Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample Collection
2.2. Preparation of Fruit Extracts
2.3. Physicochemical Characterization
2.4. Total Phenol Content (TPC)
2.5. Total Flavonoid Content (TFC)
2.6. Antioxidant Activity
2.7. Preparation of Standard Solutions
2.8. Quantification of Phenolic Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Total Phenol Content (TPC)
3.3. Total Flavonoid Content (TFC)
3.4. Antioxidant Activity
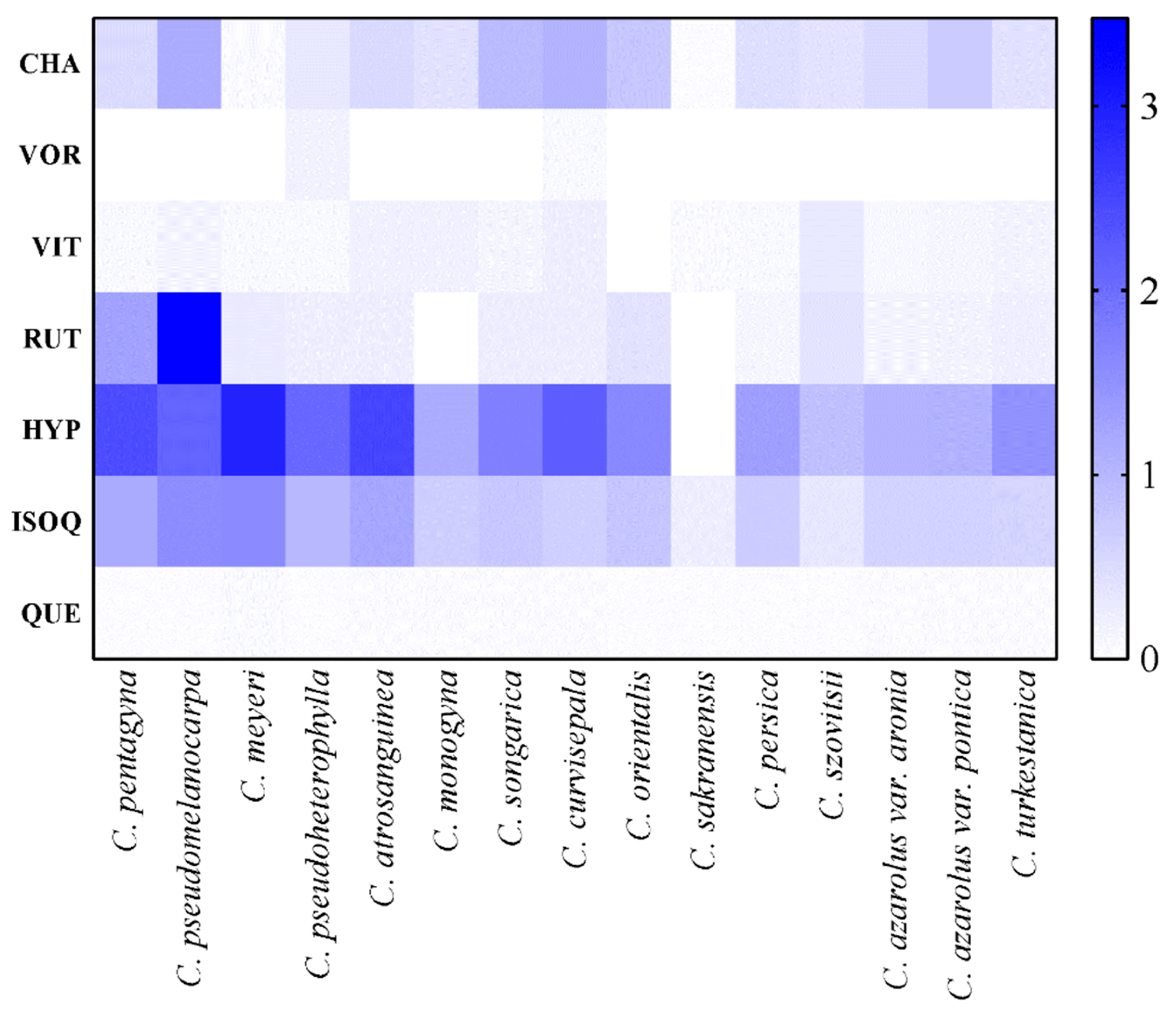
3.5. Quantification of Phenolic Compounds
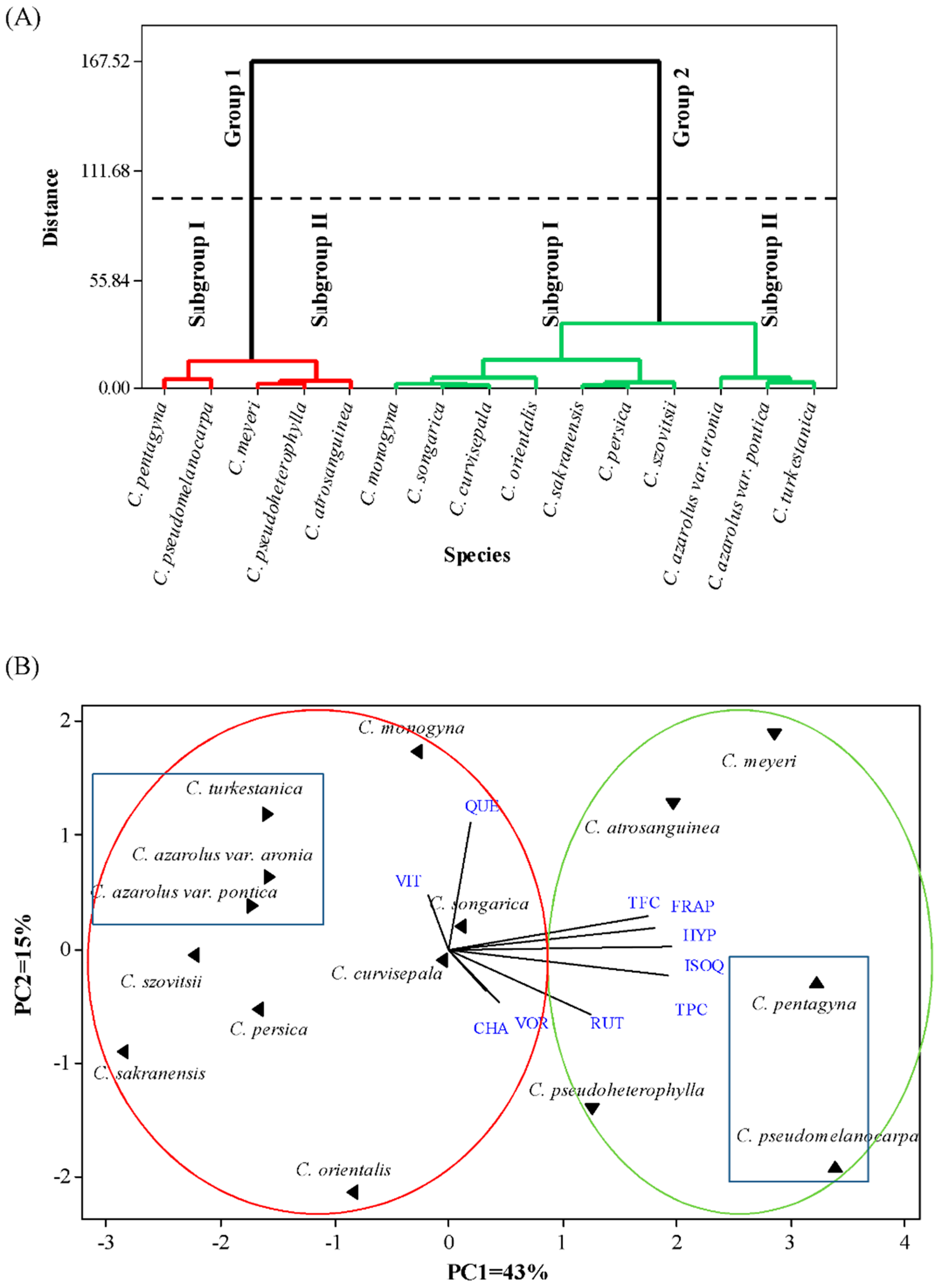
3.6. Hierarchical Cluster Analysis and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Hatami Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef]
- Kao, E.S.; Wang, C.J.; Lin, W.L.; Chu, C.Y.; Tseng, T.H. Effects of polyphenols derived from fruit of Crataegus pinnatifida on cell transformation, dermal edema and skin tumor formation by phorbol ester application. Food Chem. Toxicol. 2007, 45, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic composition of Crataegus monogyna jacq.: From chemistry to medical applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, M.H. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nutr. Res. Pract. 2011, 5, 101–106. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, X.; Feng, B. Effect of crataegus usage in cardiovascular disease prevention: An evidence-based approach. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–16. [Google Scholar]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Pugine, S.M.P.; Lima, C.G.; Lorenzo, J.M.; de Melo, M.P. Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; de Melo, M.P. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.M.; Amado, I.R.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Portnaya, I.; Cogan, U.; Azaizeh, H.; Bomzon, A. Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel. J. Ethnopharmacol. 2005, 101, 153–161. [Google Scholar] [CrossRef]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic acids present in hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evidence-based Complement. Altern. Med. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, N.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Hensel, A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Žugić, A.; Đorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crops Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Lü, D.; Zhou, C.; Yang, B. Quantitative analysis of phenolic compounds in Chinese hawthorn (Crataegus spp.) fruits by high performance liquid chromatography-electrospray ionisation mass spectrometry. Food Chem. 2011, 127, 1370–1377. [Google Scholar] [CrossRef]
