Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Treatments
2.2. Determination of the CI Index
2.3. Determination of Pericarp Color
2.4. Determination of Anthocyanin Content
2.5. Determination of REC
2.6. Determination of Malondialdehyde (MDA) Content
2.7. Analysis of Energy Status
2.8. Assay of Energy Metabolism-Related Enzyme Activities
2.9. Assay of Proline Metabolism
2.10. Statistical Analysis
3. Results
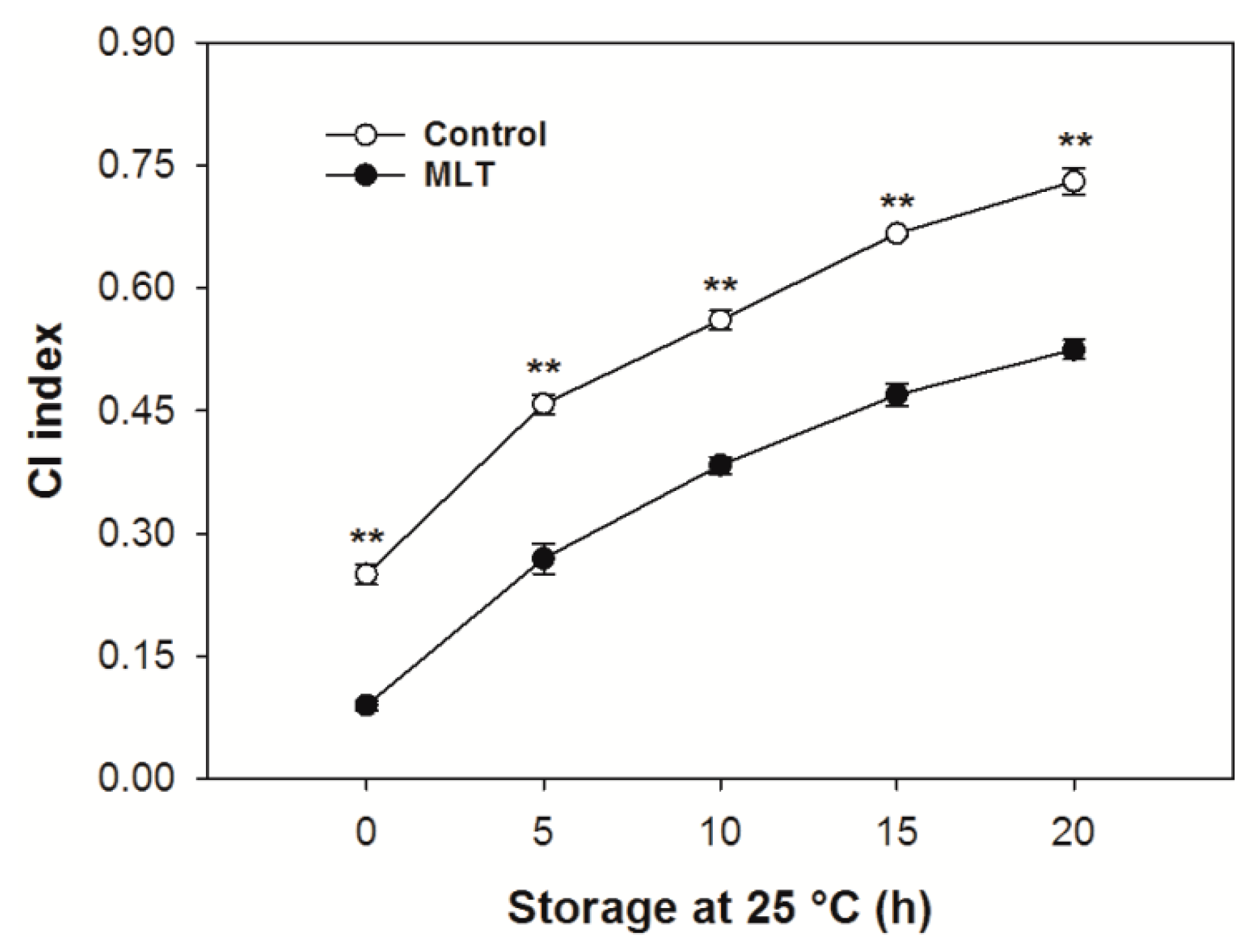
3.1. CI Index of the Pericarp
3.2. Pericarp Color
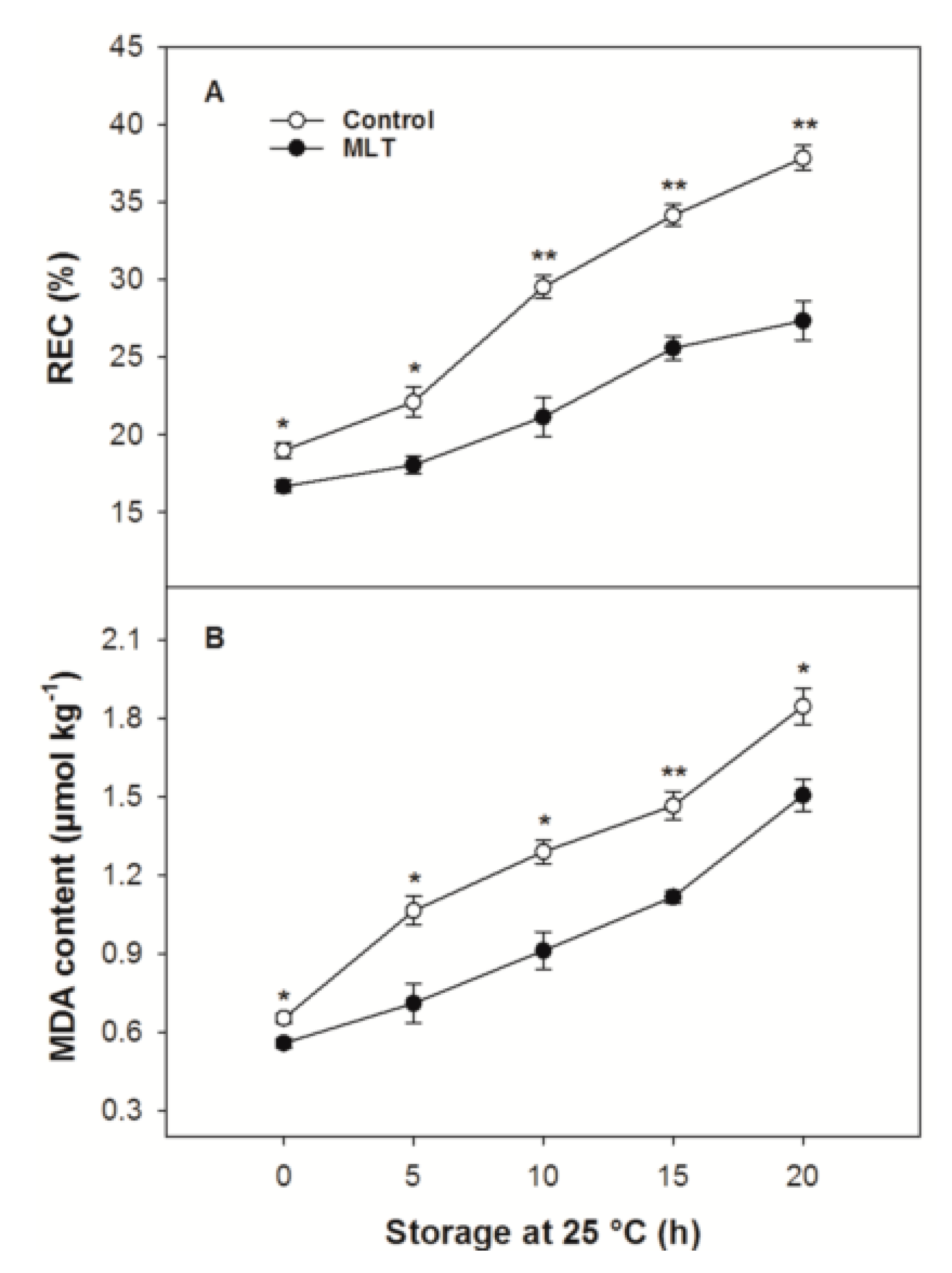
3.3. REC and MDA Content
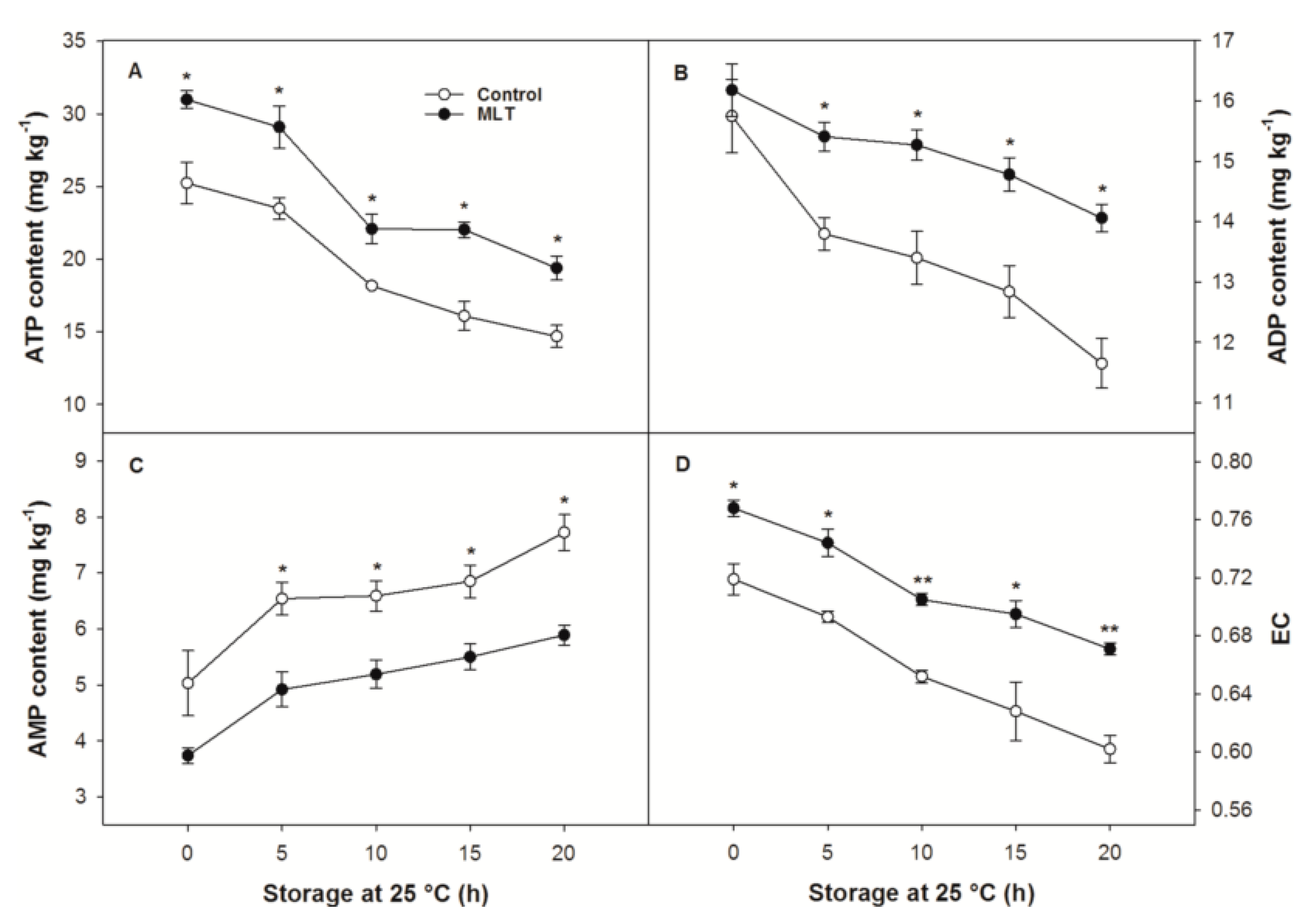
3.4. ATP, ADP and AMP Contents and EC
3.5. Enzymatic Activity Related to Energy Metabolism
3.6. The Proline Metabolic Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holcroft, D.M.; Mitcham, E.J. Postharvest physiology and handling of litchi (Litchi chinensis Sonn.). Postharvest Biol. Technol. 1996, 9, 265–281. [Google Scholar] [CrossRef]
- Sivakumar, D.; Terry, L.A.; Korsten, L. An overview on litchi fruit quality and alternative postharvest treatments to replace sulfur dioxide fumigation. Food Rev. Int. 2010, 26, 162–188. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Li, J. Effects of low temperature acclimation on browning of litchi fruit in relation to shelf life. J. Hort. Sci. Biotechnol. 2003, 78, 437–440. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; You, Y.; Li, Y.; Duan, X.; Jiang, Y.; Joyce, D.C.; Ashraf, M.; Lu, W. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, X.; Joyce, D.C.; Zhang, Z.; Li, J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Jiang, W. Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature. LWT-Food Sci. Technol. 2005, 38, 757–761. [Google Scholar] [CrossRef]
- Somboonkaew, N.; Terry, L.A. Influence of temperature and packaging on physiological and chemical profiles of imported litchi fruit. Food Res. Int. 2011, 44, 1962–1969. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anjum, M.A.; Nawaz, A.; Shah, H.M.S. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Reichel, M.; Wellhöfer, J.; Triani, R.; Sruamsiri, P.; Carle, R.; Neidhart, S. Postharvest control of litchi (Litchi chinensis Sonn.) pericarp browning by cold storage at high relative humidity after enzyme-inhibiting treatments. Postharvest Biol. Technol. 