Advances in Analysis and Detection of Major Mycotoxins in Foods
Abstract
:1. Introduction
2. Extraction Solutions, Extraction Methodologies and Clean-Up Procedures of Mycotoxins
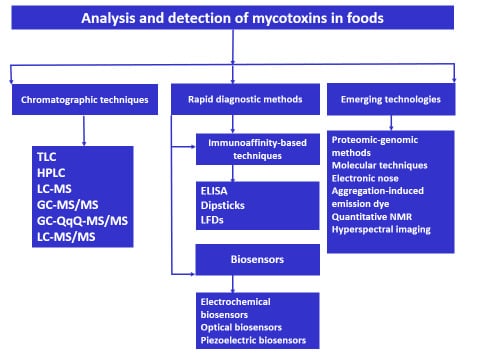
3. Analytical Techniques in Analysis and Detection of Mycotoxins
4. Rapid Diagnostic Methods for Mycotoxin Detection
4.1. Immunoassay-Based Methods
4.2. Biosensors in Mycotoxins Detection
4.2.1. Electrochemical Biosensors for Mycotoxins Detection
Impedimetric Sensors
Potentiometric Sensors
Amperometric Sensors
4.2.2. Optical Biosensors for Mycotoxins Detection
Surface Plasmon Resonance Sensors
4.2.3. Piezoelectric Biosensors for Mycotoxins Detection
Quartz Crystal Microbalance (QCM)
5. Emerging Technologies in Analysis and Detection of Mycotoxins
5.1. Proteomic and Genomic Methods
5.2. Molecular Techniques
5.3. Electronic Nose
5.4. Aggregation-Induced Emission Dye
5.5. Quantitative NMR
5.6. Hyperspectral Imaging
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An Underhand Food Problem. In Mycotoxigenic Fungi: Methods and Protocols, Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1542, pp. 154–196. [Google Scholar]
- Agriopoulou, S. Enniatins: An emerging food safety issue. EC Nutr. 2016, 3, 1142–1146. [Google Scholar]
- Barac, A. Mycotoxins and Human Disease. In Clinically Relevant Mycoses; Presterl, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 213–225. [Google Scholar]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaal, B.; Salama, S.; Al-Qasmi, N.; Jaganjac, M. Mycotoxin contamination of food and feed in the Gulf Cooperation Council countries and its detection. Toxicon 2019, 171, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kluczkovski, A.M. Fungal and mycotoxin problems in the nut industry. Curr. Opin. Food Sci. 2019, 29, 56–63. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Boil. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Coppa, C.C.; De Neeff, D.V.; Corassin, C.H.; De Oliveira, C.A.F. Mycotoxins in fruits and fruit-based products: Occurrence and methods for decontamination. Toxin Rev. 2018, 38, 263–272. [Google Scholar] [CrossRef]
- Varzakas, T. Quality and Safety Aspects of Cereals (Wheat) and Their Products. Crit. Rev. Food Sci. Nutr. 2015, 56, 2495–2510. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchis, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Viegas, S.; Assunção, R.; Twaruźek, M.; Kosicki, R.; Grajewski, J.; Viegas, C. Mycotoxins feed contamination in a dairy farm - potential implications for milk contamination and workers’ exposure in a One Health approach. J. Sci. Food Agric. 2019, 100, 1118–1123. [Google Scholar] [CrossRef]
- Gambacorta, L.; El Darra, N.; Fakhoury, R.; Logrieco, A.; Solfrizzo, M. Incidence and levels of Alternaria mycotoxins in spices and herbs produced worldwide and commercialized in Lebanon. Food Control 2019, 106, 106724. [Google Scholar] [CrossRef]
- Bessaire, T.; Perrin, I.; Tarres, A.; Bebius, A.; Reding, F.; Theurillat, V. Mycotoxins in green coffee: Occurrence and risk assessment. Food Control 2019, 96, 59–67. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.; Gámiz-Gracia, L. Solid Phase Extraction as Sample Treatment for the Determination of Ochratoxin A in Foods: A Review. Crit. Rev. Food Sci. Nutr. 2016, 57, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Kebede, H.; Liu, X.; Jin, J.; Xing, F. Current status of major mycotoxins contamination in food and feed in Africa. Food Control 2020, 110, 106975. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Koliadima, A.; Karaiskakis, G.; Kapolos, J. Kinetic study of aflatoxins’ degradation in the presence of ozone. Food Control 2016, 61, 221–226. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1993; Volume 56. [Google Scholar]
- Alshannaq, A.F.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Somsubsin, S.; Seebunrueng, K.; Boonchiangma, S.; Srijaranai, S. A simple solvent based microextraction for high performance liquid chromatographic analysis of aflatoxins in rice samples. Talanta 2018, 176, 172–177. [Google Scholar] [CrossRef]
- Leal, T.; Abrunhosa, L.; Domingues, L.; Venâncio, A.; Oliveira, C. BSA-based sample clean-up columns for ochratoxin A determination in wine: Method development and validation. Food Chem. 2019, 300, 125204. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dou, X.-W.; Zhang, C.; Logrieco, A.; Yang, M. A Review of Current Methods for Analysis of Mycotoxins in Herbal Medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Tittlemier, S.; Cramer, B.; Dall’Asta, C.; Iha, M.; Lattanzio, V.; Malone, R.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M.; Stroka, J. Developments in mycotoxin analysis: An update for 2017–2018. World Mycotoxin J. 2019, 12, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Krska, R.; Molinelli, A. Rapid test strips for analysis of mycotoxins in food and feed. Anal. Bioanal. Chem. 2008, 393, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.S.; Junior, A.G.D.S.; De Andrade, C.A.S.; Oliveira, M.D.L. Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr. Opin. Food Sci. 2019, 29, 64–79. [Google Scholar] [CrossRef]
- Neethirajan, S.; Ragavan, K.; Weng, X. Agro-defense: Biosensors for food from healthy crops and animals. Trends Food Sci. Technol. 2018, 73, 25–44. [Google Scholar] [CrossRef]
- Tothill, I.E. Biosensors and nanomaterials and their application for mycotoxin determination. World Mycotoxin J. 2011, 4, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Rodríguez, M.; Andrade, M.J.; Cordoba, M.D.G. Detection of filamentous fungi in foods. Curr. Opin. Food Sci. 2015, 5, 36–42. [Google Scholar] [CrossRef]
- El Sheikha, A.F. New Strategies for Tracing Foodstuffs: Biological Barcodes Utilising PCR-DGGE. Adv. Food Technol. Nutr. Sci. Open J. 2015, 1, S1–S7. [Google Scholar] [CrossRef]
