug, M.T.; Orsi, J.D.; Heymann, H.; Crisosto, G.M.; Crisosto, C.H. Describing the appearance and flavor profiles of fresh fig (Ficus carica L.) cultivars. J. Food Sci. 2012, 77, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, E.; Lopez-Corrales, M.; Hormaza, J.I. Optimization of the management of an ex-situ germplasm bank in common fig with SSRs. J. Am. Soc Hortic Sci. 2008, 133, 69–77. [Google Scholar] [CrossRef]
- Giraldo, E.; Viruel, M.A.; López-Corrales, M.; Hormaza, J.I. Characterisation and cross-species transferability of microsatellites in the common fig (Ficus carica L.). J. Hortic. Sci. Biotechnol. 2005, 80, 217–224. [Google Scholar] [CrossRef]
- López-Corrales, M.; Gil, M.; Pérez, F.; Cortés, J.; Serradilla, M.J.; Chome, P.M. Variedades de Higuera, Descripción y Registro de Variedades; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Span, 2011. [Google Scholar]
- Pereira, C.; López-Corrales, M.; Martín, A.; Villalobos, M.C.; Córdoba, M.G.; Serradilla, M.J. Physicochemical and nutritional characterization of brebas for fresh consumption from nine fig varieties (Ficus carica L.) grown in Extremadura (Spain). J. Food Qual. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; Córdoba, M.G. Physicochemical and sensory characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Kondjoyan, N.; Berdagué, J.L. A Compilation of Relative Retention Indices for the Analysis of Aromatic Compounds; Ed. du Laboratoire Flaveur, INRA de Theix: Saint-Gènes-Champanelle, France, 1996. [Google Scholar]
- Silva, L.C.A.S.; Harder, M.N.C.; Arthur, P.B.; Lima, R.B.; Modolo, D.M.; Arthur, V. Physical-chemical characteristics of figs (Ficus carica L.) preready to submitted to ionizing radiation. In Proceedings of the International Nuclear Atlantic Conference, Rio de Janeiro, RJ, Brazil, 27 September–2 October 2009. [Google Scholar]
- Ersoy, N.; Gozlekci, S.; Gok, V.; Yilmaz, S. Fig (Ficus carica L.) fruit, some physical and chemical properties. Acta Hortic. 2017, 1173, 329–334. [Google Scholar] [CrossRef]
- Çalişkan, O.; Polat, A.A. Fruit characteristics of fig cultivars and genotypes grown in Turkey. Sci. Hortic. 2008, 115, 360–367. [Google Scholar] [CrossRef]
- Mars, M.; Chebli, T.; Marrakchi, M. Multivariate analysis of fig (Ficus carica L.) germplasm in southern Tunisia. Acta Hortic. 1998, 480, 75–78. [Google Scholar] [CrossRef]
