Canjiqueira Fruit: Are We Losing the Best of It?
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area and Plant Material
2.2. Sample Preparation
2.3. Microwave Digestion
2.4. Elemental Analysis by Inductively Coupled Plasma Optical Emission Spectrometry (ICP OES)
2.5. Calibration Curves
2.6. Comparison Criteria
2.7. Lipid Profile Determination
2.8. Thermogravimetric Analysis (TGA)
2.9. Canjiqueira Pulp and Seed Extract Preparation and Compound Identification
2.10. Statistical Analysis
3. Results and Discussion
3.1. Mineral Content
3.2. Fatty Acids Profile
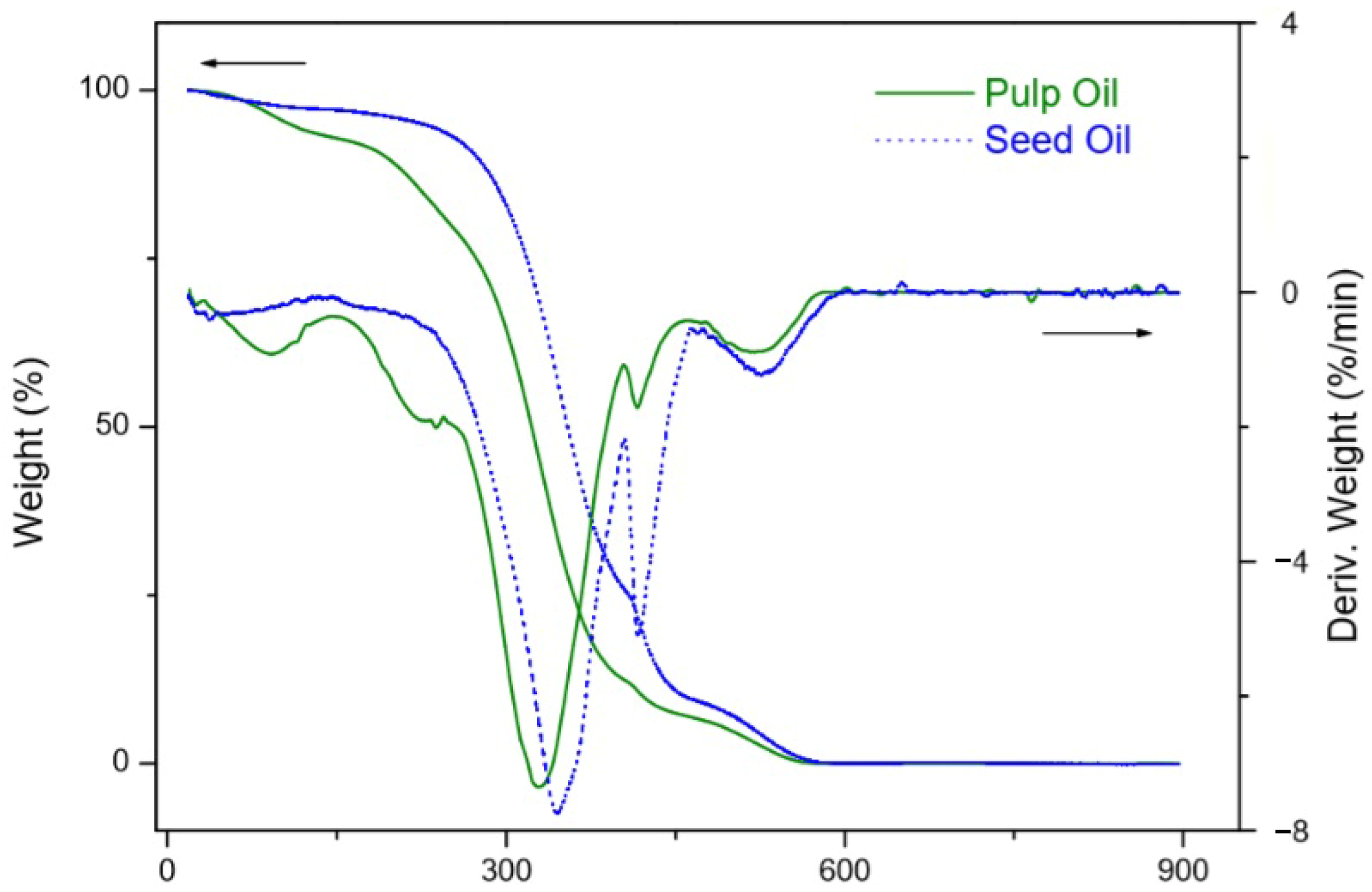
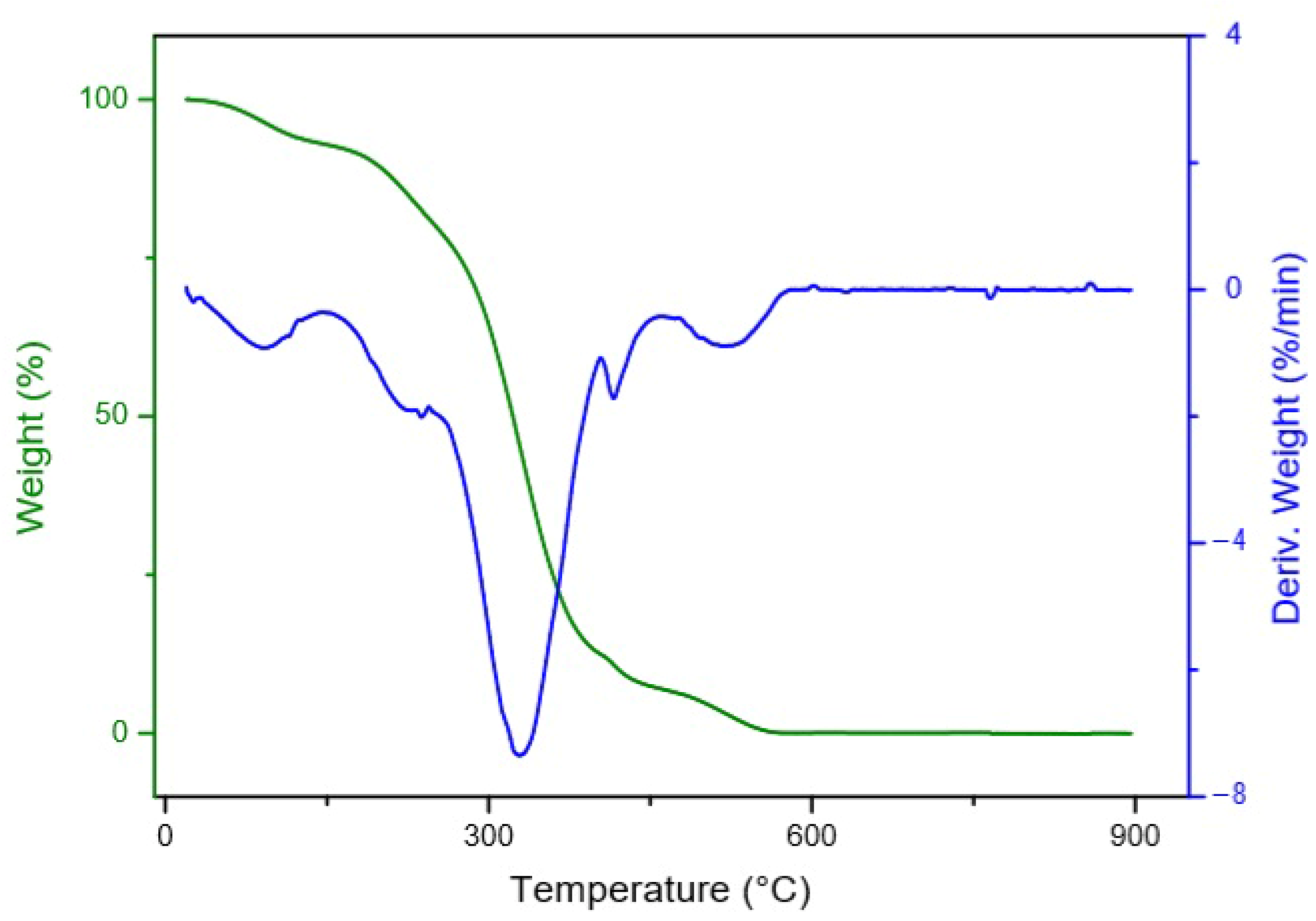
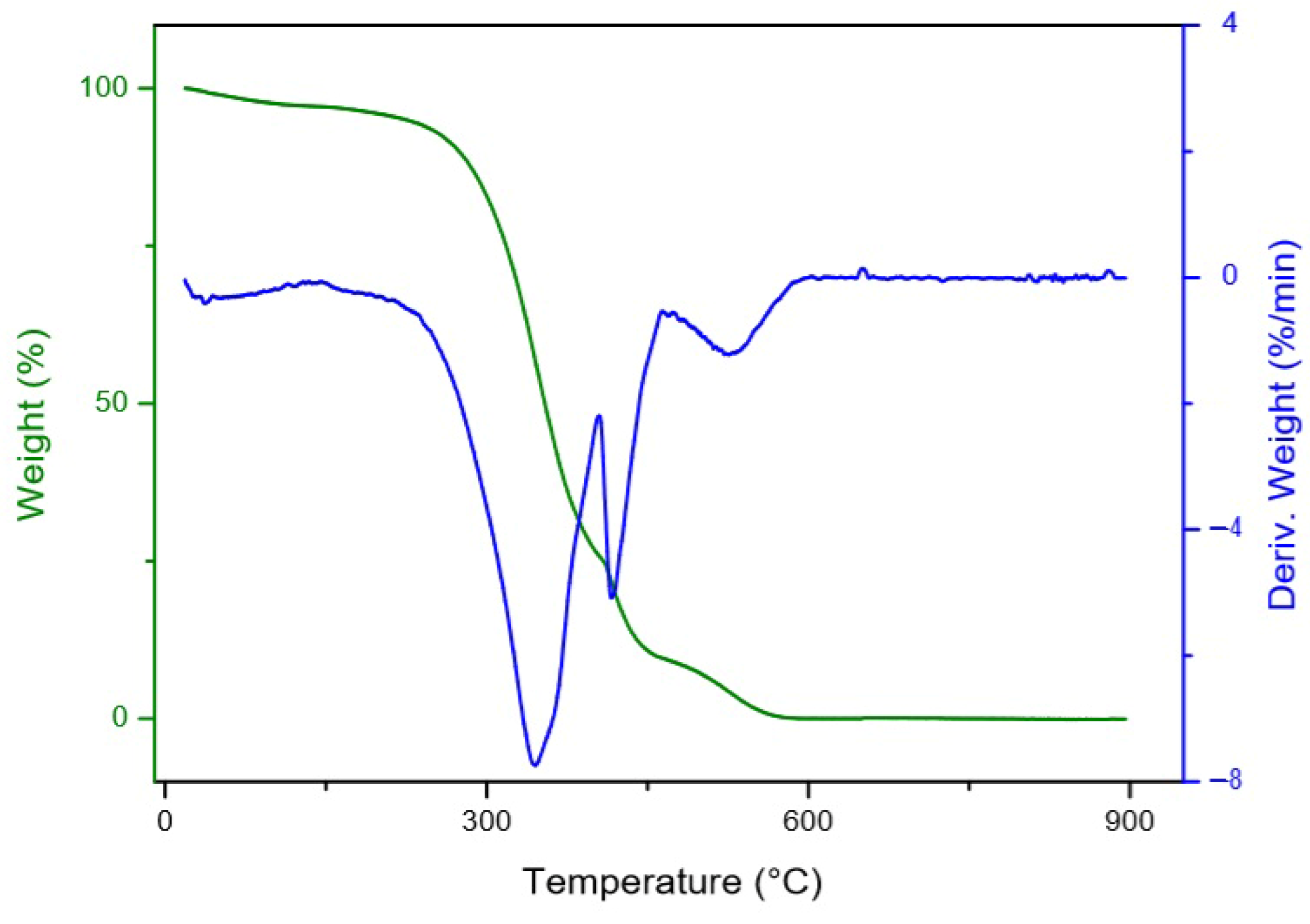