- Liang, K.; Liu, Q.X.; Xu, J.H.; Wang, Y.Q.; Okinda, C.S.; Shena, M.X. Determination and Visualization of Different Levels of Deoxynivalenol in Bulk Wheat Kernels by Hyperspectral Imaging. J. Appl. Spectrosc. 2018, 85, 953–961. [Google Scholar] [CrossRef]
- Femenias, A.; Gatius, F.; Ramos, A.J.; Sanchis, V.; Marín, S. Use of hyperspectral imaging as a tool for Fusarium and deoxynivalenol risk management in cereals: A review. Food Control 2020, 108, 106819. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize (Zea mays L.) and mycotoxins: A review on optimization and validation of analytical methods by liquid chromatography coupled to mass spectrometry. Trends Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Valenzano, S.; Lippolis, V.; Pascale, M.; De Marco, A.; Maragos, C.M.; Suman, M.; Visconti, A. Determination of Deoxynivalenol in Wheat Bran and Whole-Wheat Flour by Fluorescence Polarization Immunoassay. Food Anal. Methods 2013, 7, 806–813. [Google Scholar] [CrossRef]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A 2008, 25, 472–489. [Google Scholar] [CrossRef]
- Di Mavungu, J.D.; Monbaliu, S.; Scippo, M.-L.; Maghuin-Rogister, G.; Schneider, Y.-J.; Larondelle, Y.; Callebaut, A.; Robbens, J.; Van Peteghem, C.; De Saeger, S. LC-MS/MS multi-analyte method for mycotoxin determination in food supplements. Food Addit. Contam. Part A 2009, 26, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Delmulle, B.S.; De Saeger, S.; Adams, A.; De Kimpe, N.; Van Peteghem, C. Development of a liquid chromatography/tandem mass spectrometry method for the simultaneous determination of 16 mycotoxins on cellulose filters and in fungal cultures. Rapid Commun. Mass Spectrom. 2006, 20, 771–776. [Google Scholar] [CrossRef]
- Razzazi-Fazeli, E.; Reiter, E. Sample preparation and clean up in mycotoxin analysis: Principles, applications and recent developments. In Determining Mycotoxins and Mycotoxigenic Fungi in Food and Feed; De Saeger, S., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 37–70. [Google Scholar]
- He, T.; Zhou, T.; Wan, Y.; Tan, T. A Simple Strategy Based on Deep Eutectic Solvent for Determination of Aflatoxins in Rice Samples. Food Anal. Methods 2019, 13, 542–550. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.; Stajnbaher, D. Quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach for the determination of pesticide residues VC. In Proceedings of the 18th Annual Waste Testing and Quality Assurance Symposium Proceedings, Arlington, VA, USA, 10–15 August 2002; pp. 231–241. [Google Scholar]
- Pereira, V.; Fernandes, J.O.; Cunha, S.C. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC–MS. Food Chem. 2015, 182, 143–149. [Google Scholar] [CrossRef]
- Carballo, D.; Font, G.; Ferrer, E.; Berrada, H. Evaluation of Mycotoxin Residues on Ready-to-Eat Food by Chromatographic Methods Coupled to Mass Spectrometry in Tandem. Toxins 2018, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Koesukwiwat, U.; Sanguankaew, K.; Leepipatpiboon, N. Evaluation of a modified QuEChERS method for analysis of mycotoxins in rice. Food Chem. 2014, 153, 44–51. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Jiang, Y.; Duan, X.; Qu, H.; Yang, B.; Chen, F.; Sivakumar, D. Natural Occurrence, Analysis, and Prevention of Mycotoxins in Fruits and their Processed Products. Crit. Rev. Food Sci. Nutr. 2013, 54, 64–83. [Google Scholar] [CrossRef]
- Du, L.-J.; Chu, C.; Warner, E.; Wang, Q.-Y.; Hu, Y.-H.; Chai, K.-J.; Cao, J.; Peng, L.-Q.; Chen, Y.-B.; Yang, J.; et al. Rapid microwave-assisted dispersive micro-solid phase extraction of mycotoxins in food using zirconia nanoparticles. J. Chromatogr. A 2018, 1561, 1–12. [Google Scholar] [CrossRef]
- Turner, N.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- D’Arco, G.; Franzón, M.F.; Font, G.; Damiani, P.; Mañes, J. Analysis of fumonisins B1, B2 and B3 in corn-based baby food by pressurized liquid extraction and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2008, 1209, 188–194. [Google Scholar] [CrossRef]
- Kou, D.; Mitra, S. Sample Preparation Techniques in Analytical Chemistry; Mitra, S., Ed.; Wiley: New Jersey, NJ, USA, 2003; Chapter 3; pp. 139–183. [Google Scholar]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Woo, S.Y.; Ryu, S.; Tian, F.; Lee, S.Y.; Park, S.; Chun, H.S. Simultaneous Determination of Twenty Mycotoxins in the Korean Soybean Paste Doenjang by LC-MS/MS with Immunoaffinity Cleanup. Toxins 2019, 11, 594. [Google Scholar] [CrossRef] [Green Version]
- Ran, C.; Chen, D.; Ma, H.; Jiang, Y. Graphene oxide adsorbent based dispersive solid phase extraction coupled with multi-pretreatment clean-up for analysis of trace aflatoxins in traditional proprietary Chinese medicines. J. Chromatogr. B 2017, 1044, 120–126. [Google Scholar] [CrossRef]
- Dong, M.; Si, W.; Jiang, K.; Nie, D.; Wu, Y.; Zhao, Z.; De Saeger, S.; Han, Z. Multi-walled carbon nanotubes as solid-phase extraction sorbents for simultaneous determination of type A trichothecenes in maize, wheat and rice by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1423, 177–182. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Ciasca, B.; Powers, S.; Visconti, A. Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography–tandem mass spectrometry after multi-toxin immunoaffinity clean up. J. Chromatogr. A 2014, 1354, 139–143. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Papageorgiou, M. HPLC-DAD-FLD Method for Simultaneous Determination of Mycotoxins in Wheat Bran. J. Chromatogr. Sci. 2017, 55, 690–696. [Google Scholar] [CrossRef]
- Golge, O.; Hepsag, F.; Kabak, B. Determination of aflatoxins in walnut sujuk and Turkish delight by HPLC-FLD method. Food Control 2016, 59, 731–736. [Google Scholar] [CrossRef]
- Castegnaro, M.; Tozlovanu, M.; Wild, C.; Molinié, A.; Sylla, A.; Pfohl-Leszkowicz, A. Advantages and drawbacks of immunoaffinity columns in analysis of mycotoxins in food. Mol. Nutr. Food Res. 2006, 50, 480–487. [Google Scholar] [CrossRef]
- Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef]
- Baggiani, C.; Giovannoli, C.; Anfossi, L. Man-Made Synthetic Receptors for Capture and Analysis of Ochratoxin A. Toxins 2015, 7, 4083–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichon, V.; Combès, A. Selective tools for the solid-phase extraction of Ochratoxin A from various complex samples: Immunosorbents, oligosorbents, and molecularly imprinted polymers. Anal. Bioanal. Chem. 2016, 408, 6983–6999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Chen, X.; Duan, N.; Wu, S.; Wang, Z.; Wei, X.; Wang, Y. Selection and characterization of DNA aptamers against Staphylococcus aureus enterotoxin C1. Food Chem. 2015, 166, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, J.; Yang, Q.; McDermott, J.; Scott, A.; Vukovich, M.; Lagrois, R.; Gong, Q.; Greenleaf, W.; Eisenstein, M.; et al. Multiparameter Particle Display (MPPD): A Quantitative Screening Method for the Discovery of Highly Specific Aptamers. Angew. Chem. 2016, 129, 762–765. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhang, Z.; Zhang, Q.; Li, P. Mycotoxin Determination in Foods Using Advanced Sensors Based on Antibodies or Aptamers. Toxins 2016, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Huang, Y.; Duan, N.; Wu, S.; Xia, Y.; Ma, X.; Zhu, C.; Jiang, Y.; Ding, Z.; Wang, Z. Selection and characterization of single stranded DNA aptamers recognizing fumonisin B1. Microchim. Acta 2014, 181, 1317–1324. [Google Scholar] [CrossRef]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.; Marty, J.L. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Wang, Y.; Yang, H.; Dong, Y.; Zhang, K.; Lu, Y.; Deng, R.; He, Q. Enzyme-free amplified and ultrafast detection of aflatoxin B1 using dual-terminal proximity aptamer probes. Food Chem. 2019, 283, 32–38. [Google Scholar] [CrossRef]
- Pereira, V.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Ricelli, A.; Fanelli, C.; Caputo, D.; De Cesare, G.; Chu, V.; Aires-Barros, M.R.; Conde, J.P. Advances, challenges and opportunities for point-of-need screening of mycotoxins in foods and feeds. Analyst 2018, 143, 1015–1035. [Google Scholar] [CrossRef]
- Stroka, J.; Maragos, C.M. Challenges in the analysis of multiple mycotoxins. World Mycotoxin J. 2016, 9, 847–861. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Wang, P.; Wang, Y.; Chen, J. Thin-layer chromatography of mycotoxins and comparison with other chromatographic methods. J. Chromatogr. A 1998, 815, 3–20. [Google Scholar] [CrossRef]
- Aiko, V.; Mehta, A. Prevalence of toxigenic fungi in common medicinal herbs and spices in India. 3 Biotech 2016, 6, 159. [Google Scholar] [CrossRef] [Green Version]
- Ezekwesili-Ofili, J.; Onyemelukwe, N.; Agwaga, P.; Orji, I. The Bioload and Aflatoxin Content of Herbal Medicines from Selected States in Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.; Homem-De-Mello, M.; França, J.; Caldas, E.D. Aflatoxins in food products consumed in Brazil: A preliminary dietary risk assessment. Food Addit. Contam. Part A 2013, 30, 127–136. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Zohri, A.A. Fungal flora and mycotoxins of six kinds of nut seeds for human consumption in Saudi Arabia. Mycopathologia 1993, 124, 55–64. [Google Scholar] [CrossRef]
- Ahmad, B.; Ashiq, S.; Hussain, A.; Bashir, S.; Hussain, M. Evaluation of mycotoxins, mycobiota, and toxigenic fungi in selected medicinal plants of Khyber Pakhtunkhwa, Pakistan. Fungal Boil. 2014, 118, 776–784. [Google Scholar] [CrossRef]
- Caldas, E.D.; Silva, A.C.S. Mycotoxins in Corn-Based Food Products Consumed in Brazil: An Exposure Assessment for Fumonisins. J. Agric. Food Chem. 2007, 55, 7974–7980. [Google Scholar] [CrossRef]
- Jeleń, H.; Wasowicz, E. Determination of trichothecenes in wheat grain without sample cleanup using comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 2008, 1215, 203–207. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Berrada, H.; Font, G.; Mañes, J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 28–40. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Nie, D.; Meng, J.; Huang, Q.; Yang, J.; Shen, Y.; Tangni, E.K.; Zhao, Z.; Wu, Y.; et al. Development of a QuEChERS-Based UHPLC-MS/MS Method for Simultaneous Determination of Six Alternaria Toxins in Grapes. Toxins 2019, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Valenta, H. Chromatographic methods for the determination of ochratoxin A in animal and human tissues and fluids. J. Chromatogr. A 1998, 815, 75–92. [Google Scholar] [CrossRef]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Ma, L.; Xu, W.; He, X.; Huang, K.; Wang, Y.; Luo, Y. Determination of fumonisins B1and B2in Chinese rice wine by HPLC using AQC precolumn derivatisation. J. Sci. Food Agric. 2012, 93, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Myresiotis, C.K.; Testempasis, S.; Vryzas, Z.; Karaoglanidis, G.S.; Papadopoulou-Mourkidou, E. Determination of mycotoxins in pomegranate fruits and juices using a QuEChERS-based method. Food Chem. 2015, 182, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pascale, M.; De Girolamo, A.; Lippolis, V.; Stroka, J.; Mol, H.G.J.; Lattanzio, V.M.T. Performance evaluation of LC-MS methods for multimycotoxin determination. J. AOAC Int. 2019, 102, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Bessaire, T.; Mujahid, C.; Mottier, P.; Desmarchelier, A. Multiple Mycotoxins Determination in Food by LC-MS/MS: An International Collaborative Study. Toxins 2019, 11, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Taher, F.; Cappozzo, J.; Zweigenbaum, J.; Lee, H.; Jackson, L.S.; Ryu, D. Detection and quantitation of mycotoxins in infant cereals in the U.S. market by LC-MS/MS using a stable isotope dilution assay. Food Control 2017, 72, 27–35. [Google Scholar] [CrossRef] [Green Version]