- Khapre, A.P.; Satwadhar, P.N.; Syed, H.M. Studies on processing technology and cost estimation of fig (Ficus carica L.) fruit powder enriched Burfi (Indian cookie). IASET 2015, 7, 621–624. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of color and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, L.R.; Andrade, P.B.; Valentão, P.; Silva, B.M.; Pereira, J.A.; de Pinho, P.G. Determination of low molecular weight volatiles in Ficus carica using HS-SPME and GC/FID. Food Chem. 2010, 121, 1289–1295. [Google Scholar] [CrossRef]
- Li, J.; Tian, Y.; Sun, B.; Yang, D.; Chen, J.P.; Men, Q.M. Analysis on volatile constituents in leaves and fruits of Ficus carica by GC-MS. Chin. Herb Med. 2012, 4, 63–69. [Google Scholar]
- Trad, M.; Ginies, C.; Gaaliche, B.; Renard, C.M.; Mars, M. Does pollination affect aroma development in ripened fig (Ficus carica L.) fruit. Sci. Hortic. 2012, 134, 93–99. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles, hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant. Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Werkhoff, P.; Güntert, M.; Krammer, G.; Sommer, H.; Kaulen, J. Vacuum headspace method in aroma research, flavor chemistry of yellow passion fruits. J. Agric. Food Chem. 1998, 46, 1076–1093. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant. J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Mujić, I.; Kralj, M.B.; Jokić, S.; Jug, T.; Šubarić, D.; Vidović, S.; Jarni, K. Characterisation of volatiles in dried white varieties figs (Ficus carica L.). Int. J. Food Sci. Technol. 2014, 51, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.; Moo-Huchin, V.; Sosa-Moguel, O.; Sauri-Duch, E.; Cuevas-Glory, L. Characterization of aroma-active compounds in choch (Lucuma hypoglauca Standley) fruit. Int. J. Food Prop. 2017, 20, 444–448. [Google Scholar] [CrossRef]
- Siegmund, B.; Murkovic, M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (part 2, volatile compounds). Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]
- Elss, S.; Grünewald, L.; Richling, E.; Schreier, P. Occurrence of 2-ethylhexanoic acid in foods packed in glass jars. Food Addit. Contam. 2004, 21, 811–814. [Google Scholar] [CrossRef]
- Spence, C. Multi-sensory integration & the psychophysics of flavour perception. In Food Oral Processing Fundamentals of Eating and Sensory Perception; Chen, J., Engelen, L., Eds.; Blackwell Publishing: Oxford, UK, 2012; pp. 203–219. [Google Scholar]
- Rosianski, Y.; Freiman, Z.E.; Cochavi, S.M.; Yablovitz, Z.; Kerem, Z.; Flaishman, M.A. Advanced analysis of developmental and ripening characteristics of pollinated common-type fig (Ficus carica L.). Sci. Hortic. 2016, 198, 98–106. [Google Scholar] [CrossRef]