2017, 125, 77–90. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U. Postharvest L-cysteine application delayed pericarp browning, suppressed lipid peroxidation and maintained antioxidative activities of litchi fruit. Postharvest Biol. Technol. 2016, 121, 135–142. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Khan, A.S.; Ali, S. Pre-storage kojic acid application delays pericarp browning and maintains antioxidant activities of litchi fruit. Postharvest Biol. Technol. 2017, 132, 154–161. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shaheen, T.; Shahid, M. Pre-storage methionine treatment inhibits postharvest enzymatic browning of cold stored ‘Gola’ litchi fruit. Postharvest Biol. Technol. 2018, 140, 100–106. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Cabrera, J.; D’Arpa, D.; Sainz, R.M.; Mayo, J.C.; Ramos, S. The oxidant/antioxidant network: Role of melatonin. Biol. Signals Recept. 1999, 8, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.D.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in medicinal and food plants: Occurrence, bioavailability, and health potential for humans. Cells. 2019, 8, 681. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Cao, S.; Song, C.; Shao, J.; Bian, K.; Chen, W.; Yang, Z. Exogenous melatonin treatment increases chilling tolerance and induces defense response in harvested peach fruit during cold storage. J. Agric. Food Chem. 2016, 64, 5215–5222. [Google Scholar] [CrossRef]
- Cao, S.; Bian, K.; Shi, L.; Chung, H.; Chen, W.; Yang, Z. Role of melatonin in cell-wall disassembly and chilling tolerance in cold-stored peach fruit. J. Agric. Food Chem. 2018, 66, 5663–5670. [Google Scholar] [CrossRef]
- Jannatizadeh, A.; Aghdamm, M.S.; Luo, Z.; Razavi, F. Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food Bioprocess Technol. 2019, 12, 741–750. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J.R.; Razavi, F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 2019, 275, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Jannatizadeh, A. Exogenous melatonin applying confers chilling tolerance in pomegranate fruit during cold storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z.; Li, L.; Jannatizadeh, A.; Fard, J.R.; Pirzad, F. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chem. 2020, 303, 125385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huber, D.J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of postharvest browning in litchi fruit by melatonin via the enhancing of antioxidative processes and oxidation repair. J. Agric. Food Chem. 2018, 66, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Floresco, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Faliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Yun, Z.; Wang, J.; Feng, G.; Gao, Z.; Shi, X.; Jiang, Y. Effect of tea seed oil treatment on browning of litchi fruit in relation to energy status and metabolism. Postharvest Biol. Technol. 2017, 132, 97–104. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Ji, Z.; Duan, X.; Ji, Z. Change of anthocyanin content and it’s determination during lychee pericarp browning. J. South China Agric. Uni. 2002, 23, 16–19, (In Chinese with English abstract). [Google Scholar]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Duan, X.; Liu, T.; Zhang, D.; Su, X.; Lin, H.; Jiang, Y. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Liu, T.; Wang, H.; Kuang, J.; Sun, C.; Shi, J.; Duan, X.; Qu, H.; Jiang, Y. Short-term anaerobic, pure oxygen and refrigerated storage conditions affect the energy status and selective gene expression in litchi fruit. LWT-Food Sci. Technol. 2015, 60, 1254–1261. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, J.; Chen, J.; Wang, X.; Zheng, Y. Effect of methyl jasmonate on energy metabolism in peach fruit during chilling stress. J. Sci. Food Agric. 2013, 93, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Q.; Hu, M.; Gao, Z.; An, F.; Li, M.; Jiang, Y. Low-temperature conditioning induces chilling tolerance in stored mango fruit. Food Chem. 2017, 219, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Roosens, N.H.C.J.; Thu, T.T.; Iskandar, H.M.; Jacobs, M. Isolation of ornithine-aminotransferase cDNA and effect of salt stress on its expression in Arabidopsis thaliana. Plant Physiol. 1998, 117, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Rho, H.W.; Park, J.W.; Park, B.H.; Kim, J.S.; Lee, M.W. Assay of ornithine aminotransferase with ninhydrin. Anal. Biochem. 1994, 223, 205–207. [Google Scholar] [CrossRef]