- EN 16924:2017. Foodstuffs—Determination of Zearalenone in Edible Vegetable Oils by LC-FLD or LC-MS/MS; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- EN 16923:2017. Foodstuffs—Determination ofT-2 Toxin and HT-2 Toxin in Cereals and Cereal Products for Infants and Young Children by LC-MS/MS after SPE Cleanup; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- Habler, K.; Gotthardt, M.; Schüler, J.; Rychlik, M. Multi-mycotoxin stable isotope dilution LC—MS/MS method for Fusarium toxins in beer. Food Chem. 2017, 218, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Bolechová, M.; Benešová, K.; Běláková, S.; Čáslavský, J.; Pospíchalová, M.; Mikulíková, R. Determination of seventeen mycotoxins in barley and malt in the Czech Republic. Food Control 2015, 47, 108–113. [Google Scholar] [CrossRef]
- Han, Z.; Ren, Y.; Zhu, J.; Cai, Z.; Chen, Y.; Luan, L.; Wu, Y. Multianalysis of 35 Mycotoxins in Traditional Chinese Medicines by Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry Coupled with Accelerated Solvent Extraction. J. Agric. Food Chem. 2012, 60, 8233–8247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-P.; Zhao, Y.-G.; Chen, X.-H.; Pan, S.-D.; Jin, M.-C. Simultaneous determination of four aflatoxins in walnut kernel using dispersive solid-phase extraction combined with ultra fast liquid chromatography-tandem mass spectrometry. Chin. J. Health Lab. Technol. 2014, 24, 2647–2650. [Google Scholar]
- Tölgyesi, Á.; Stroka, J.; Tamošiūnas, V.; Zwickel, T. Simultaneous analysis of Alternaria toxins and citrinin in tomato: An optimised method using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A 2015, 32, 1512–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Han, X.; Li, F.; Zhang, L. Natural Occurrence of Alternaria Toxins in the 2015 Wheat from Anhui Province, China. Toxins 2016, 8, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Ruiz, J.L.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. A rapid method for the determination of mycotoxins in edible vegetable oils by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2019, 288, 22–28. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; González-Peñas, E. An LC–MS/MS method for multi-mycotoxin quantification in cow milk. Food Chem. 2017, 218, 378–385. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Shen, C.; Qu, B. Determination of 16 mycotoxins in vegetable oils using a QuEChERS method combined with high performance liquid chromatography tandem mass spectrometry. Food Addit. Contam. Part A 2016, 34, 1–10. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS Method for the Multi-Mycotoxin Determination in Composite Cereal-Based Samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-H.; Hong, S.-Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous Determination of Multi-Mycotoxins in Cereal Grains Collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Urusov, A.; Zherdev, A.V.; Petrakova, A.V.; Sadykhov, E.G.; Koroleva, O.V.; Dzantiev, B.B. Rapid Multiple Immunoenzyme Assay of Mycotoxins. Toxins 2015, 7, 238–254. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Elliott, C.T.; Campbell, K. Current trends in rapid tests for mycotoxins. Food Addit. Contam. Part A 2019, 36, 800–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oplatowska-Stachowiak, M.; Sajic, N.; Xu, Y.; Haughey, S.A.; Mooney, M.H.; Gong, Y.Y.; Verheijen, R.; Elliott, C.T. Fast and sensitive aflatoxin B1 and total aflatoxins ELISAs for analysis of peanuts, maize and feed ingredients. Food Control 2016, 63, 239–245. [Google Scholar] [CrossRef]
- Tripathi, P.; Upadhyay, N.; Nara, S. Recent advancements in lateral flow immunoassays: A journey for toxin detection in food. Crit. Rev. Food Sci. Nutr. 2017, 58, 1715–1734. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Spano, G.; Speranskaya, E.S.; Goryacheva, I.; Baggiani, C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim. Acta 2018, 185, 94. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Russo, A.; Cavalera, S.; Giovannoli, C.; Spano, G.; Baumgartner, S.; Lauter, K.; Baggiani, C. Silver and gold nanoparticles as multi-chromatic lateral flow assay probes for the detection of food allergens. Anal. Bioanal. Chem. 2018, 411, 1905–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, H.; Wei, M.; Gu, H.; Xu, Q.; Zhu, W. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Mater. Sci. Eng. C 2009, 29, 714–718. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Wen, K.; Li, C.; Mari, G.M.; Jiang, H.; Shi, W.; Shen, J.; Wang, Z. Multiplex Lateral Flow Immunoassays Based on Amorphous Carbon Nanoparticles for Detecting Three Fusarium Mycotoxins in Maize. J. Agric. Food Chem. 2017, 65, 8063–8071. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.; Tessier, M.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a Rainbow Lateral Flow Immunoassay for the Simultaneous Detection of Four Mycotoxins. J. Agric. Food Chem. 2016, 65, 7121–7130. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Wu, A.-B.; Ediage, E.N.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef]
- Yu, Q.; Li, H.; Li, C.; Zhang, S.; Shen, J.; Wang, Z. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim. Acta 2019, 186, 748. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Chen, X.; Xu, W.; Fu, J.; Xiong, Y.; Wang, A. Quantum-DoT submicrobead-based immunochromatographic assay for quantitative and sensitive detection of zearalenone. Talanta 2015, 132, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pirinçci, Ş.; Ertekin, Ö.; Laguna, D.E.; Özen, F.Ş.; Ozturk, Z.Z.; Öztürk, S. Label-Free QCM Immunosensor for the Detection of Ochratoxin A. Sensors 2018, 18, 1161. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, G. Current Advances in Biosensor Design and Fabrication. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2018; pp. 1–25. [Google Scholar]
