Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study
Abstract
1. Introduction
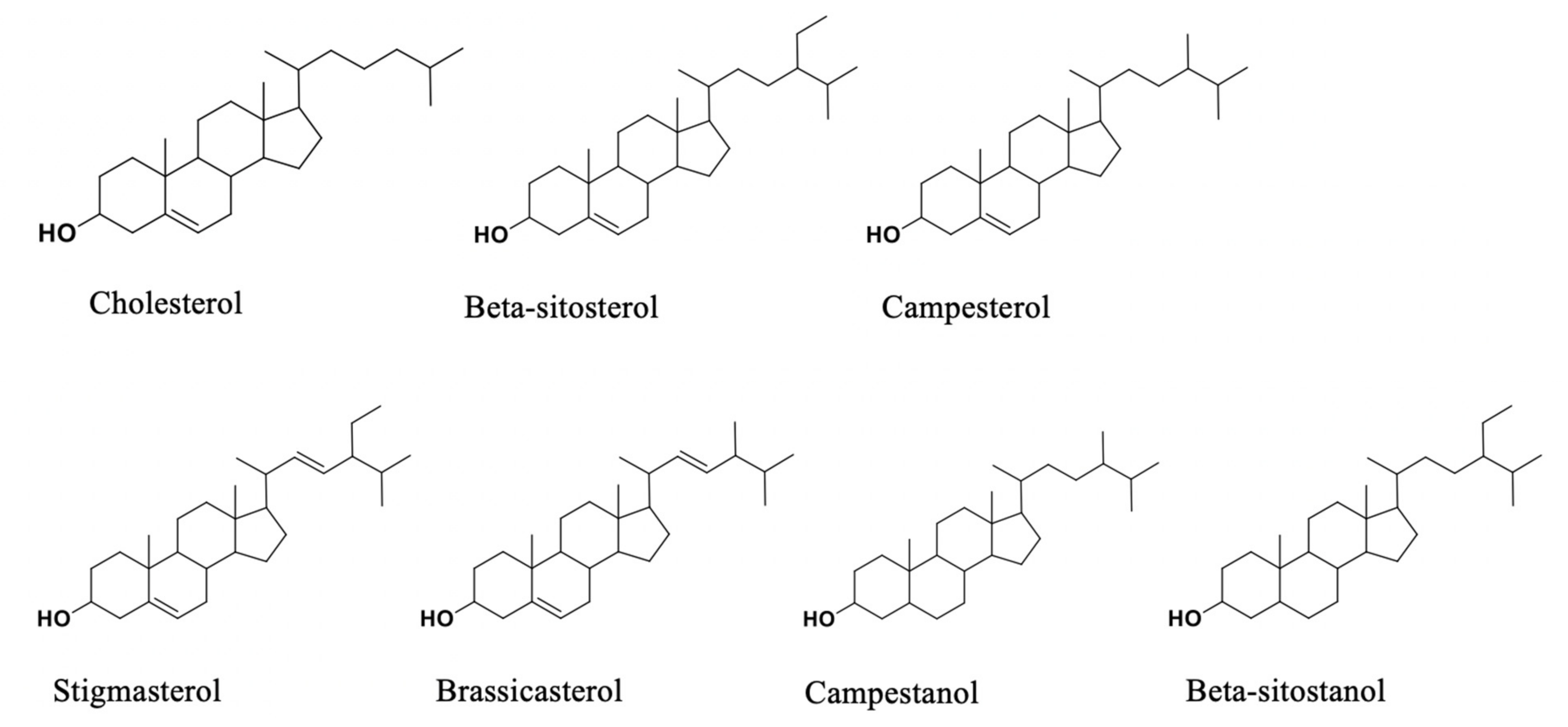
2. Effects and Action Mechanisms of Phytosterols
3. Phytosterols as Natural Source or Added in Foods
4. Stability of Phytosterols in Foods
5. Micro/Nanoencapsulation of Phytosterols
6. Use of Microencapsulated Phytosterols for Functional Foods’ Production
7. Legislation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merah, O.; Mouloungui, Z. Tetraploid wheats: Valuable source of phytosterols and phytostanols. Agronomy 2019, 9, 201. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. The emerging use of mycosterols in food industry along with the current trend of extended use of bioactive phytosterols. Trends Food Sci. Technol. 2017, 67, 19–35. [Google Scholar] [CrossRef]
- Racette, S.B.; Lin, X.; Lefevre, M.; Spearie, C.A.; Most, M.M.; Ma, L.; Ostlund, R.E., Jr. Dose effects of dietary phytosterols on cholesterol metabolism: A controlled feeding study. Am. J. Clin. Nutr. 2009, 91, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tolve, R.; Condelli, N.; Can, A.; Tchuenbou-Magaia, F.L. Development and characterization of phytosterol-enriched oil microcapsules for foodstuff application. Food Bioproc. Tech. 2018, 11, 152–163. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food ingredients that inhibit cholesterol absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar] [PubMed]
- Hasler, C.M. Functional foods: Their role in disease prevention and health promotion. Food Technol.-Champaign Chicago 1998, 52, 63–147. [Google Scholar]
- Dias, M.I.; Ferreira, I.C.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef]
- da Silva, B.V.; Barreira, J.C.; Oliveira, M.B.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Abbas, S.; Da Wei, C.; Hayat, K.; Xiaoming, Z. Ascorbic acid: Microencapsulation techniques and trends—A review. Food Rev. Int. 2012, 28, 343–374. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Berger, A.; Jones, P.J.H.; Abumweis, S.S. Plant sterols: Factors affecting their efficacy and safety as functional food ingredients. Lipid Health Dis. 2004, 3, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E., Jr. Phytosterols and cholesterol metabolism. Curr. Opin. Lipidol. 2004, 15, 37–41. [Google Scholar] [CrossRef] [PubMed]
- MacKay, D.S.; Jones, P.J.H. Phytosterols in human nutrition: Type, formulation, delivery, and physiological function. Eur. J. Lipid Sci. Tech. 2011, 113, 1427–1432. [Google Scholar] [CrossRef]
- de Jong, A.; Plat, J.; Mensink, R.P. Metabolic effects of plant sterols and stanols. J. Nutr. Biochem. 2003, 14, 362–369. [Google Scholar] [CrossRef]
- Izar, M.C.; Tegani, D.M.; Kasmas, S.H.; Fonseca, F.A. Phytosterols and phytosterolemia: Gene–diet interactions. Genes Nutr. 2011, 6, 17–26. [Google Scholar] [CrossRef]
- Bain, B.J.; Chakravorty, S. Phytosterolemia. Am. J. Hematol. 2016, 91, 643. [Google Scholar] [CrossRef]
- Tzavella, E.; Hatzimichael, E.; Kostara, C.; Bairaktari, E.; Elisaf, M.; Tsimihodimos, V. Sitosterolemia: A multifaceted metabolic disorder with important clinical consequences. J. Clin. Lipidol. 2017, 11, 1095–1100. [Google Scholar] [CrossRef]