- Kalt, W. Effects of Production and Processing Factors on Major Fruit and Vegetable Antioxidants. J. Food Sci. 2005, 70, R11–R19. [Google Scholar] [CrossRef]
- Özcan, M.; Haciseferoǧullari, H.; Marakoǧlu, T.; Arslan, D. Hawthorn (Crataegus spp.) fruit: Some physical and chemical properties. J. Food Eng. 2005, 69, 409–413. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, C.T.; Wang, S.Y. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009, 117, 426–431. [Google Scholar] [CrossRef]
- Mraihi, F.; Hidalgo, M.; de Pascual-Teresa, S.; Trabelsi-Ayadi, M.; Chérif, J.K. Wild grown red and yellow hawthorn fruits from Tunisia as source of antioxidants. Arab. J. Chem. 2015, 8, 570–578. [Google Scholar] [CrossRef]
- Serçe, S.; Şimşek, Ö.; Toplu, C.; Kamiloǧlu, Ö.; Çalişkan, O.; Gündüz, K.; Özgen, M.; Kaçar, Y.A. Relationships among Crataegus accessions sampled from Hatay, Turkey, as assessed by fruit characteristics and RAPD. Genet. Resour. Crop Evol. 2011, 58, 933–942. [Google Scholar] [CrossRef]
- Calişkan, O.; Gündüz, K.; Serçe, S.; Toplu, C.; Kamiloğlu, O.; Sengül, M.; Ercişli, S. Phytochemical characterization of several hawthorn (Crataegus spp.) species sampled from the Eastern Mediterranean region of Turkey. Pharmacogn. Mag. 2012, 8, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Chen, H.S.; Shen, Y.C.; Lai, G.H.; Lin, P.K.; Wang, C.M. Phytochemical composition, antioxidant activity and neuroprotective effect of Crataegus pinnatifida fruit. S. Afr. J. Bot. 2013, 88, 432–437. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020. In press. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M.J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Ortega-Ramirez, L.A.; Rodriguez-Garcia, I.; Leyva, J.M.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Siddiqui, M.W.; Ayala-Zavala, J.F. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: A hypothesis. J. Food Sci. 2014, 79, 129–137. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Kim, S.J.; Min, S.C.; Shin, H.J.; Lee, Y.J.; Cho, A.R.; Kim, S.Y.; Han, J. Evaluation of the antioxidant activities and nutritional properties of ten edible plant extracts and their application to fresh ground beef. Meat Sci. 2013, 93, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Hesari, J.; Eskandari, M.H.; Valizadeh, H.; Sirousazar, M. Effect of green tea, stinging nettle and olive leaves extracts on the quality and shelf life stability of frankfurter type sausage. J. Food Process. Preserv. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z.; Tang, S.; Jin, P. Control of anthracnose rot and quality deterioration in loquat fruit with methyl jasmonate. J. Sci. Food Agric. 2008, 88, 1598–1602. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analisys of total phenols and other oxidation sobstrates and antioxidants by means of Folin Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, Q.; Zuo, Z.; Harrison, F.; Chow, M.S.S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef]
- Cui, T.; Li, J.Z.; Kayahara, H.; Ma, L.; Wu, L.X.; Nakamura, K. Quantification of the polyphenols and triterpene acids in Chinese hawthorn fruit by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef] [PubMed]
- Albarouki, E.; Peterson, A. Molecular and morphological characterization of Crataegus L. species (Rosaceae) in southern Syria. Bot. J. Linn. Soc. 2007, 153, 255–263. [Google Scholar] [CrossRef]
- Orhan, I.; Özçelik, B.; Kartal, M.; Özdeveci, B.; Duman, H. HPLC Quantification of Vitexine-2″-O-rhamnoside and Hyperoside in Three Crataegus Species and Their Antimicrobial and Antiviral Activities. Chromatographia 2007, 66, 153–157. [Google Scholar] [CrossRef]
- Li, W.Q.; Hu, Q.P.; Xu, J.G. Changes in physicochemical characteristics and free amino acids of hawthorn (Crataegus pinnatifida) fruits during maturation. Food Chem. 2015, 175, 50–56. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell Online 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.K.; Chen, Z.Y. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Masteikova, R.; Majiene, D.; Savickas, A.; Kevelaitis, E.; Bernatoniene, R.; Dvořáčkovâ, K.; Civinskiene, G.; Lekas, R.; Vitkevičius, K.; et al. Free radical-scavenging activities of crataegus monogyna extracts. Medicina 2008, 44, 706–712. [Google Scholar] [CrossRef]
- Liu, T.; Cao, Y.; Zhao, M. Extraction optimization, purification and antioxidant activity of procyanidins from hawthorn (C. pinnatifida Bge. var. major) fruits. Food Chem. 2010, 119, 1656–1662. [Google Scholar] [CrossRef]