3.3. Thermal Stability
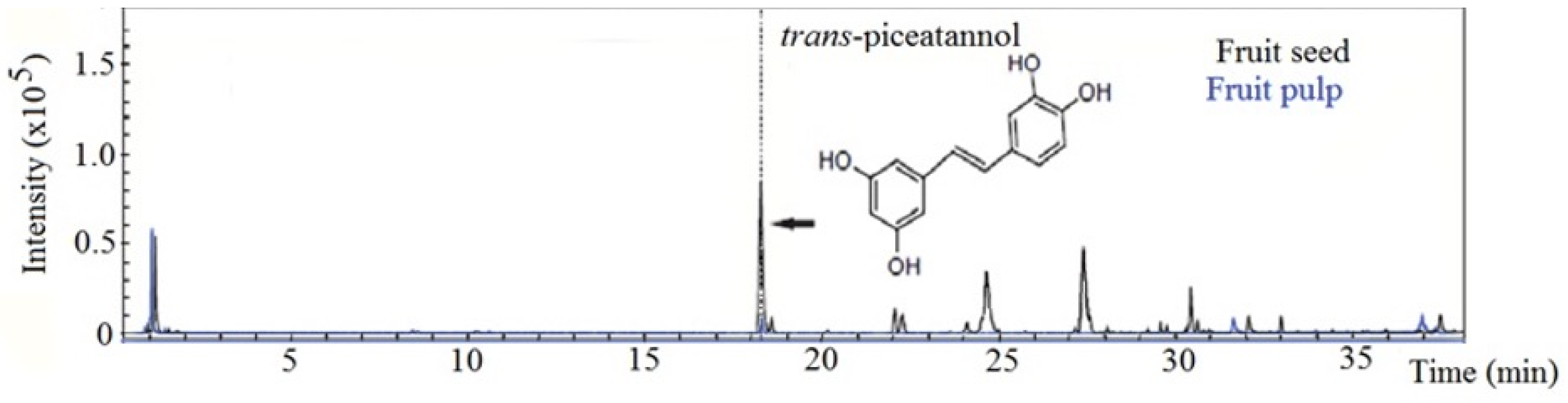
3.4. Piceatannol in B. cydoniifolia Pulp and Seed
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO|Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. Available online: http://www.who.int/elena/titles/fruit_vegetables_ncds/en/ (accessed on 21 December 2018).