- Vergès, B.; Fumeron, F. Potential risks associated with increased plasma plant-sterol levels. Diabetes Metab. 2015, 41, 76–81. [Google Scholar] [CrossRef]
- Fardet, A.; Morise, A.; Kalonji, E.; Margaritis, I.; Mariotti, F. Influence of phytosterol and phytostanol food supplementation on plasma liposoluble vitamins and provitamin A carotenoid levels in humans: An updated review of the evidence. Crit. Rev. Food Sci. 2017, 57, 1906–1921. [Google Scholar] [CrossRef]
- Penchala Raju, M.; Aravind Babu, D.G.; Rakesh Kumar, B.; Rajashekar, C.H. The role of phytosterols enriched foods-A review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 7, 40–47. [Google Scholar] [CrossRef]
- Sanclemente, T.; Marques-Lopes, I.; Fajó-Pascual, M.; Cofán, M.; Jarauta, E.; Ros, E.; Puzo, J.; García-Otín, A.L. Naturally-occurring phytosterols in the usual diet influence cholesterol metabolism in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A. Composition of plant sterols and stanols in supplemented food products. J. AOAC Int. 2015, 98, 685–690. [Google Scholar] [CrossRef]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous dose-response relationship of the LDL-cholesterol–lowering effect of phytosterol intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.C. Occurrence and levels of phytosterols in food. In Phytosterols as Functional Food Components and Nutraceuticals; Dutta, P.C., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 1–27. [Google Scholar]
- Vaghini, A.S.; Cilla, A.; Garcia-Llatas, G.; Lagarda, M.J. Bioaccessibility study of plant sterol-enriched fermented milks. Food Funct. 2016, 7, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, A.; Garcia-Llatas, G.; Cilla, A.; Barberá, R.; Sánchez-Siles, L.M.; Lagarda, M.J. Impact of lipid components and emulsifiers on plant sterols bioaccessibility from milk-based fruit beverages. J. Agric. Food Chem. 2016, 64, 5686–5691. [Google Scholar] [CrossRef] [PubMed]
- Izadi, Z.; Nasirpour, A.; Garoosi, G.A.; Tamjidi, F. Rheological and physical properties of yogurt enriched with phytosterol during storage. J. Food Sci. Technol. 2014, 52, 5341–5346. [Google Scholar] [CrossRef]
- Duong, S.; Strobel, N.; Buddhadasa, S.; Stockham, K.; Auldist, M.; Wales, B.; Orbell, J.; Cran, M. Rapid measurement of phytosterols in fortified food using gas chromatography with flame ionization detection. Food Chem. 2016, 211, 570–576. [Google Scholar] [CrossRef]
- Grasso, S.; Brunton, N.P.; Monahan, F.J.; Harrison, S.M. Development of a method for the analysis of sterols in sterol-enriched deli-style turkey with GC-FID. Food Anal. Methods 2016, 9, 724–728. [Google Scholar] [CrossRef]
- Menéndez-Carreño, M.; Knol, D.; Janssen, H.G. Development and validation of methodologies for the quantification of phytosterols and phytosterol oxidation products in cooked and baked food products. J. Chromatogr. A 2016, 1428, 316–325. [Google Scholar] [CrossRef]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of phytosterols in foods. J. Pharmaceut. Biomed. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- González-Larena, M.; Garcia-Llatas, G.; Clemente, G.; Barberá, R.; Lagarda, M.J. Plant sterol oxides in functional beverages: Influence of matrix and storage. Food Chem. 2015, 173, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Guth, S.; Engel, K.H.; Steinberg, P. Phytosterol oxidation products in enriched foods: Occurrence, exposure, and biological effects. Mol. Nutr. Food Res. 2015, 59, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Vanmierlo, T.; Husche, C.; Schött, H.F.; Pettersson, H.; Lütjohann, D. Plant sterol oxidation products—Analogs to cholesterol oxidation products from plant origin? Biochimie 2013, 95, 464–472. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol oxidation products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur. J. Lipid Sci. Tech. 2016, 118, 1423–1438. [Google Scholar] [CrossRef]
- González-Larena, M.; Cilla, A.; García-Llatas, G.; Barberá, R.; Lagarda, M.J. Plant sterols and antioxidant parameters in enriched beverages: Storage stability. J. Agric. Food Chem. 2012, 60, 4725–4734. [Google Scholar] [CrossRef]
- Botelho, P.B.; Galasso, M.; Dias, V.; Mandrioli, M.; Lobato, L.P.; Rodriguez-Estrada, M.T.; Castro, I.A. Oxidative stability of functional phytosterol-enriched dark chocolate. LWT- Food Sci. Technol. 2014, 55, 444–451. [Google Scholar] [CrossRef]
- Rudzińska, M.; Przybylski, R.; Wąsowicz, E. Degradation of phytosterols during storage of enriched margarines. Food Chem. 2014, 142, 294–298. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Cardenia, V.; Mandrioli, M.; Muste, S.; Borsari, A.; Rodriguez-Estrada, M.T. Stability of flavoured phytosterol-enriched drinking yogurts during storage as affected by different packaging materials. J. Sci. Food Agric. 2016, 96, 2782–2787. [Google Scholar] [CrossRef]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.L.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of health-promoting ingredients: Applications in foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Chen, Q.; McGillivray, D.; Wen, J.; Zhong, F.; Quek, S.Y. Co-encapsulation of fish oil with phytosterol esters and limonene by milk proteins. J. Food Eng. 2013, 117, 505–512. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chen, B.H.; Chiu, C.P.; Lu, Y.F. The influence of phytosterols on the encapsulation efficiency of cholesterol liposomes. Int. J. Food Sci. Tech. 2004, 39, 985–995. [Google Scholar] [CrossRef]
- Alexander, M.; Lopez, A.A.; Fang, Y.; Corredig, M. Incorporation of phytosterols in soy phospholipids nanoliposomes: Encapsulation efficiency and stability. LWT- Food Sci. Technol. 2012, 47, 427–436. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Leong, W.F.; Lai, O.M.; Long, K.; Che Man, Y.B.; Misran, M.; Tan, C.P. Preparation and characterisation of water-soluble phytosterol nanodispersions. Food Chem. 2011, 129, 77–83. [Google Scholar] [CrossRef]
- Auweter, H.; Bohn, H.; Hasselwander, O.; Runge, F. Process for Producing Pulvurulent Phytosterols Formulations. U.S. Patent 2009/0047355 A1, 19 February 2009. [Google Scholar]
- Choi, M.J.; Briançon, S.; Bazile, D.; Royere, A.; Min, S.G.; Fessi, H. Effect of cryoprotectant and freeze-drying process on the stability of W/O/W emulsions. Dry. Technol. 2007, 25, 809–819. [Google Scholar] [CrossRef]