- Sahin, S.; Samli, R.; Birteks, Z.; Tan, A.S.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of drying method on oleuropein, total phenolic content, flavonoid content, and antioxidant activity of olive (Olea europaea) leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar] [CrossRef]
- Froehlicher, T.; Hennebelle, T.; Martin-Nizard, F.; Cleenewerck, P.; Hilbert, J.L.; Trotin, F.; Grec, S. Phenolic profiles and antioxidative effects of hawthorn cell suspensions, fresh fruits, and medicinal dried parts. Food Chem. 2009, 115, 897–903. [Google Scholar] [CrossRef]
- Prinz, S.; Ring, A.; Huefner, A.; Pemp, E.; Kopp, B. 42“-Acetylvitexin-2” -O-rhamnoside, isoorientin, orientin, and 8-methoxykaempferol-3-O-glucoside as markers for the differentiation of Crataegus monogyna and Crataegus pentagyna from Crataegus laevigata (Rosaceae). Chem. Biodivers. 2007, 4, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Bignami, C.; Paolocci, M.; Scossa, A.; Bertazza, G. Preliminary evaluation of nutritional and medicinal components of Crataegus azarolus fruits. Acta Hortic. 2003, 597, 95–100. [Google Scholar] [CrossRef]
- Bahri-Sahloul, R.; Ammar, S.; Grec, S.; Harzallah-Skhiri, F. Chemical characterisation of Crataegus azarolus l. Fruit from 14 genotypes found in Tunisia. J. Hortic. Sci. Biotechnol. 2009, 84, 23–28. [Google Scholar] [CrossRef]
- Simirgiotis, M.J. Antioxidant capacity and HPLC-DAD-MS profiling of chilean peumo (Cryptocarya alba) fruits and comparison with german peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef]
- Agregan, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Muchenje, V.; Barba, F.J.; & Lorenzo, J.M. Phenolic content and antioxidant activity of extracts from bifurcaria bifurcata alga, obtained by diverse extraction conditions using three different techniques (hydrothermal, ultrasounds and supercritical CO2). Environ. Eng. Manag. J. 2019, 18, 1535–1542. [Google Scholar]
- Roselló-Soto, E.; Barba, F.J.; Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Moltó, J.C. Phenolic profile of oils obtained from “horchata” by-products assisted by supercritical-CO2and its relationship with antioxidant and lipid oxidation parameters: Triple TOF-LC-MS-MS characterization. Food Chem. 2019, 274, 865–871. [Google Scholar] [CrossRef]