| Volatile Compounds | CD † | KI ‡ | ID § | RT ¶ | AAU | | Percentage of Area (%) # | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | ||||||
| Hydrocarbons | H | 452 | 3.18 | 3.20 | 0.32 | 15.15 | |||
| Pentane, 2-methyl- | H1 | 570 | A | 7.0 | 2 | 0.02 | 0.10 | 0.00 | 0.52 |
| Pentane, 3-methyl- | H2 | 585 | A | 7.7 | 6 | 0.05 | 0.20 | 0.00 | 1.01 |
| Hexane ¤ | H3 | 600 | A | 8.9 | 241 | 1.84 | 1.64 | 0.23 | 7.29 |
| Cyclopentane, methyl- | H4 | 635 | B | 10.3 | 4 | 0.03 | 0.13 | 0.00 | 0.67 |
| Heptane | H5 | 700 | A | 14.7 | 10 | 0.08 | 0.18 | 0.00 | 0.55 |
| Toluene | H6 | 779 | B | 18.8 | 106 | 0.66 | 1.32 | 0.00 | 5.57 |
| Ethylbenzene | H7 | 864 | B | 23.6 | 17 | 0.10 | 0.21 | 0.00 | 0.86 |
| p-Xylene | H8 | 869 | B | 23.8 | 52 | 0.29 | 0.64 | 0.00 | 2.39 |
| Tetradecane | H9 | 1400 | A | 39.4 | 14 | 0.10 | 0.27 | 0.00 | 1.16 |
| Alcohols | OL | 378 | 2.50 | 1.18 | 0.63 | 5.71 | |||
| 3-Buten-1-ol, 3-methyl- | OL1 | 726 | B | 16.7 | 15 | 0.11 | 0.13 | 0.00 | 0.53 |
| 3-Heptanol | OL2 | 894 | B | 24.7 | 44 | 0.40 | 0.71 | 0.00 | 2.66 |
| Branched alcohol | OL3 | 1028 | D | 29.8 | 26 | 0.30 | 0.49 | 0.00 | 1.90 |
| Aromatic alcohol | OL4 | 1080 | D | 31.7 | 292 | 1.69 | 0.81 | 0.40 | 3.40 |
| Aldehydes | AL | 2407 | 18.25 | 10.34 | 4.99 | 58.98 | |||
| Propanal | AL1 | C | 5.2 | 90 | 0.60 | 0.42 | 0.00 | 1.95 | |
| 2-Butenal, (E)- | AL2 | 640 | B | 11.6 | 5 | 0.03 | 0.09 | 0.00 | 0.39 |
| Butanal, 3-methyl- | AL3 | 645 | A | 11.9 | 6 | 0.04 | 0.10 | 0.00 | 0.39 |
| Butanal, 2-methyl- | AL4 | 660 | A | 12.5 | 83 | 0.42 | 0.64 | 0.00 | 2.51 |
| 2-Butenal, 2-methyl- | AL5 | 745 | B | 17.5 | 98 | 0.94 | 1.09 | 0.00 | 3.59 |
| 2-Pentanal, (E)- | AL6 | 750 | B | 18.0 | 27 | 0.24 | 0.45 | 0.00 | 1.89 |
| 2-Butenal, 3-methyl- | AL7 | 783 | B | 19.4 | 53 | 0.37 | 0.22 | 0.00 | 0.72 |
| Hexanal | AL8 | 800 | A | 20.2 | 221 | 1.76 | 1.44 | 0.22 | 5.98 |
| 2-Hexenal, (E)- | AL9 | 853 | A | 22.8 | 305 | 2.65 | 2.97 | 0.50 | 15.15 |
| Heptanal | AL10 | 902 | A | 25.1 | 58 | 0.28 | 0.40 | 0.00 | 1.72 |
| 2,4-Hexadienal (E,E)- | AL11 | 910 | B | 25.4 | 1 | 0.02 | 0.07 | 0.00 | 0.37 |
| 2-Heptenal | AL12 | 952 | B | 27.4 | 4 | 0.04 | 0.06 | 0.00 | 0.23 |
| Benzaldehyde | AL13 | 956 | A | 27.8 | 985 | 7.13 | 6.33 | 1.45 | 32.44 |
| Octanal | AL14 | 1004 | A | 29.1 | 50 | 0.35 | 0.20 | 0.00 | 0.76 |
| 2,4-Heptadienal | AL15 | 1010 | B | 29.4 | 14 | 0.11 | 0.24 | 0.00 | 1.09 |
| Benzeneacetaldehyde | AL16 | 1051 | B | 30.7 | 20 | 0.14 | 0.38 | 0.00 | 1.89 |
| 2-Octenal, (E)- | AL17 | 1062 | C | 31.1 | 16 | 0.14 | 0.22 | 0.00 | 0.91 |
| Nonanal | AL18 | 1106 | A | 32.6 | 294 | 2.41 | 1.74 | 0.00 | 8.22 |