- Alvim, I.D.; Souza, F.D.S.D.; Koury, I.P.; Jurt, T.; Dantas, F.B.H. Use of the spray chilling method to deliver hydrophobic components: Physical characterization of microparticles. LWT- Food Sci. Technol. 2013, 33, 34–39. [Google Scholar] [CrossRef]
- Ling, H.W.; Lin, N.K. In Vitro release study of freeze-dried and oven-dried microencapsulated kenaf seed oil. Malays. J. Nutr. 2017, 23, 139–149. [Google Scholar]
- Lim, W.T.; Nyam, K.L. Characteristics and controlled release behaviour of microencapsulated kenaf seed oil during in-vitro digestion. J. Food Eng. 2016, 182, 26–32. [Google Scholar] [CrossRef]
- Khalid, N.; Kobayashi, I.; Neves, M.A.; Uemura, K.; Nakajima, M.; Nabetani, H. Encapsulation of β-sitosterol plus γ-oryzanol in O/W emulsions: Formulation characteristics and stability evaluation with microchannel emulsification. Food Bioprod. Process. 2017, 102, 222–232. [Google Scholar] [CrossRef]
- Comunian, T.A.; Nogueira, M.; Scolaro, B.; Thomazini, M.; Ferro-Furtado, R.; de Castro, I.A.; Favaro-Trindade, C.S. Enhancing stability of echium seed oil and beta-sitosterol by their coencapsulation by complex coacervation using different combinations of wall materials and crosslinkers. Food Chem. 2018, 252, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Jessie, L.Y.L.; Tan, C.P.; Long, K.; Nyam, K.L. Effect of accelerated storage on microencapsulated kenaf seed oil. J. Am. Oil Chem. Soc. 2013, 90, 1023–1029. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramiréz-Rigo, M.V.; Piña, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Winkler, J.K.; Warner, K. The effect of phytosterol concentration on oxidative stability and thermal polymerization of heated oils. Eur. J. Lipid Sci. Technol. 2008, 110, 455–464. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Caruso, M.C.; Genovese, F.; Di Renzo, G.C.; Mauriello, G.; Galgano, F. Preparation and characterization of microencapsulated phytosterols for the formulation of functional foods: Scale up from laboratory to semi-technical production. Food Res. Int. 2019, 116, 1274–1281. [Google Scholar] [CrossRef]
- Bagherpour, S.; Alizadeh, A.; Ghanbarzadeh, S.; Mohammadi, M.; Hamishehkar, H. Preparation and characterization of betasitosterol-loaded nanostructured lipid carriers for butter enrichment. Food Biosci. 2017, 20, 51–55. [Google Scholar] [CrossRef]
- Comunian, T.A.; Chaves, I.E.; Thomazini, M.; Moraes, I.C.F.; Ferro-Furtado, R.; de Castro, I.A.; Favaro-Trindade, C.S. Development of functional yogurt containing free and encapsulated echium oil, phytosterol and sinapic acid. Food Chem. 2017, 237, 948–956. [Google Scholar] [CrossRef]
- Tolve, R.; Condelli, N.; Caruso, M.C.; Barletta, D.; Favati, F.; Galgano, F. Fortification of dark chocolate with microencapsulated phytosterols: Chemical and sensory evaluation. Food Funct. 2018, 9, 1265–1273. [Google Scholar] [CrossRef]
- Regulation No 258/97 of the European Parliament and of the Council of 27 January 1997. O J E U 1997, L43, 14.2.
- Commission Regulation No 608/2004/EC of 31 March 2004. O J E U 2004, L15, 288.
- Commission Regulation No 2013/718/EU of 25 July 2013. O J E U 2013, L201, 49.
- Commission Decision No 2004/336/EC of 31 March 2004. O J E U 2004, L105, 49.