- Yin, M.; Cheng, W. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003, 63, 23–28. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Cardinali, R.; Cullere, M.; Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C.; Trabalza Marinucci, M.; Dalle Zotte, A. Oregano, rosemary and vitamin E dietary supplementation in growing rabbits: Effect on growth performance, carcass traits, bone development and meat chemical composition. Livest. Sci. 2015, 175, 83–89. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Zhang, M.; Chen, J.J.; He, Z.; Qin, F.; Xu, Z.; Cao, D.; Chen, J.J. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018, 239, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mojtahed Zadeh Asl, R.; Niakousari, M.; Hashemi Gahruie, H.; Saharkhiz, M.J.; Mousavi Khaneghah, A. Study of two-stage ohmic hydro-extraction of essential oil from Artemisia aucheri Boiss.: Antioxidant and antimicrobial characteristics. Food Res. Int. 2018, 107, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Bunce, J.A.; Maas, J.L. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, M.; Julkunen-Tiitto, R.; Mutikainen, P.; Walls, M.; Ovaska, J.; Vapaavuori, E. Trade-offs in phenolic metabolism of silver birch: Effects of fertilization, defoliation, and genotype. Ecology 1999, 80, 1970–1986. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; Mcewen, J.; Brien, C.O.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium Species. J. Agric. Food Chem. 1998, 8561, 2686–2693. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]

| Province | Species | Height | Latitude | Longitude |
|---|---|---|---|---|
| Semnan | C. pentagyna | 1540 | 36° 02′N | 53° 28′E |
| Mazandaran | C. monogyna | 1081 | 36° 25′N | 51° 52′E |
| Mazandaran | C. pseudomelanocarpa | 1371 | 36° 23′N | 51° 32′E |
| Mazandaran | C. songarica | 1123 | 36° 25′N | 51° 31′E |
| Bakhtiari | C. azarolus var. aronia | 1913 | 31° 33′N | 51° 12′E |
| Alborz | C. azarolus var. pontica | 1846 | 36° 09′N | 50° 42′E |
| East Azerbaijan | C. sakranensis | 1694 | 38° 14′N | 45° 42′E |
| East Azerbaijan | C. turkestanica | 1690 | 38° 14′N | 45° 42′E |
| East Azerbaijan | C. meyeri | 1281 | 38° 49′N | 47° 03′E |
| East Azerbaijan | C. orientalis | 1277 | 38° 49′N | 47° 03′E |
| East Azerbaijan | C. curvisepala | 1196 | 38° 50′N | 47° 02′E |
| Kurdistan | C. atrosanguinea | 1633 | 35° 23′N | 46° 55′E |
| Kurdistan | C. persica | 1637 | 35° 23′N | 46° 55′E |
| Kurdistan | C. szovitsii | 1506 | 36° 06′N | 46° 20′E |
| West Azerbaijan | C. pseudoheterophylla | 1488 | 37° 27′N | 44° 56′E |
| Species | Color parameters | ||||
|---|---|---|---|---|---|