- Silva, L.M.R.; Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Tiekink, E.R. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 0-470-86404-4. [Google Scholar]
- Snyder, S.M.; Reber, J.D.; Freeman, B.L.; Orgad, K.; Eggett, D.L.; Parker, T.L. Controlling for sugar and ascorbic acid, a mixture of flavonoids matching navel oranges significantly increases human postprandial serum antioxidant capacity. Nutr. Res. 2011, 31, 519–526. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Ferrentino, G.; Asaduzzaman, M.; Scampicchio, M.M. Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Crit. Rev. Food Sci. Nutr. 2018, 58, 386–404. [Google Scholar] [CrossRef]
- Glew, R.S.; VanderJagt, D.J.; Bosse, R.; Huang, Y.-S.; Chuang, L.-T.; Glew, R.H. The nutrient content of three edible plants of the Republic of Niger. J. Food Compost. Anal. 2005, 18, 15–27. [Google Scholar] [CrossRef]
- Luna, L.C.; Pigni, N.B.; Torras-Claveria, L.; Monferran, M.V.; Maestri, D.; Wunderlin, D.A.; Feresin, G.E.; Bastida, J.; Tapia, A. Ramorinoa girolae Speg (Fabaceae) seeds, an Argentinean traditional indigenous food: Nutrient composition and antioxidant activity. J. Food Compost. Anal. 2013, 31, 120–128. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
- Chen, L.; Shen, M.; Ma, A.; Han, W. Investigation of Trace Element Content in the Seeds, Pulp, and Peel of Mashui Oranges Using Microwave Digestion and ICP-MS Analysis. Biol. Trace Elem. Res. 2018, 182, 152–158. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, Md.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Rufino, M.; Sampaio, C.; Alves, R.; de Brito, E. Antioxidant Activity Measurement in Tropical Fruits: A Case Study with Acerola. Acta Hortic. 2008, 1, 299–305. [Google Scholar]
- Pott, A.; Pott, V.J. Plantas Do Pantanal; EMBRAPA: Brasilia, Brazil, 1994. [Google Scholar]
- Alves, G.L.; Franco, M.R.B. Headspace gas chromatography-mass spectrometry of volatile compounds in murici (Byrsonima crassifolia l. Rich). J. Chromatogr. A 2003, 985, 297–301. [Google Scholar] [CrossRef]
- Rezende, C.M.; Fraga, S.R.G. Chemical and aroma determination of the pulp and seeds of murici (Byrsonima crassifolia L.). J. Braz. Chem. Soc. 2003, 14, 425–428. [Google Scholar] [CrossRef]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding Value to Fruit Processing Waste: Innovative Ways to Incorporate Fibers from Berry Pomace in Baked and Extruded Cereal-based Foods—A SUSFOOD Project. Foods 2015, 4, 690–697. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Takasawa, R.; Akahane, H.; Tanaka, H.; Shimada, N.; Yamamoto, T.; Uchida-Maruki, H.; Sai, M.; Yoshimori, A.; Tanuma, S. Piceatannol, a natural trans-stilbene compound, inhibits human glyoxalase I. Bioorg. Med. Chem. Lett. 2017, 27, 1169–1174. [Google Scholar] [CrossRef]
- Sano, S.; Sugiyama, K.; Ito, T.; Katano, Y.; Ishihata, A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011, 59, 6209–6213. [Google Scholar] [CrossRef]
- Viganó, J.; Aguiar, A.C.; Moraes, D.R.; Jara, J.L.P.; Eberlin, M.N.; Cazarin, C.B.B.; Maróstica, M.R.; Martínez, J. Sequential high pressure extractions applied to recover piceatannol and scirpusin B from passion fruit bagasse. Food Res. Int. 2016, 85, 51–58. [Google Scholar] [CrossRef]
- Marcelino, G.; Donadon, J.R.; Caires, A.R.; Michels, F.S.; Oliveira, L.C.; Cortes, M.R.; Maldonade, I.R.; Cavalheiro, L.F.; Nazário, C.E.; Maróstica Júnior, M.R.; et al. Characterization and oxidative stability of oils and bioactive compounds of the fruits of Byrsonima cydoniifolia A. Juss. at different ripening stages. J. Sci. Food Agric. 2019, 99, 2855–2864. [Google Scholar] [CrossRef]