- Schulz, K.; Pöhlmann, C.; Dietrich, R.; Märtlbauer, E.; Elßner, T. Electrochemical Biochip Assays Based on Anti-idiotypic Antibodies for Rapid and Automated On-Site Detection of Low Molecular Weight Toxins. Front. Chem. 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly(o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B Chem. 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Yagati, A.K.; Chavan, S.G.; Baek, C.; Lee, M.-H.; Min, J. Label-Free Impedance Sensing of Aflatoxin B1 with Polyaniline Nanofibers/Au Nanoparticle Electrode Array. Sensors 2018, 18, 1320. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.; Singh, J.; Solanki, P.R.; Manaka, T.; Iwamoto, M.; Basu, T.; Malhotra, B.D. Label-free piezoelectric immunosensor decorated with gold nanoparticles: Kinetic analysis and biosensing application. Sens. Actuators B Chem. 2016, 222, 804–814. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, D.-Y.; Zhang, J.; Tang, D. Novel quartz crystal microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay. Anal. Chim. Acta 2018, 1031, 161–168. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.-R.; Wang, W.-C.; Xue, J.; Huang, Y.-L.; Yang, X.-X.; Tan, B.; Zhou, X.-P.; Shao, C.; Ding, S.-J.; et al. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem. 2016, 192, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Busman, M.; Maragos, C.M. Immunoassay utilizing imaging surface plasmon resonance for the detection of cyclopiazonic acid (CPA) in maize and cheese. Anal. Bioanal. Chem. 2019, 411, 3543–3552. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Maragos, C.M. Gold nanoparticle-enhanced multiplexed imaging surface plasmon resonance (iSPR) detection of Fusarium mycotoxins in wheat. Biosens. Bioelectron. 2018, 101, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; McCormick, S.; Maragos, C.M. An Imaging Surface Plasmon Resonance Biosensor Assay for the Detection of T-2 Toxin and Masked T-2 Toxin-3-Glucoside in Wheat. Toxins 2018, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, M.; Bi, Y.; Xue, H.; Xue, S.; Long, H.; Pu, L.; Fu, G. Rapid Determination of Ochratoxin A in Grape and Its Commodities Based on a Label-Free Impedimetric Aptasensor Constructed by Layer-by-Layer Self-Assembly. Toxins 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Feng, M.; Zuo, L.; Zhu, Z.; Wang, F.; Chen, L.; Li, J.; Shan, G.; Luo, S.-Z. An aptamer based surface plasmon resonance biosensor for the detection of ochratoxin A in wine and peanut oil. Biosens. Bioelectron. 2015, 65, 320–326. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Y.; Cheng, Y.; Huang, L.; Zhuang, J.; Tang, D. Two-dimensional MoS2 as a nano-binder for ssDNA: Ultrasensitive aptamer based amperometric detection of Ochratoxin A. Microchim. Acta 2018, 185, 162. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Haupt, K.; Feller, K.-H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef]
- Rehmat, Z.; Mohammed, W.; Sadiq, M.B.; Somarapalli, M.; Anal, A.K. Ochratoxin A detection in coffee by competitive inhibition assay using chitosan-based surface plasmon resonance compact system. Colloids Surf. B Biointerfaces 2019, 174, 569–574. [Google Scholar] [CrossRef]
- Xiang, Y.; Camarada, M.B.; Wen, Y.; Wu, H.; Chen, J.; Li, M.; Liao, X. Simple voltammetric analyses of ochratoxin A in food samples using highly-stable and anti-fouling black phosphorene nanosensor. Electrochim. Acta 2018, 282, 490–498. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.-H. Rapid and sensitive inhibition-based assay for the electrochemical detection of Ochratoxin A and Aflatoxin M1 in red wine and milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Dubiak-Szepietowska, M.; Vorobii, M.; Rodriguez-Emmenegger, C.; Dostalek, J.; Feller, K.-H. Sensitive and rapid detection of aflatoxin M1 in milk utilizing enhanced SPR and p(HEMA) brushes. Biosens. Bioelectron. 2016, 81, 159–165. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Dong, X. Aptamer based voltammetric patulin assay based on the use of ZnO nanorods. Microchim. Acta 2018, 185, 462. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ben Aissa, S.; Sherazi, T.A.; Catanante, G.; Hayat, A.; Marty, J.L. Development of an Impedimetric Aptasensor for Label Free Detection of Patulin in Apple Juice. Molcules 2019, 24, 1017. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Yan, X. An amperometric zearalenone aptasensor based on signal amplification by using a composite prepared from porous platinum nanotubes, gold nanoparticles and thionine-labelled graphene oxide. Microchim. Acta 2019, 186, 383. [Google Scholar] [CrossRef]
- Liu, N.; Nie, D.; Tan, Y.; Wu, A.-B.; Liao, Y.; Wang, H.; Sun, C.; Wu, A. An ultrasensitive amperometric immunosensor for zearalenones based on oriented antibody immobilization on a glassy carbon electrode modified with MWCNTs and AuPt nanoparticles. Microchim. Acta 2016, 184, 147–153. [Google Scholar] [CrossRef]
- He, B.; Yan, X. A “signal-on” voltammetric aptasensor fabricated by hcPt@AuNFs/PEI-rGO and Fe3O4NRs/rGO for the detection of zearalenone. Sens. Actuators B Chem. 2019, 290, 477–483. [Google Scholar] [CrossRef]
- Joshi, S.; Segarra-Fas, A.; Peters, J.; Zuilhof, H.; Van Beek, T.A.; Nielen, M.W.F. Multiplex surface plasmon resonance biosensing and its transferability towards imaging nanoplasmonics for detection of mycotoxins in barley. Analyst 2016, 141, 1307–1318. [Google Scholar] [CrossRef]
- Ram, Y.; Yoetz-Kopelman, T.; Dror, Y.; Freeman, A.; Shacham-Diamand, Y. Impact of Molecular Surface Charge on Biosensing by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2016, 200, 161–167. [Google Scholar] [CrossRef]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.A.P.; Venturini, C.; Castilho, M.D.S.; Canaverolo, N.; Vinicius, M.; Dos Santos, G.P.; Sadao, C.; Vicente, A.; Yamanak, H. Amperometric Biosensor for Diagnosis of Disease; InTech: Rijeka, Croatia, 2013; pp. 253–289. [Google Scholar]