- Shang, H.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Effect of exogenous gamma-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, S.; Yuan, M.; Song, H.; Wang, T.; Zhang, W.; Zhang, Z. Effect of glycine betaine on chilling injury in relation to energy metabolism in papaya fruit during cold storage. Food Sci. Nutr. 2019, 7, 1123–1130. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Zhou, X.; Shi, F.; Fu, W.; Ji, S. Effect of ATP treatment on enzymes involved in energy and lipid metabolisms accompany peel browning of ‘Nanguo’ pears during shelf life after low temperature. Sci. Hortic. 2018, 240, 446–452. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, Y.; Shan, T.; Huang, Y.; Xu, J.; Zheng, Y. Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J. Agric. Food Chem. 2015, 63, 3654–3659. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Wang, J.; Zhang, W.; Jiang, Y.; Lv, M. 1-Methylcyclopropene alleviates peel browning of ‘Nanguo’ pears by regulating energy, antioxidant and lipid metabolisms after long term refrigeration. Sci. Hortic. 2019, 247, 254–263. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Du, R.; Luo, Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016, 208, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Khan, Z.U.; Mao, L.; Yin, T. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Sondergaard, T.E.; Schulz, A.; Palmgren, M.G. Energization of transport processes in plants. roles of the plasma membrane H+-ATPase. Plant Physiol. 2004, 136, 2475–2482. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, M.; Zhang, W.; Zhang, Z. Effect of low temperatures on chilling injury in relation to energy status in papaya fruit during storage. Postharvest Biol. Technol. 2017, 125, 181–187. [Google Scholar] [CrossRef]
- Dhanraj, K.M.; Veerakumari, L. Effect of ethanol extract of Areca catechu on fumarate reductase and succinate dehydrogenase of Cotylophoron cotylophorum. Int. J. Res. Dev. Pharm. Life Sci. 2016, 5, 2117–2123. [Google Scholar]
- Millar, A.H.; Atkin, O.K.; Lambers, H.; Wiskich, J.T.; Day, D.A. A critique of the use of inhibitors to estimate partitioning of electrons between mitochondrial respiratory pathways in plants. Physiol. Plant. 1995, 95, 523–532. [Google Scholar] [CrossRef]