- Prates, M.F.O.; Campos, R.P.; Silva, M.M.B. da; Macedo, M.L.R.; Hiane, P.A.; Ramos Filho, M.M.; Prates, M.F.O.; Campos, R.P.; Silva, M.M.B. da; Macedo, M.L.R.; et al. Nutritional and antioxidant potential of canjiqueira fruits affected by maturity stage and thermal processing. Cienc. Rural 2015, 45, 399–404. [Google Scholar] [CrossRef]
- Santos, V.S. dos; Nascimento, T.V.; Felipe, J.L.; Boaretto, A.G.; Damasceno-Junior, G.A.; Silva, D.B.; Toffoli-Kadri, M.C.; Carollo, C.A. Nutraceutical potential of Byrsonima cydoniifolia fruits based on chemical composition, anti-inflammatory, and antihyperalgesic activities. Food Chem. 2017, 237, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; da Silva, A.F.; Visentainer, J.V.; Matsushita, M.; de Souza, N.E. Proximate compositions, mineral contents and fatty acid compositions of native Amazonian fruits. Food Res. Int. 2015, 77, 441–449. [Google Scholar] [CrossRef]
- Morzelle, M.C.; Bachiega, P.; Souza, E.C.D.; Boas, V.; Barros, E.V.D.; Lamounier, M.L. Caracterização química e física de frutos de curriola, gabiroba e murici provenientes do cerrado brasileiro. Rev. Bras. Frutic. 2015, 37, 96–103. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.54 (accessed on 5 February 2020).
- Figueiredo, P.S.; Candido, C.J.; Jaques, J.A.; Nunes, Â.A.; Caires, A.R.; Michels, F.S.; Almeida, J.A.; Filiú, W.F.; Hiane, P.A.; Nascimento, V.A. Oxidative stability of sesame and flaxseed oils and their effects on morphometric and biochemical parameters in an animal model. J. Sci. Food Agric. 2017, 97, 3359–3364. [Google Scholar] [CrossRef]
- Mariod, A.A.; Elkheir, S.; Ahmed, Y.M.; Matthäus, B. Annona squamosa and Catunaregam nilotica Seeds, the Effect of the Extraction Method on the Oil Composition. J. Am. Oil Chem. Soc. 2010, 87, 763–769. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Kumssa, D.B.; Joy, E.J.M.; Ander, E.L.; Watts, M.J.; Young, S.D.; Walker, S.; Broadley, M.R. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 2015, 5, 10974. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese is essential for neuronal health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef] [PubMed]
- Miller-Ihli, N.J. Atomic Absorption and Atomic Emission Spectrometry for the Determination of the Trace Element Content of Selected Fruits Consumed in the United States. J. Food Compost. Anal. 1996, 9, 301–311. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M. P6—Selenium Intake and Status In Health & Disease. Free Radic. Biol. Med. 2017, 112, 5. [Google Scholar]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Prasad, A.S. Chapter 20—Discovery of Zinc for Human Health and Biomarkers of Zinc Deficiency. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 241–260. ISBN 978-0-12-802168-2. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca raton, FL, USA, 2010; ISBN 1-4200-9370-3. [Google Scholar]
- Ha, N.N.; Agusa, T.; Ramu, K.; Tu, N.P.C.; Murata, S.; Bulbule, K.A.; Parthasaraty, P.; Takahashi, S.; Subramanian, A.; Tanabe, S. Contamination by trace elements at e-waste recycling sites in Bangalore, India. Chemosphere 2009, 76, 9–15. [Google Scholar] [CrossRef]
- Jung, M.C. Heavy metal concentrations in soils and factors affecting metal uptake by plants in the vicinity of a Korean Cu-W mine. Sensors 2008, 8, 2413–2423. [Google Scholar] [CrossRef]