- FSANZ, Food Standards Australia New Zealand. Food Standards Code. Commonwealth of Australia Gazette No. FSC 31; Food Standards Australia New Zealand: Canberra, Australia, 2006. [Google Scholar]
- Health Canada. Notice of Assessment of Certain Categories of Foods Containing Added Phytosterols; Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch Health Canada: Montreal, QC, Canada, 2010. [Google Scholar]
- FOSHU (Food for Specified Health Uses) Japanese Ministry of Health, Labour and Welfare. 2006. Available online: http://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html (accessed on 24 February 2018).
- Raisio, PLC. Approval by the Chinese Ministry of Health Administration for Benecol Ingredient. 2008. Available online: https://www.globenewswire.com/news-release/2008/12/04/24962/0/en/APPROVAL-BY-THE-CHINESE-MINISTRY-OF-HEALTH-FOR-BENECOL-INGREDIENT.html (accessed on 7 December 2014).
- Kim, J.Y.; Dai, B.K.; Hyong, J.L. Regulations on Health/functional Foods in Korea; Nutrition and Functional Food Headquarters, Korea Food and Drug Administration: Seoul, Korea, 2005. [Google Scholar]
- Zawistowski, J.; Jones, P. Regulatory aspects related to plant sterol and stanol supplemented foods. J. AOAC Int. 2015, 98, 750–756. [Google Scholar] [CrossRef] [PubMed]

| Phytosterol Food Sources | Phytosterols Content (mg/kg of Fresh Weight) | Reference |
|---|---|---|
| Oils | ||
| Corn | 7150–9520 | [23] |
| Olive | 1140–1150 | [25] |
| Palm | 490–610 | [25] |
| Peanut | 1670–2290 | [25] |
| Rice bran | 10,550 | [23] |
| Soybean | 2210–3280 | [23] |
| Sunflower | 2030–3280 | [25] |
| Vegetables | ||
| Broccoli | 367–390 | [25] |
| Carrot | 153–160 | [25] |
| Cauliflower | 310–400 | [25] |
| Onion | 84–93 | [25] |
| Potato | 38–73 | [25] |
| Tomato | 47–148 | [25] |
| Fruits | ||
| Apple | 130–183 | [25] |
| Banana | 116–161 | [25] |
| Grapes | 40–200 | [25] |
| Orange | 228–240 | [25] |
| Nuts | ||
| Almond | 1380–1430 | [25] |
| Peanuts | 600–1608 | [25] |
| Cereals | ||
| Barley | 720–801 | [25] |
| Buckwheat | 963–1980 | [25] |
| Corn | 662–1205 | [25] |
| Oats | 350–491 | [25] |
| Rye | 707–1134 | [25] |
| Wheat | 447–830 | [25] |
| Core Material | Shell Material | Encapsulation Technique | Food Inclusion | Principal Outcomes | Reference |
|---|---|---|---|---|---|
| Fish oil, phytosterol esters and limonene | Whey protein isolate and sodium caseinate | Spray drying | N.R. | Higher protection from oxidation than non-encapsulated phytosterols | [43] |
| Phytosterols mixture | Lipid mixture of low trans hydrogenated vegetable fats and stearic acid | Spray chilling | N.R. | Good quality microcapsules with a mean diameter varied between 13.8 and 32.2 μm | [51] |
| Kenaf seed oil containing phytosterols | Alginate with high methoxy pectin and chitosan | Oven-dried | N.R. | Increase in phytosterols bioavailability evaluated through in vitro release | [52] |
| Kenaf seed oil containing phytosterols | Carboxymethyl-cellulose, maltodextrin and soy lecithin | Spray drying | N.R. | Increase in phytosterols bioavailability evaluated through in vitro release | [53] |
| Beta-sitosterol and γ-oryzanol | Medium chain triglycerice oil | O/W microchannel emulsification | N.R. | Phytosterols retention ranged from 50 to 80%, according to the use of tween 20 or decaglycerol monolaurate as surfactant agent, when stored for 30 days at 4 and 25 °C | [54] |
| Beta-sitosterol and echium oil | Arabic gum, cashew gum | Complex coacervation | N.R. | Phytosterols retention ranged from 70.74 to 73.78% depending upon the absence or the use of sinapic acid as crosslinking when stored for 30 days at 37 °C | [55] |
| Kenaf seed oil containing phytosterols | Sodium caseinate and of maltodextrin | Spray drying | N.R. | Phytosterols concentration was stable when microcapsules were stored at 65 °C for 24 days | [56] |
| Phytosterol mixture | Arabic gum, maltodextrin | Spray drying | N.R. | The microcapsules particle size was lower than 25 μm, which is required to ensure the phytosterols inclusion in the intestinal micellar phase | [57] |
| Phytosterol mixture | Whey protein isolate, inulin and chitosan | O/W emulsion + spray drying | N.R. | Unexpected, the peroxide values of the obtained microcapsules were relatively high even just after the production | [4] |