- Gomes, F.P.; Oliva, M.A.; Mielke, M.S.; Almeida, A.A.F.; Aquino, L.A. Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Sci. Hortic. 2010, 126, 379–384. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-epibrassinolide on chilling injury of peach fruit in relation to phenolic and proline metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Delauney, A.J.; Hu, C.A.; Kishor, P.B.; Verma, D.P. Cloning of ornithine delta-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coil and regulation of proline biosynthesis. J. Biol. Chem. 1993, 268, 18673–18678. [Google Scholar] [PubMed]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Wei, B.; Cheng, S.; Ji, S. Exogenous glycine betaine treatment alleviates low temperature-induced pericarp browning of ‘Nanguo’ pears by regulating antioxidant enzymes and proline metabolism. Food Chem. 2020, 306, 125626. [Google Scholar] [CrossRef]
- Cao, S.; Cai, Y.; Yang, Z.; Zheng, Y. MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chem. 2012, 133, 1466–1470. [Google Scholar] [CrossRef]
- Li, P.; Zheng, X.; Liu, Y.; Zhu, Y. Pre-storage application of oxalic acid alleviates chilling injury in mango fruit by modulating proline metabolism and energy status under chilling stress. Food Chem. 2012, 142, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Du, R.; Liu, Y.; Ying, T.; Mao, L. Effect of nitric oxide on antioxidative response and proline metabolism in banana during cold storage. J. Agric. Food Chem. 2013, 61, 8880–8887. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z. Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit. Foods 2020, 9, 454. https://doi.org/10.3390/foods9040454
Liu G, Zhang Y, Yun Z, Hu M, Liu J, Jiang Y, Zhang Z. Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit. Foods. 2020; 9(4):454. https://doi.org/10.3390/foods9040454
Chicago/Turabian StyleLiu, Gangshuai, Yuxin Zhang, Ze Yun, Meijiao Hu, Jialiang Liu, Yueming Jiang, and Zhengke Zhang. 2020. "Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit" Foods 9, no. 4: 454. https://doi.org/10.3390/foods9040454
APA StyleLiu, G., Zhang, Y., Yun, Z., Hu, M., Liu, J., Jiang, Y., & Zhang, Z. (2020). Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit. Foods, 9(4), 454. https://doi.org/10.3390/foods9040454