- Morgan, R. Soil, heavy metals, and human health. In Soils and Human Health; CRC Press: Boca Raton, FL, USA, 2012; pp. 74–97. [Google Scholar]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Zelman, K. The great fat debate: A closer look at the controversy-questioning the validity of age-old dietary guidance. J. Am. Diet Assoc. 2011, 111, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Katan, M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am. J. Med. 2002, 113 (Suppl. 9B), 13S–24S. [Google Scholar] [CrossRef]
- Hernández, M.L.; Sicardo, M.D.; Martínez-Rivas, J.M. Differential Contribution of Endoplasmic Reticulum and Chloroplast ω-3 Fatty Acid Desaturase Genes to the Linolenic Acid Content of Olive (Olea europaea) Fruit. Plant Cell Physiol. 2016, 57, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.S.; Narain, N.; Rocha, R.V.M.; Paulo, M.Q. Characterization of the oils from the pulp and seeds of avocado (cultivar: Fuerte) fruits. Grasas Aceites 2001, 52, 171–174. [Google Scholar]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter Enteral Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High-monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabaté, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J. Nutr. 2008, 138, 1746S–1751S. [Google Scholar] [CrossRef]
- Guo, Z.; Jia, X.; Zheng, Z.; Lu, X.; Zheng, Y.; Zheng, B.; Xiao, J. Chemical composition and nutritional function of olive (Olea europaea L.): A review. Phytochem. Rev. 2018, 17, 1091–1110. [Google Scholar] [CrossRef]
- Whelan, J.; Fritsche, K. Linoleic Acid1. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef]
- Lai, T.N.H.; André, C.; Rogez, H.; Mignolet, E.; Nguyen, T.B.T.; Larondelle, Y. Nutritional composition and antioxidant properties of the sim fruit (Rhodomyrtus tomentosa). Food Chem. 2015, 168, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.Y.H.; Gay, G.M.W.; Su, J.; Tai, E.-S.; Yuan, J.-M.; Koh, W.-P.; Ong, C.N.; Van Dam, R.M. Consumption of Red Meat, but Not Cooking Oils High in Polyunsaturated Fat, Is Associated with Higher Arachidonic Acid Status in Singapore Chinese Adults. Nutrients 2017, 9, 101. [Google Scholar] [CrossRef]
- Endo, Y.; Hoshizaki, S.; Fujimoto, K. Oxidation of synthetic triacylglycerols containing eicosapentaenoic and docosahexaenoic acids: Effect of oxidation system and triacylglycerol structure. J. Am. Oil Chem. Soc. 1997, 74, 1041. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Li, J.; Tang, L.; Hu, J.; Deng, Z. Evaluating and predicting the oxidative stability of vegetable oils with different fatty acid compositions. J. Food Sci. 2013, 78, H633–H641. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and Off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Dweck, J.; Sampaio, C. Analysis of the thermal decomposition of commercial vegetable oils in air by simultaneous TG/DTA. J. Therm Anal. Calorim. 2004, 75, 385–391. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Arvanitidis, V.; Stratis, J.A. On-line emulsion formation and multi-element analysis of edible oils by inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2005, 537, 271–278. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Franco, P.; Zuppa, T.; Antoniosi Filho, N.; Leles, M. Thermal stability studies of some cerrado plant oils. J. Therm Anal. Calorim. 2007, 87, 645–648. [Google Scholar] [CrossRef]