| Phytosterol mixture | Whey protein isolate, inulin and chitosan | Spray drying | N.R. | Possibility to scale up the production of microcapsules without affect their features using a laboratory dryer or a spray dryer for semi- technical production | [59] |
| Beta-sitosterol | Lipid mixture of Precirol and Miglyol | Hot melt homogenization method | Butter | Beta-sitosterol loaded lipid nanocarriers, showed good stability during three months’ storage period Moreover, the use of this technique does not alter the texture and the organoleptic characteristics of the product | [60] |
| Echium oil and beta-sitosterol | Arabic gum and gelatin | Complex coacervation | Yogurt | Yogurt containing microcapsules did not show a significant difference in terms of physicochemical, rheological and sensorial properties with respect to control | [61] |
| Phytosterols mixture | Whey protein isolate | Spray drying | Dark chocolates | No matter the microencapsulated phytosterols concentration, fortified dark chocolate was widely accepted by consumers | [62] |
| Country | Current Legislation | Health Claim |
|---|---|---|
| European Union (EU) | Novel Food Regulations (EC 258/97) | “Plant sterols (stanols) have been shown to lower/reduce blood cholesterols. High cholesterol is a risk factor in the development of coronary disease” “Plant sterols/stanols contribute to the maintenance of normal blood cholesterol levels” |
| United States of America | GRAS notification and self-GRAS regulation; Dietary Supplement Health and Education ACT (DSHEA) | “Helps maintain normal cholesterol levels”; “May reduce the risk of heart disease” |
| Australia and New Zealand | Novel Food Standard; Food Standards Australia New Zealand (FSANZ) | “Reduces blood cholesterol” |
| Canada | Part B, Division 28 (Novel Foods) of the Food and Drug Regulations | “Plant sterols help reduce/lower cholesterol. High cholesterol is a risk factor for heart disease” |
| Japan | Food for Specified Health Uses (FOSHU) | “Good for those concerned about serum cholesterol” “Good for those having relatively high serum cholesterol and triglycerides with mild obesity” |
| China | State Food and Drug Administration (SFDA) | “This product is not a substitute for medicine” |
| Taiwan | Health Food Control Act | “Regulating blood lipids”; “An animal study shows that consumption of this product may help lower blood total cholesterol” |
| South Korea | Korea Health Functional Food Act (HFFA) by Korean Food and Drug Agency (KFDA) | “Phytosterols may reduce the risk of coronary heart disease” |
| Malaysia | Food Safety and Quality Division under Malaysian Regulations of the Food Act | “Helps lower or reduce cholesterol” |
| Indonesia | Indonesian National Agency for Drug and Food Control (NADFC) | “May reduce the risk of coronary heart disease” |
| Thailand | Thai Food and Drug Administration | “May help lower cholesterol” |
| Philippines | Philippine Food Fortification Act | “This product contains plant sterols that help lower cholesterol” |
| Singapore | Implemented by Agri-Food and Veterinary Authority with the Health Promotion Board | “Plant sterols/stanols have been shown to lower/reduce blood cholesterol. High blood cholesterol is a risk factor in the development of coronary heart disease”; “Intended exclusively for people who want to lower their blood cholesterol level” |
| Brazil | National Health Surveillance Agency | “Helps to maintain healthy level of cholesterol when associated with a healthy diet and life style” |
| Mexico | Mexican General Health Law | “Proven to reduce cholesterol” |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolve, R.; Cela, N.; Condelli, N.; Di Cairano, M.; Caruso, M.C.; Galgano, F. Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods 2020, 9, 470. https://doi.org/10.3390/foods9040470
Tolve R, Cela N, Condelli N, Di Cairano M, Caruso MC, Galgano F. Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods. 2020; 9(4):470. https://doi.org/10.3390/foods9040470
Chicago/Turabian StyleTolve, Roberta, Nazarena Cela, Nicola Condelli, Maria Di Cairano, Marisa C. Caruso, and Fernanda Galgano. 2020. "Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study" Foods 9, no. 4: 470. https://doi.org/10.3390/foods9040470
APA StyleTolve, R., Cela, N., Condelli, N., Di Cairano, M., Caruso, M. C., & Galgano, F. (2020). Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods, 9(4), 470. https://doi.org/10.3390/foods9040470