| 2-Nonenal | AL19 | 1164 | B | 34.5 | 7 | 0.04 | 0.07 | 0.00 | 0.27 |
| Decanal | AL20 | 1204 | A | 35.9 | 70 | 0.54 | 0.60 | 0.00 | 2.67 |
| Ketones | K | 495 | 3.24 | 1.70 | 0.71 | 6.09 | |||
| 3-Heptanone | K1 | 889 | B | 24.3 | 373 | 2.52 | 1.41 | 0.67 | 6.09 |
| 1,3-Cyclopentanone | K2 | 992 | C | 28.8 | 106 | 0.64 | 0.57 | 0 | 1.89 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- (Corylon) | K3 | 1029 | C | 30.0 | 15 | 0.08 | 0.10 | 0 | 0.34 |
| Acid | AC | 594 | 3.12 | 1.77 | 0.02 | 7.28 | |||
| Acetic acid | AC1 | 8.3 | 10 | 0.05 | 0.12 | 0 | 0.51 | ||
| Hexanoic acid, 2-ethyl- | AC2 | 1123 | 32.8 | 520 | 2.66 | 1.62 | 0 | 6.15 | |
| Nonanoic acid | AC3 | 1277 | 37.3 | 64 | 0.41 | 0.35 | 0 | 1.13 | |
| Ester | ES | 3545 | 26.93 | 20.31 | 0.69 | 65.61 | |||
| Acetic acid, methyl ester | ES1 | 554 | A | 6.2 | 42 | 0.38 | 0.30 | 0.01 | 1.15 |
| Acetic acid, ethyl ester | ES2 | 628 | A | 9.6 | 3496 | 26.53 | 20.11 | 0.67 | 65.26 |
| Butanoic acid, methyl ester | ES3 | 723 | B | 16.4 | 7 | 0.02 | 0.10 | 0 | 0.52 |
| Furans | F | 5831 | 23.05 | 13.72 | 1.83 | 57.33 | |||
| Furfural | F1 | 830 | A | 21.9 | 966 | 4.24 | 2.32 | 0.51 | 8.21 |
| 2-Furanmethanol | F2 | 859 | B | 23.0 | 903 | 4.39 | 2.06 | 0.97 | 8.2 |
| 2(3H)-Furanone, 5-methyl- | F3 | 930 | B | 26.0 | 336 | 1.69 | 0.83 | 0.1 | 2.88 |
| Furaneol | F4 | 1058 | B | 30.8 | 49 | 0.30 | 0.60 | 0 | 2.31 |
| Unknown furan 1 | F5 | 1098 | D | 32.2 | 156 | 0.45 | 0.64 | 0 | 2.31 |
| 5-(Hydroxymethyl)-2(5H)-furanone | F6 | 1178 | B | 34.9 | 65 | 0.33 | 0.57 | 0 | 2.34 |
| Unknown furan 2 | F7 | 1197 | D | 35.5 | 509 | 2.14 | 1.34 | 0 | 4.8 |
| 5-Hydroxymethylfurfural | F8 | 1224 | B | 36.5 | 2847 | 9.52 | 8.41 | 0 | 33.38 |
| Pyranones | P | 3041 | 12.65 | 7.15 | 0.81 | 26.64 | |||
| 2H-Pyran, 3,4-dihydro- | P1 | 25.5 | 313 | 1.59 | 0.76 | 0.4 | 2.97 | ||
| 3-Hydroxy-2,3-dihydromaltol | P2 | 1140 | B | 34.2 | 2727 | 11.06 | 6.54 | 0.36 | 25.06 |
| Monoterpenes | T | 156 | 1.17 | 1.46 | 0.00 | 4.72 | |||
| α-Pinene | T1 | 940 | B | 26.7 | 9 | 0.05 | 0.14 | 0 | 0.65 |
| Unknown monoterpene | T2 | 1006 | D | 29.2 | 9 | 0.04 | 0.11 | 0 | 0.39 |
| Limonene | T3 | 1030 | A | 30.3 | 38 | 0.19 | 0.29 | 0 | 1.18 |
| Linalool | T4 | 1098 | B | 32.6 | 101 | 0.89 | 1.41 | 0 | 4.35 |
| Miscellaneous | |||||||||
| Ethyl ether | ET1 | A | 5.3 | 10 | 0.07 | 0.19 | 0 | 0.91 | |
| Thymine | M1 | 1075 | B | 31.6 | 855 | 5.85 | 3.28 | 0.58 | 11.5 |