- Reda, S.Y. Evaluation of antioxidants stability by thermal analysis and its protective effect in heated edible vegetable oil. Food Sci. Technol. (Campinas) 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First Study on the Oxidative Stability and Elemental Analysis of Babassu (Attalea speciosa) Edible Oil Produced in Brazil Using a Domestic Extraction Machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Gruczyńska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.O.; Santos, I.M.G.D.; Souza, A.G.D.; Prasad, S.; Santos, A.V. dos Thermal Stability and Kinetic Study on Thermal Decomposition of Commercial Edible Oils by Thermogravimetry. J. Food Sci. 2002, 67, 1393–1398. [Google Scholar] [CrossRef]
- Rangel-Zuñiga, O.A.; Haro, C.; Tormos, C.; Perez-Martinez, P.; Delgado-Lista, J.; Marin, C.; Quintana-Navarro, G.M.; Cerdá, C.; Sáez, G.T.; Lopez-Segura, F.; et al. Frying oils with high natural or added antioxidants content, which protect against postprandial oxidative stress, also protect against DNA oxidation damage. Eur. J. Nutr. 2017, 56, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, B.; Nuñez, V.; Monagas, M.; Gómez-Cordovés, C. In vitro antioxidant activity of red grape skins. Eur. Food Res. Technol. 2004, 218, 173–177. [Google Scholar] [CrossRef]
- Dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Smith, G.D. Reduction in saturated fat intake for cardiovascular disease. Cochrane DB Syst. Rev. 2015, 6. [Google Scholar] [CrossRef]
- Vincenzi, S.; Tomasi, D.; Gaiotti, F.; Lovat, L.; Giacosa, S.; Torchio, F.; Segade, S.R.; Rolle, L. Comparative study of the resveratrol content of twenty-one Italian red grape varieties. S. Afr. J. Enol. Vitic. 2013, 34, 30–35. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of Passion Fruit (Passiflora edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I.; et al. The Effect of Piceatannol from Passion Fruit (Passiflora edulis) Seeds on Metabolic Health in Humans. Nutrients 2017, 9, 1142. [Google Scholar] [CrossRef]
- Sham, T.-T.; Li, M.-H.; Chan, C.-O.; Zhang, H.; Chan, S.-W.; Mok, D.K.-W. Cholesterol-lowering effects of piceatannol, a stilbene from wine, using untargeted metabolomics. J. Funct. Foods 2017, 28, 127–137. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, Y.; Nii, R.; Kakizaki, N. Resveratrol analogues like piceatannol are potent antioxidants as quantitatively demonstrated through the high scavenging ability against reactive oxygen species and methyl radical. Bioorg. Med. Chem. Lett. 2017, 27, 5203–5206. [Google Scholar] [CrossRef] [PubMed]
| Analyte | R2 | Equation External Calibration | LOD mg/kg | LOQ mg/kg |
|---|---|---|---|---|
| Al | 0.9996 | y = 457.76x + 8.7836 | 0.056 | 0.187 |
| Ca | 0.9993 | y = 70225x + 1953.4 | 0.003 | 0.011 |
| Co | 0.9998 | y = 4161.7x + 5.8914 | 0.003 | 0.008 |
| Cu | 0.9997 | y = 13021x + 107.6 | 0.003 | 0.010 |
| Fe | 0.9996 | y = 3451.9x + 31.784 | 0.007 | 0.022 |
| K | 0.9969 | y = 290.72x + 18.667 | 0.345 | 1.149 |
| Mg | 0.9997 | y =284979x + 3438.5 | 0.001 | 0.002 |
| Mo | 0.9998 | y = 2201.2x + 4.468 | 0.001 | 0.005 |
| Mn | 0.9999 | y = 37724x + 281.01 | 0.0004 | 0.001 |
| Na | 0.9995 | y = 1167.7x + 7.7392 | 0.086 | 0.287 |
| Se | 0.9999 | y = 365.94x + 1.7592 | 0.011 | 0.037 |
| Zn | 0.9999 | y = 8849.5x + 74.424 | 0.0005 | 0.002 |
| Analyte | Accuracy | Precision | ||
|---|---|---|---|---|
| Expected Value mg/L | Obtained Value mg/L | % Recovery | % RSD | |
| Al | 1.00 | 1.09 ± 0.003 | 109.17 | 0.27 |
| Ca | 1.00 | 0.89 ± 0.139 | 88.98 | 15.61 |
| Co | 1.00 | 0.95 ± 0.000 | 94.75 | 0.00 |
| Cu | 1.00 | 1.08 ± 0.003 | 108.28 | 0.27 |
| Fe | 1.00 | 1.11 ± 0.001 | 110.98 | 0.09 |
| K | 1.00 | 1.18 ± 0.390 | 118.50 | 33.05 |
| Mg | 1.00 | 0.90 ± 0.069 | 90.13 | 7.66 |
| Mo | 1.00 | 1.01 ± 0.001 | 101.29 | 0.10 |