- Ricci, F.; Volpe, G.; Micheli, L.; Palleschi, G. A review on novel developments and applications of immunosensors in food analysis. Anal. Chim. Acta 2007, 605, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Švitel, J.; Katrlík, J.; Vitel, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.T. Plasmonic biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 7, 152–168. [Google Scholar] [CrossRef] [Green Version]
- Montagut, Y.; Garcia, J.; Jiménez, Y.; March, C.; Montoya, A.; Arnau, A. QCM Technology in Biosensors. In Biosensors—Emerging Materials and Applications; IntechOpen: Rijeka, Croatia, 2011; pp. 153–178. [Google Scholar]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernandez, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef]
- Rameil, S.; Schubert, P.; Grundmann, P.; Dietrich, R.; Märtlbauer, E. Use of 3-(4-hydroxyphenyl)propionic acid as electron donating compound in a potentiometric aflatoxin M1-immunosensor. Anal. Chim. Acta 2010, 661, 122–127. [Google Scholar] [CrossRef]
- Spinella, K.; Mosiello, L.; Palleschi, G.; Vitali, F. Development of a QCM (Quartz Crystal Microbalance) Biosensor to Detection of Mycotoxins. Lect. Notes Electr. Eng. 2013, 268, 195–198. [Google Scholar] [CrossRef]
- Rodrigues, P.; Santos, C.; Venâncio, A.; Lima, N. Species identification of Aspergillus section Flavi isolates from Portuguese almonds using phenotypic, including MALDI-TOF ICMS, and molecular approaches. J. Appl. Microbiol. 2011, 111, 877–892. [Google Scholar] [CrossRef]
- Panda, A.; Ghosh, A.K.; Mirdha, B.R.; Xess, I.; Paul, S.; Samantaray, J.C.; Srinivasan, A.; Khalil, S.; Rastogi, N.; Dabas, Y. MALDI-TOF mass spectrometry for rapid identification of clinical fungal isolates based on ribosomal protein biomarkers. J. Microbiol. Methods 2015, 109, 93–105. [Google Scholar] [CrossRef]
- Hleba, L.; Císarová, M.; Shariati, M.A.; Tancinová, D. Detection of mycotoxins using MALDI-TOF Mass SpectrometryJ. Microbiol. Biotechnol. Food Sci. 2017, 7, 181–185. [Google Scholar]
- Sivagnanam, K.; Komatsu, E.; Rampitsch, C.; Perreault, H.; Gräfenhan, T. Rapid screening of Alternaria mycotoxins using MALDI-TOF mass spectrometry. J. Sci. Food Agric. 2016, 97, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Bintvihok, A.; Treebonmuang, S.; Srisakwattana, K.; Nuanchun, W.; Patthanachai, K.; Usawang, S. A Rapid and Sensitive Detection of Aflatoxin-producing Fungus Using an Optimized Polymerase Chain Reaction (PCR). Toxicol. Res. 2016, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Paniel, N.; Rhouati, A.; Marty, J.-L.; Barthelmebs, L. Recent advances in ochratoxin A-producing fungi detection based on PCR methods and ochratoxin A analysis in food matrices. Food Control 2012, 26, 401–415. [Google Scholar] [CrossRef]
- Midorikawa, G.E.O.; Miller, R.N.G.; Bittencourt, D.M.d.C. Molecular Techniques in Food Biology: Safety, Biotechnology, Authenticity and Traceability, 1st ed.; El Sheikha, A.F., Levin, R., Xu, J., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 3, pp. 385–407. [Google Scholar]
- Luo, J.; Vogel, R.F.; Niessen, L. Development and application of a loop-mediated isothermal amplification assay for rapid identification of aflatoxigenic molds and their detection in food samples. Int. J. Food Microbiol. 2012, 159, 214–224. [Google Scholar] [CrossRef]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic Nose for Microbiological Quality Control of Food Products. Int. J. Electrochem. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Casalinuovo, I.A.; Di Pierro, N.; Coletta, M.; Di Francesco, P. Application of Electronic Noses for Disease Diagnosis and Food Spoilage Detection. Sensors 2006, 6, 1428–1439. [Google Scholar] [CrossRef] [Green Version]
- Sanaeifar, A.; Zakidizaji, H.; Jafari, A.; De La Guardia, M. Early detection of contamination and defect in foodstuffs by electronic nose: A review. TrAC Trends Anal. Chem. 2017, 97, 257–271. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic Nose-Based Technique for Rapid Detection and Recognition of Moldy Apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef] [Green Version]
- Gruber, J.; Nascimento, H.M.; Yamauchi, E.Y.; Li, R.W.; Esteves, C.H.; Rehder, G.P.; Gaylarde, C.; Shirakawa, M.A. A conductive polymer based electronic nose for early detection of Penicillium digitatum in post-harvest oranges. Mater. Sci. Eng. C 2013, 33, 2766–2769. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, K.; Zhao, N.; Yang, J.; Zhang, Y.; Ma, C.; Pan, L.; Tu, K. Information fusion of hyperspectral imaging and electronic nose for evaluation of fungal contamination in strawberries during decay. Postharvest Boil. Technol. 2019, 153, 152–160. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, G. Advances in luminescent materials with aggregation-induced emission (AIE) properties for biomedical applications. J. Mater. Chem. B 2018, 6, 4029–4042. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Gao, H.; Wang, Y. A pH-responsive drug delivery system with an aggregation-induced emission feature for cell imaging and intracellular drug delivery. Polym. Chem. 2015, 6, 4715–4718. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Yang, B.; Deng, F.; Huang, Z.; Yang, Y.; Li, Z.; Zhang, X.; Wei, Y. Ultrabright and biocompatible AIE dye based zwitterionic polymeric nanoparticles for biological imaging. RSC Adv. 2014, 4, 35137–35143. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, X.; Deng, S.; Yan, B.; Dong, Y.; Zhang, K.; Deng, R.; He, Q. Label-free fluorescent aptasensing of mycotoxins via aggregation-induced emission dye. Dye. Pigment. 2019, 170, 107572. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Wang, C.; Ho, W.E.; Ong, C.N. Recent developments and applications of metabolomics in microbiological investigations. TrAC Trends Anal. Chem. 2014, 56, 37–48. [Google Scholar] [CrossRef]
- Serkova, N.J.; Brown, M.S. Quantitative analysis in magnetic resonance spectroscopy: From metabolic profiling to in vivo biomarkers. Bioanalysis 2012, 4, 321–341. [Google Scholar] [CrossRef]