| a* | b* | L* | C | h° | |
| C. pentagyna | 0.36 ± 0.12h | 0.14 ± 0.06j | 0.17 ± 0.09h | 0.39 ± 0.10j | 18.98 ± 1.13fg |
| C. monogyna | 33.95 ± 1.49bc | 12.55 ± 1.30fg | 7.37 ± 0.11e | 36.21 ± 1.34e | 20.18 ± 1.51f |
| C. pseudomelanocarpa | 0.42 ± 0.23h | 0.10 ± 0.13j | 0.15 ± 0.02h | 0.43 ± 0.11j | 12.95 ± 0.34i |
| C. songarica | 6.59 ± 1.29g | 1.62 ± 0.15j | 1.03 ± 0.32h | 6.79 ± 0.27i | 13.83 ± 1.76i |
| C. azarolus var. aronia | 12.33 ± 0.19f | 56.93 ± 1.17a | 37.36 ± 1.97a | 58.25 ± 1.29a | 77.78 ± 1.21a |
| C. azarolus var. pontica | 22.85 ± 1.82e | 56.71 ± 1.43a | 35.44 ± 1.87a | 61.15 ± 1.55a | 68.03 ± 1.85b |
| C. sakranensis | 7.07 ± 0.46g | 1.73 ± 0.07j | 1.10 ± 0.27h | 7.28 ± 0.38i | 13.72 ± 1.65i |
| C. turkestanica | 39.43 ± 1.67a | 18.79 ± 1.34e | 10.98 ± 1.29d | 43.68 ± 1.98cd | 25.45 ± 0.45e |
| C. meyeri | 22.23 ± 1.36e | 5.68 ± 0.19i | 3.41 ± 0.13g | 22.95 ± 1.83h | 14.32 ± 0.92hi |
| C. orientalis | 27.74 ± 1.76d | 42.73 ± 1.92b | 26.89 ± 1.32b | 50.97 ± 1.87b | 57.06 ± 0.26c |
| C. curvisepala | 31.45 ± 1.43c | 9.67 ± 0.39gh | 5.70 ± 0.32ef | 32.91 ± 1.10f | 17.04 ± 1.18gh |
| C. atrosanguinea | 40.63 ± 1.27a | 23.53 ± 1.20c | 13.74 ± 1.10c | 46.96 ± 1.29b | 30.04 ± 0.87d |
| C. persica | 39.49 ± 1.45a | 22.22 ± 1.49cd | 12.98 ± 1.37cd | 45.31 ± 1.19bc | 29.36 ± 1.35d |
| C. szovitsii | 36.01 ± 2.07b | 19.53 ± 1.59de | 11.60 ± 1.15d | 40.99 ± 1.72d | 28.46 ± 1.13d |
| C. pseudoheterophylla | 26.40 ± 1.13d | 7.07 ± 0.27hi | 4.19 ± 0.23fg | 27.33 ± 1.20g | 14.99 ± 0.24hi |
| Significant level | *** | *** | *** | *** | *** |
| Species | pH | TA a (%) | TSS b (°Brix) | TSC c (%) | TCC d (μg/g) |
|---|---|---|---|---|---|
| C. pentagyna | 3.07 ± 0.07i | 0.75 ± 0.10i | 14.99 ± 0.11f | 7.21 ± 0.43i | 281.44 ± 6.54g |
| C. monogyna | 3.93 ± 0.15c | 0.97 ± 0.04d | 19.58 ± 0.21cd | 5.27 ± 0.53j | 282.74 ± 6.66g |
| C. pseudomelanocarpa | 3.12 ± 0.13h | 0.79 ± 0.12g | 15.65 ± 0.31f | 10.11 ± 0.44g | 205.77 ± 4.24h |
| C. songarica | 3.15 ± 0.16g | 0.76 ± 0.07hi | 15.73 ± 0.13f | 10.91 ± 0.26g | 141.63 ± 4.13i |
| C. azarolus var. aronia | 3.14 ± 0.10gh | 1.02 ± 0.15c | 20.34 ± 0.22c | 19.43 ± 0.32a | 359.79 ± 6.15b |
| C. azarolus var. pontica | 3.16 ± 0.17g | 1.17 ± 0.14a | 23.43 ± 0.19a | 15.35 ± 1.23e | 405.79 ± 5.64a |
| C. sakranensis | 3.52 ± 0.17e | 0.89 ± 0.07ef | 17.59 ± 0.98de | 19.12 ± 0.86a | 322.75 ± 1.13d |
| C. turkestanica | 3.63 ± 0.12d | 0.91 ± 0.08e | 18.15 ± 1.21de | 6.58 ± 0.54i | 86.84 ± 2.65j |