| Mn | 1.00 | 0.94 ± 0.016 | 94.04 | 1.70 |
| Na | 1.00 | 1.09 ± 0.036 | 109.54 | 3.30 |
| Se | 1.00 | 0.90 ± 0.002 | 89.79 | 0.22 |
| Zn | 1.00 | 0.93 ± 0.001 | 92.94 | 0.10 |
| Canjiqueira mg/100 g | ||||||
|---|---|---|---|---|---|---|
| Element | Pulp | % Regarding DRIs | Seed | % Regarding DRIs | DRI (RDA/AI *) | * p |
| Al | <LOD | NA | <LOD | NA | NA | NA |
| Ca | 295.56 ± 3.92 | 24.63–29.56 | 381.36 ± 3.92 | 31.78–38.14 | 1000–1200 | 0.6667 † |
| Co | <LOD | NA | <LOD | NA | NA | NA |
| Cu | 0.30 ± 0.02 | 33.33 | 0.78 ± 0.05 | 86.66 | 0.9 | 0.0057 |
| Fe | 1.89 ± 0.09 | 10.50–23.63 | 4.24 ± 0.79 | 23.56–53 | 8–18 | 0.0527 |
| K * | 1507.15 ± 100.70 | 44.33–57.97 | 891.16 ± 128.67 | 26.21–34.28 | 2600–3400 * | 0.0334 |
| Mg | 143.24 ± 0.40 | 34.10–46.21 | 151.90 ± 32.44 | 36.17–49 | 310–420 | >0.9999 † |
| Mo | <LOD | NA | <LOD | NA | NA | NA |
| Mn | 0.63 ± 0.04 | 27.39–35.00 | 0.90 ± 0.09 | 39.13–50 | 1.8–2.3 | 0.0575 |
| Na * | 1.08 ± 0.13 | 0.07 | 0.74 ± 0.19 | 0.05 | 1500 * | 0.3333 † |
| Se | 0.16 ± 0.03 | NA | 0.18 ± 0.01 | NA | NA | 0.3349 |
| Zn | 0.48 ± 0.04 | 4.36–6 | 1.28 ± 0.31 | 11.64–16 | 8–11 | 0.3333 † |
| Fatty Acid | Pulp | Seed | * p |
|---|---|---|---|
| Saturated fatty acids | |||
| C10:0 –Capric acid | 0.2 ± 0.02 | 0.1 ± 0.01 | 0.0015 |
| C12:0 –Lauric acid | 0.4 ± 0.03 | 0.3 ± 0.02 | 0.0086 |
| C14:0 –Miristic acid | 0.6 ± 0.02 | 0.4 ± 0.02 | 0.0001 |
| C16:0 –Palmitic acid | 30.8 ± 0.31 | 18.2 ± 0.34 | <0.0001 |
| C18:0 –Estearic acid | 4.2 ± 0.04 | 4.9 ± 0.10 | 0.0004 |
| C20:0 –Araquidonic acid | 0.8 ± 0.03 | 0.5 ± 0.04 | 0.0005 |
| C22:0 –Behenic acid | 0.2 ± 0.01 | 0.1 ± 0.02 | 0.0015 |
| Total (%) | 37.1 | 24.5 | |
| Monounsaturated fatty acids | |||
| C16:1 –Palmitoleic acid | 1.4 ± 0.06 | 0.65 ± 0.02 | <0.0001 |
| C18:1 –Oleic acid | 47.6 ± 0.35 | 39.3 ± 0.30 | <0.0001 |
| C20:1 - Cis-11-Eicosenoic acid | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.0003 |
| Total (%) | 49.2 | 40.3 | |
| Polyunsaturatedfatty acids | |||
| C18:2 –Linoleic acid | 8.5 ± 0.00 | 31.7 ± 0.11 | <0.0001 |
| C18:3n3 –Linolenic acid | 0.5 ± 0.01 | 0.2 ± 0.01 | <0.0001 |
| Total (%) | 9.0 | 31.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakaki, D.G.; Samúdio dos Santos, V.; Melo, E.P.d.; Pereira, H.; Silva Figueiredo, P.; Rodrigues Cortês, M.; Alexandre Carollo, C.; Oliveira, L.C.S.d.; Tschinkel, P.; Reis, F.; et al. Canjiqueira Fruit: Are We Losing the Best of It? Foods 2020, 9, 521. https://doi.org/10.3390/foods9040521
Arakaki DG, Samúdio dos Santos V, Melo EPd, Pereira H, Silva Figueiredo P, Rodrigues Cortês M, Alexandre Carollo C, Oliveira LCSd, Tschinkel P, Reis F, et al. Canjiqueira Fruit: Are We Losing the Best of It? Foods. 2020; 9(4):521. https://doi.org/10.3390/foods9040521
Chicago/Turabian StyleArakaki, Daniela G., Vanessa Samúdio dos Santos, Elaine Pádua de Melo, Hugo Pereira, Priscila Silva Figueiredo, Mário Rodrigues Cortês, Carlos Alexandre Carollo, Lincoln Carlos Silva de Oliveira, Paula Tschinkel, Francisco Reis, and et al. 2020. "Canjiqueira Fruit: Are We Losing the Best of It?" Foods 9, no. 4: 521. https://doi.org/10.3390/foods9040521
APA StyleArakaki, D. G., Samúdio dos Santos, V., Melo, E. P. d., Pereira, H., Silva Figueiredo, P., Rodrigues Cortês, M., Alexandre Carollo, C., Oliveira, L. C. S. d., Tschinkel, P., Reis, F., Souza, I., Rosa, R., Sanches, F., Freitas dos Santos, E., & Aragão do Nascimento, V. (2020). Canjiqueira Fruit: Are We Losing the Best of It? Foods, 9(4), 521. https://doi.org/10.3390/foods9040521