- Kleigrewe, K.; Aydin, F.; Hogrefe, K.; Piecuch, P.; Bergander, K.; Würthwein, E.-U.; Humpf, H.-U. Structure Elucidation of New Fusarins Revealing Insights in the Rearrangement Mechanisms of the Fusarium Mycotoxin Fusarin C. J. Agric. Food Chem. 2012, 60, 5497–5505. [Google Scholar] [CrossRef]
- López-Ruiz, R.; Romero-González, R.; Frenich, A.G. Metabolomics approaches for the determination of multiple contaminants in food. Curr. Opin. Food Sci. 2019, 28, 49–57. [Google Scholar] [CrossRef]
- ElMasry, G.; Sun, D.W. Principles of hyperspectral imaging technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Sun, D.W., Ed.; Elsevier: London, UK, 2010; pp. 3–43. [Google Scholar]
- Qin, J.; Kim, M.; Chao, K.; Chan, D.E.; Delwiche, S.R.; Cho, B.-K. Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. Appl. Sci. 2017, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.-W.; Allen, P. Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. [Google Scholar] [CrossRef] [PubMed]
- Tekle, S.; Måge, I.; Segtnan, V.H.; Bjørnstad, A. Near-Infrared Hyperspectral Imaging of Fusarium-Damaged Oats (Avena sativa L.). Cereal Chem. J. 2015, 92, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Barbedo, J.G.; Tibola, C.S.; Lima, M.I.P. Deoxynivalenol screening in wheat kernels using hyperspectral imaging. Biosyst. Eng. 2017, 155, 24–32. [Google Scholar] [CrossRef]
- Ropelewska, E.; Zapotoczny, P. Classification of Fusarium-infected and healthy wheat kernels based on features from hyperspectral images and flatbed scanner images: A comparative analysis. Eur. Food Res. Technol. 2018, 244, 1453–1462. [Google Scholar] [CrossRef] [Green Version]
| Extraction Methods | Extraction Solvents | Limits | Benefits | Reference |
|---|---|---|---|---|
| QuEChERS | Organic solvents or mixtures (CH3CN, MeOH, MeOH/CH3CN) | Modifications of the original procedure, need of an additional enrichment step | Economical, fast, simple, detection of low ppb levels, better reproducibility and accuracy | [5] |
| LLE | Mixture of organic solvents (hexane, cyclohexane) with diluted acids or water | Time consuming, the sample can be absorbed by the glass equipment depending on the matrix and the determined compounds | Effective, for small-scale preparations | [40] |
| SLE | Mixture of organic solvents with diluted acids or water | Matrix effects | Smaller volumes of solvent | [32,45] |
| ASE or PLE | Mixture of organic solvents (MeOH/CH3CN, CH3CN/water) | Expensive instruments, matrix components excessively coextracted | Fully automated, faster extraction compared to the conventional ones, minimal solvents, higher extraction efficiency | [22,46] |
| SFE | Supercritical fluids (CO2), MeOH, ethanol, acetone | Need for specialized and very expensive equipment, not suitable for routine analysis | Fast technique, small solvent volumes, preconcentration effect, extraction of temperature sensible analytes | [22,45,47] |
| MAE | Aqueous solution | Only applicable for thermally stable compounds, costly instruments | Reduced extraction time and extraction solvent | [48] |
| VALDS–ME | Mixture of organic solvents dispersive solvent and water | Optimization after control a lot of parameters | Use of low density solvents, simple, fast, effective | [20] |
| Sample | Origin | Number of Samples | Mycotoxins | LOD | LOQ | References |
|---|---|---|---|---|---|---|
| Herbs and herbal products | India | 63 | AFB1, AFB2, AFG1, AFG2, CIT | 10 ng/mL for AFB1 NA for others | NA | [69] |
| Herbal medicines | Nigeria | 210 | AFB1, AFB2, AFG1, AFG2 | NA | NA | [70] |
| Brazil nuts | Brazil | 67 | AFB1, AFB2, AFG1, AFG2 | NA | 2 mg/kg | [71] |
| Almonds, cashew nuts, chestnuts, hazelnuts, pistachio nuts, walnuts | Saudi Arabia | 5 | AFB1, AFB2, AFG1, AFG2, CIT, OTs, PAT, T-2, ZEA, ST, DAS | NA | 5 μg/kg (for AFs), NA for others | [72] |
| Medicinal plants | Pakistan | 30 | AFB1, AFB2, AFG1, AFG2, OTA | NA | NA | [73] |
| Corn-based food products | Brazil | 208 | AFB1, AFB2, AFG1, AFG2 | NA | 2 µg/kg | [74] |
| Mycotoxin | Year of Publication | Country | Sample | Extraction Solution | Extraction Method | Clean-Up | LOD | LOQ * | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AFs | 2014 | China | Walnut kernel | Methanol–water (70:30, v/v) | Sonicating | Self-made amino-function nanometer Fe3O4 magnetic polymer SPE | 0.004–0.013 µg kg−1 | 0.012–0.042 µg kg−1 | [90] |
| AFs, OTA Fusarium mycotoxins | 2014 | Italy | Cereals and derived products | Methanol–water (60:40, v/v) | Blending | IAC | 1 µg kg−1 for AFs and OTA 5–30 µg kg−1 for Fusarium toxins | Nd | [52] |
| 5 Alternaria mycotoxins, CIT | 2015 | Belgium | Tomato and tomato juice | Methanol 2,4-dinitrophenylhydrazine | Vortex | SPE cartridge | 1–20 µg kg−1 | 2–50 µg kg−1 | [91] |
| 4 Alternaria mycotoxins | 2016 | China | Wheat kernel | Acetonitrile–water–methanol (45:45:10, v/v/v) | Sonicating | SPE cartridge | 0.04–1.3 µg kg−1 | 0.1–4.2 | [92] |
| AFs, FB1, FB2, DON, OTA, ZEA | 2016 | Thailand | Brown rice | Acetonitrile with 10% (v/v) acetic acid | Vortex | QuEChERS | 1.4–25 µg kg−1 | 4.1–75 µg kg−1 | [93] |
| 15 mycotoxins | 2016 | Spain | Cow milk | Acetonitrile (2% formic acid) | Shaking | Sodium acetate | 0.02−10.14 ng mL−1 | Nd | [94] |
| 16 mycotoxins | 2017 | China | Vegetable oils | 85% Acetonitrile | Shaking | QuEChERS | 0.04–2.9 ng g−1 | Nd | [95] |
| 11 mycotoxins | 2017 | USA | Infant cereals | Acetonitrile/water/formic acid, (80:19.9:0.1, v/v/v) | Shaking | Nd | 0.01−10.0 ng g−1 | 0.05–50 ng g–1 | [84] |
| 12 Fusarium mycotoxins | 2017 | Germany | Beer | Acetonitrile/water (70:30, v/v) Acetonitrile/water (84:16, v/v) | Vortex | SPE cartridge | 0.05–6.9 µg L−1 | 0.15–20 µg L−1 | [87] |
| AFB1 OTA FB1 DON T2 HT-2 ZEA | 2017 | Italy | Cereal-based samples | Acetonitrile–water–acetic acid (79:20:1, v/v/v) | Shaking | Nd | 0.06–0.13 µg kg−1fo r AFB1 0.4–0.8 µg kg−1 for OTA 8−16 µg kg−1 for FB1 20 µg kg−1 for DON 4–8 µg kg−1 for T–2 20 µg kg−1 for HT–2 1.6–3.2 µg kg−1 for ZEA | Nd | [96] |