| External Appearance | Skin Colour | Flesh Colour | Firmness | Sweetness | Acid | Bitter | Juiciness | Seeds | Fruit Flavour | Overall Acceptability | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivars | |||||||||||
| DR | 6.30 | 5.42 | 5.58 | 6.35 | 3.76 | 1.30 | 2.93 | 5.18 | 3.33 | 5.84 | 6.11 |
| CDB | 6.53 | 6.18 | 5.97 | 6.80 | 3.11 | 1.41 | 5.14 | 4.71 | 4.93 | 6.70 | 6.71 |
| BT | 6.16 | 5.04 | 4.02 | 5.82 | 3.97 | 1.61 | 4.10 | 5.00 | 4.48 | 4.05 | 4.37 |
| SA | 5.62 | 5.85 | 5.67 | 5.54 | 4.09 | 1.22 | 3.67 | 5.46 | 3.81 | 6.25 | 6.65 |
| CDN | 7.12 † | 7.09 | 5.23 | 7.24 | 3.38 | 2.32 | 3.95 | 4.39 | 2.64 | 4.35 | 5.01 |
| BN | 4.92 | 6.43 | 5.22 | 4.97 | 3.46 | 2.17 | 3.80 | 5.49 | 3.63 | 4.83 | 5.08 |
| CE | 6.88 | 6.27 | 5.09 | 6.77 | 3.25 | 2.11 | 3.98 | 4.74 | 3.67 | 4.81 | 5.68 |
| TV | 4.97 ‡ | 6.24 | 5.71 | 5.19 | 2.86 | 1.61 | 3.69 | 4.39 | 3.32 | 5.91 | 4.90 |
| BB | 5.59 | 5.15 | 5.03 | 5.26 | 4.28 | 1.84 | 3.00 | 5.56 | 4.88 | 5.21 | 5.57 |
| Ripening stage | |||||||||||
| 2 | 6.24 | 6.09 | 5.23 | 6.19 | 3.37 | 1.69 | 3.61 | 4.95 | 3.77 | 5.10 | 5.40 |
| 3 | 5.77 | 5.84 | 5.33 | 5.80 | 3.77 | 1.77 | 4.00 | 5.03 | 3.93 | 5.55 | 5.73 |
| PC § | 0.000 | 0.000 | 0.006 | 0.000 | 0.389 | 0.032 | 0.008 | 0.130 | 0.000 | 0.000 | 0.000 |
| Tukey CI ¶ | ± 1.60 | ±1.49 | ±1.64 | ±1.52 | ±2.26 | ±1.10 | ±1.89 | ±1.75 | ±1.77 | ±1.73 | ±1.45 |
| PS# | 0.037 | 0.230 | 0.684 | 0.075 | 0.210 | 0.651 | 0.143 | 0.738 | 0.526 | 0.064 | 0.117 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.; Martín, A.; López-Corrales, M.; Córdoba, M.d.G.; Galván, A.I.; Serradilla, M.J. Evaluation of the Physicochemical and Sensory Characteristics of Different Fig Cultivars for the Fresh Fruit Market. Foods 2020, 9, 619. https://doi.org/10.3390/foods9050619
Pereira C, Martín A, López-Corrales M, Córdoba MdG, Galván AI, Serradilla MJ. Evaluation of the Physicochemical and Sensory Characteristics of Different Fig Cultivars for the Fresh Fruit Market. Foods. 2020; 9(5):619. https://doi.org/10.3390/foods9050619
Chicago/Turabian StylePereira, Cristina, Alberto Martín, Margarita López-Corrales, María de Guía Córdoba, Ana Isabel Galván, and Manuel Joaquín Serradilla. 2020. "Evaluation of the Physicochemical and Sensory Characteristics of Different Fig Cultivars for the Fresh Fruit Market" Foods 9, no. 5: 619. https://doi.org/10.3390/foods9050619
APA StylePereira, C., Martín, A., López-Corrales, M., Córdoba, M. d. G., Galván, A. I., & Serradilla, M. J. (2020). Evaluation of the Physicochemical and Sensory Characteristics of Different Fig Cultivars for the Fresh Fruit Market. Foods, 9(5), 619. https://doi.org/10.3390/foods9050619