| C. meyeri | 3.93 ± 0.14c | 0.98 ± 0.10d | 19.65 ± 0.97c | 19.22 ± 0.55a | 285.86 ± 5.54fg |
| C. orientalis | 3.03 ± 0.11j | 0.77 ± 0.09ghi | 15.15 ± 0.87f | 17.55 ± 0.86b | 340.86 ± 6.76c |
| C. curvisepala | 4.35 ± 0.10a | 1.07 ± 0.05b | 21.75 ± 0.54b | 8.21 ± 0.45h | 295.15 ± 7.76ef |
| C. atrosanguinea | 3.14 ± 0.18gh | 0.79 ± 0.14g | 15.67 ± 1.10f | 15.63 ± 0.75de | 355.73 ± 2.76b |
| C. persica | 3.48 ± 0.07f | 0.87 ± 0.07f | 17.40 ± 0.58e | 16.36 ± 0.35cd | 282.82 ± 5.54g |
| C. szovitsii | 3.12 ± 0.14h | 0.78 ± 0.21gh | 15.59 ± 0.76f | 17.19 ± 0.87bc | 366.22 ± 6.76b |
| C. pseudoheterophylla | 4.03 ± 0.18b | 1.04 ± 0.09c | 20.24 ± 0.41c | 12.61 ± 0.35f | 316.95 ± 8.23d |
| Significant level | *** | *** | *** | *** | *** |
| Species | Phytochemical Traits | ||
|---|---|---|---|
| TPC a (mg GAE/g DW) | TFC b (mg QUE/g DW) | Antioxidant Activity (mmol Fe++/g DW) | |
| C. pentagyna | 69.12 ± 0.83a | 5.64 ± 0.14bc | 1.84 ± 0.21a |
| C. monogyna | 35.85 ± 0.25fg | 5.77 ± 0.09b | 0.93 ± 0.08e |
| C. pseudomelanocarpa | 65.06 ± 0.67b | 5.18 ± 0.17d | 1.10 ± 0.21d |
| C. songarica | 36.87 ± 0.52f | 5.47 ± 0.19c | 0.73 ± 0.12f |
| C. azarolus var. aronia | 27.09 ± 0.63i | 2.89 ± 0.14g | 0.79 ± 0.10ef |
| C. azarolus var. pontica | 23.89 ± 0.08j | 2.74 ± 0.12g | 0.72 ± 0.06f |
| C. sakranensis | 31.22 ± 0.11h | 3.47 ± 0.08f | 0.51 ± 0.09gh |
| C. turkestanica | 21.19 ± 0.10j | 3.21 ± 0.14f | 0.65 ± 0.03fg |
| C. meyeri | 58.17 ± 0.82d | 6.08 ± 0.25a | 1.27 ± 0.21c |
| C. orientalis | 40.04 ± 0.25e | 3.29 ± 0.17f | 0.45 ± 0.14hi |
| C. curvisepala | 36.87 ± 0.57f | 4.29 ± 0.11e | 0.68 ± 0.12f |
| C. atrosanguinea | 61.60 ± 0.52c | 4.37 ± 0.04e | 1.44 ± 0.21b |
| C. persica | 31.09 ± 0.88h | 3.21 ± 0.17f | 0.32 ± 0.18i |
| C. szovitsii | 33.50 ± 0.33gh | 2.44 ± 0.12h | 0.66 ± 0.08fg |
| C. pseudoheterophylla | 59.25 ± 0.64cd | 4.42 ± 0.28e | 1.12 ± 0.18cd |
| Significant level | *** | *** | *** |
| Fruit Phenolic Compounds (mg/g DW) | |||||||
|---|---|---|---|---|---|---|---|
| Species | Chlorogenic acid | Vitexin 2-O-rhamnoside | Vitexin | Rutin | Hyperoside | Isoquercetin | Quercetin |
| C. pentagyna | 0.50 ± 0.06de | - | 0.07 ± 0.02e-g | 1.27 ± 0.08b | 2.41 ± 0.04b | 1.18 ± 0.07b | 0.04 ± 0.00a |
| C. monogyna | 0.40 ± 0.04ef | - | 0.18 ± 0.05bc | - | 1.15 ± 0.07i | 0.68 ± 0.04d-f | 0.05 ± 0.00a |