| 13 mycotoxins | 2017 | Korea | Cereal grains | Methanol 80%, containing 0.5% acetic acid | Shaking | IAC | 0.1−18.1 ng/g | 0.4–54.8 ng/g | [97] |
| 20 mycotoxins | 2019 | Korea | Soybean Paste | Methanol–water (60:40, v/v) and PBS | Blending | IAC | 0.06–4.68 µg kg−1 | 0.17−13.24 | [49] |
| 6 Alternaria toxins | 2019 | China | Grapes | Acetonitrile and dispersive solid phase extraction | Shaking | QuEChERS | 0.03–0.21 µg kg−1 | 0.09–0.48 µg kg−1 | [77] |
| AFs, ZEA, α-ZOL | 2019 | Spain | Vegetable oils | Acetonitrile | Shaking | QuEChERS | Nd | 0.5 μg kg−1 for AFs 1 μg kg−1 for ZEA and α-ZOL | [93] |
| Mycotoxin | Label Used | Commodity | Sensitivity | Reference |
|---|---|---|---|---|
| Deoxynivalenol (DON) Zearalenone (ZEA) T-2/H-T2-toxin | Epoxy-functionalized silica coated QDs | Barley | 1000 µg/kg 80 µg/kg 80 µg/kg | [106] |
| Aflatoxin B1 (AFB1) Zearalenone (ZEA) Deoxynivalenol (DON) | Monoclonal antibodies (mAbs) with the conjugates bovine serum albumin (BSA) | Wheat and maize | 0.05 μg/kg 1 μg/kg 3 μg/kg | [107] |
| Fumonisin B1 (FB1) Deoxynivalenol (DON) | Gold nanoparticles (AuNPs) | Maize | 2.0 ng mL−1 40 ng mL−1 | [108] |
| Deoxynivalenol (DON) T-2 toxin (T-2) Zearalenone (ZEN) | Amorphous carbon nanoparticles (ACNPs) | Maize | 20 µg/kg 13 µg/kg 1 µg/kg | [109] |
| Aflatoxin B1 (AFB1) Zearalenone (ZEN) Deoxynivalenol (DON) | CdSe/SiO2 quantum dot microbeads | Feedstuff | 10 pg mL−1 80 pg mL−1 500 pg mL−1 | [110] |
| Zearalenone (ZEN) | Antibody-labeled quantum dot sumicro beads | Corn | 3.6 mg mL−1 | [111] |
| Fumonisins (FUs) | CdSe/ZnS QD + GNP | Maize | 62.5 μg/kg | [102] |
| Mycotoxin | Recognition Element | Transducer/Technique | Food | Detection Limit | Reference |
|---|---|---|---|---|---|
| AFB1 | Organic framework composite | Piezoelectric (QCM) | Peanut, pistachio, rice, and wheat | 2.8 pg mL−1 | [116] |
| AFB1 | Antibody | Impedimetric (EIS) | Corn | 0.05 ng mL−1 | [117] |
| AFB1 | Antibody | Piezoelectric (EQCM) | Cereal | 8 pg mL−1 | [118] |
| AFB1 | Antibody | Piezoelectric (QCM) | Peanut | 0.83 ng kg−1 | [119] |
| AFB1 | Antibody | Potentiometric (DPV) | Corn powder | 3.5 pg mL−1 | [120] |
| Cyclopiazonic acid | Antibody | Optical (SPR) | Maize and cheese | 0.29 mg mL−1 | [121] |
| DON, ZEN, T-2toxin | Antibody | Optical (SPR) | Wheat | 15μg/kg−1 24 μg/kg−1 12 μg/kg−1 | [122] |
| HT-2 toxin, T-2 toxin, AFM1 | Antibody | Amperometric (CV) | Human urine | 0.4 ng mL−1 1 ng mL−1 0.3 ng mL−1 | [113] |
| T-2 toxin, T-2 toxin-3-glucoside (T2-G) | Antibody | Optical (iSPR) | Wheat | 1.2 ng mL−1 | [123] |
| OTA | Aptamer | Impedimetric (EIS) | Grape and commodities | 0.030 ng mL−1 | [124] |
| OTA | Aptamer | SPR | Wine and peanut oil | 0.005 ng mL−1 | [125] |
| OTA | Antibody | Piezoelectric (QCM) | Buffer | 17.2 ng mL−1 | [111] |
| OTA | Aptamer | Amperometric (CV) | Red wine | 0.23 pg mL−1 | [126] |
| OTA | Antibody | Piezoelectric (QCM) | Red wine | 0.16 ng mL−1 | [127] |
| OTA | Antibody | Optical (SPR) | Coffee | 3.8 ng mL−1 | [128] |
| OTA | Black phosphorene | Potentiometric (DPV) | Grape juice and red wine | 0.18 μg mL−1 | [129] |
| OTA | Antibody | Piezoelectric (QCM-D) | Red wine | 0.16 ng mL−1 | [127] |
| OTA, AFM1 | Antibody | Potentiometric (CV) | Red wine and milk | 0.15 ng mL−1 3.04 ng mL−1 | [130] |
| AFM1 | Antibody | Optical (SPR) | Milk | 18 pg mL−1 | [131] |
| PAT | Aptamer | Potentiometric (EIS/DPV) | Juice | 0.27 pg mL−1 | [132] |
| PAT | Aptamer | Impedimetric (EIS) | Apple juice | 2.8 ng L−1 | [133] |
| ZEN | Aptamer | Amperometric (CV/DPV) | Maize | 0.17 pg mL−1 | [134] |
| ZEN | Antibody | Amperometric (CV/DPV) | Corn and corn products | 1.5 pg mL−1 | [135] |
| ZEN | Aptamer | Potentiometric (CV/DPV) | Maize | 0.105 pg mL−1 | [136] |
| DON, T-2, ZEA, FB1 | Antibody | Optical (iSPR) | Barley | 64 µg kg−1, 26 µg kg−1, 96 µg kg−1, 13 µg kg−1 | [137] |
| Biosensors | Advantages | Limitations | Reference |
|---|---|---|---|
| Impedimetric | High sensitivity and selectivity, time-efficient, simple operation, fast response, mobility due to portable instrumentation, miniaturization | Complex construction, expensive labeling markers | [25,124,147] |
| Potentiometric | Reduced analysis time, mobility due to portable instrumentation, miniaturization, high sensitivity and selectivity, use without sample treatment | The sensitivity and lifetime are seriously influenced by factors such as temperature, pH, immobilization support, and immunological cross-reaction | [129,147,148] |
| Amperometric | Mobility due to portable instrumentation, miniaturization, high sensitivity and selectivity | Regeneration between measurements | [147] |
| Surface plasmon resonance | High specificity and sensitivity, small size and cost-efficiency, direct, real-time analysis and detection without label, development of portable devices | The broad practical application is still under development | [25,144] |
| Quartz crystal microbalance | Low cost with high sensitivity, selectivity, and possibility of reuse, real-time output, and label- or radiation-free entities, development of portable devices | Requirement of a relatively high background signal relative to the signal-on assay formation | [119,149] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9, 518. https://doi.org/10.3390/foods9040518
Agriopoulou S, Stamatelopoulou E, Varzakas T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods. 2020; 9(4):518. https://doi.org/10.3390/foods9040518
Chicago/Turabian StyleAgriopoulou, Sofia, Eygenia Stamatelopoulou, and Theodoros Varzakas. 2020. "Advances in Analysis and Detection of Major Mycotoxins in Foods" Foods 9, no. 4: 518. https://doi.org/10.3390/foods9040518
APA StyleAgriopoulou, S., Stamatelopoulou, E., & Varzakas, T. (2020). Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods, 9(4), 518. https://doi.org/10.3390/foods9040518