| C. pseudomelanocarpa | 1.16 ± 0.06a | - | 0.15 ± 0.04b-e | 2.68 ± 0.07a | 2.11 ± 0.06cd | 1.56 ± 0.07a | 0.04 ± 0.01a |
| C. songarica | 0.94 ± 0.03b | - | 0.13 ± 0.05c-f | 0.24 ± 0.05de | 1.74 ± 0.03e | 0.76 ± 0.08d | 0.05 ± 0.01a |
| C. azarolus var. aronia | 0.51 ± 0.05d | - | 0.09 ± 0.01d-f | 0.15 ± 0.04fg | 1.05 ± 0.07i | 0.59 ± 0.06ef | 0.05 ± 0.02a |
| C. azarolus var. pontica | 0.71 ± 0.04c | - | 0.11 ± 0.00 c-f | 0.16 ± 0.07e-g | 1.07 ± 0.05i | 0.61 ± 0.04ef | 0.05 ± 0.02a |
| C. sakranensis | 0.03 ± 0.02i | - | 0.06 ± 0.02fg | - | - | 0.24 ± 0.08g | 0.03 ± 0.02a |
| C. turkestanica | 0.40 ± 0.05ef | - | 0.16 ± 0.07b-d | 0.21 ± 0.07d-f | 1.50 ± 0.08g | 0.58 ± 0.05f | 0.05 ± 0.01a |
| C. meyeri | 0.06 ± 0.00i | - | 0.08 ± 0.06d-g | 0.28 ± 0.08d | 2.94 ± 0.02a | 1.59 ± 0.06a | 0.06 ± 0.01a |
| C. orientalis | 0.77 ± 0.06c | - | - | 0.39 ± 0.03c | 1.62 ± 0.04f | 0.78 ± 0.08d | 0.03 ± 0.02a |
| C. curvisepala | 1.04 ± 0.05b | 0.08 ± 0.02b | 0.22 ± 0.05b | 0.24 ± 0.03de | 2.21 ± 0.05c | 0.65 ± 0.06ef | 0.05± 0.02a |
| C. atrosanguinea | 0.51 ± 0.03d | - | 0.19 ± 0.07bc | 0.23 ± 0.04d-f | 2.51 ± 0.03b | 1.22 ± 0.03b | 0.05 ± 0.02a |
| C. persica | 0.44 ± 0.06ef | - | 0.08 ± 0.09d-g | 0.12 ± 0.07g | 1.32 ± 0.08h | 0.70 ± 0.05de | 0.04 ± 0.01a |
| C. szovitsii | 0.39 ± 0.08fg | - | 0.31 ± 0.06a | 0.38 ± 0.06c | 0.87 ± 0.09j | 0.34 ± 0.07g | 0.04 ± 0.02a |
| C. pseudoheterophylla | 0.32 ± 0.04fg | 0.17 ± 0.09a | 0.07 ± 0.03e-g | 0.23 ± 0.03d-f | 2.05 ± 0.05d | 0.98 ± 0.04c | 0.04 ± 0.01a |
| Significant level | *** | *** | *** | *** | *** | *** | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. https://doi.org/10.3390/foods9040436
Alirezalu A, Ahmadi N, Salehi P, Sonboli A, Alirezalu K, Mousavi Khaneghah A, Barba FJ, Munekata PES, Lorenzo JM. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods. 2020; 9(4):436. https://doi.org/10.3390/foods9040436
Chicago/Turabian StyleAlirezalu, Abolfazl, Nima Ahmadi, Peyman Salehi, Ali Sonboli, Kazem Alirezalu, Amin Mousavi Khaneghah, Francisco J. Barba, Paulo E.S. Munekata, and Jose M. Lorenzo. 2020. "Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications" Foods 9, no. 4: 436. https://doi.org/10.3390/foods9040436
APA StyleAlirezalu, A., Ahmadi, N., Salehi, P., Sonboli, A., Alirezalu, K., Mousavi Khaneghah, A., Barba, F. J., Munekata, P. E. S., & Lorenzo, J. M. (2020). Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods, 9(4), 436. https://doi.org/10.3390/foods9040436