Antimicrobial Activity of Selected Banana Cultivars Against Important Human Pathogens, Including Candida Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extract Preparation
2.3. Antimicrobial Activity
2.3.1. Microorganisms
2.3.2. Antibacterial Assay (Broth Microdilution Method) and Determination of Inhibitory Concentration (IC50)
2.3.3. Anti-Candida Activity and Determination of Biofilm Inhibitory Concentration (BIC50)
2.4. Determination of Total Phenolic Content
2.5. Genetic Relationship Analysis Using Distance-Based Methods
2.6. Statistical Analysis
3. Results
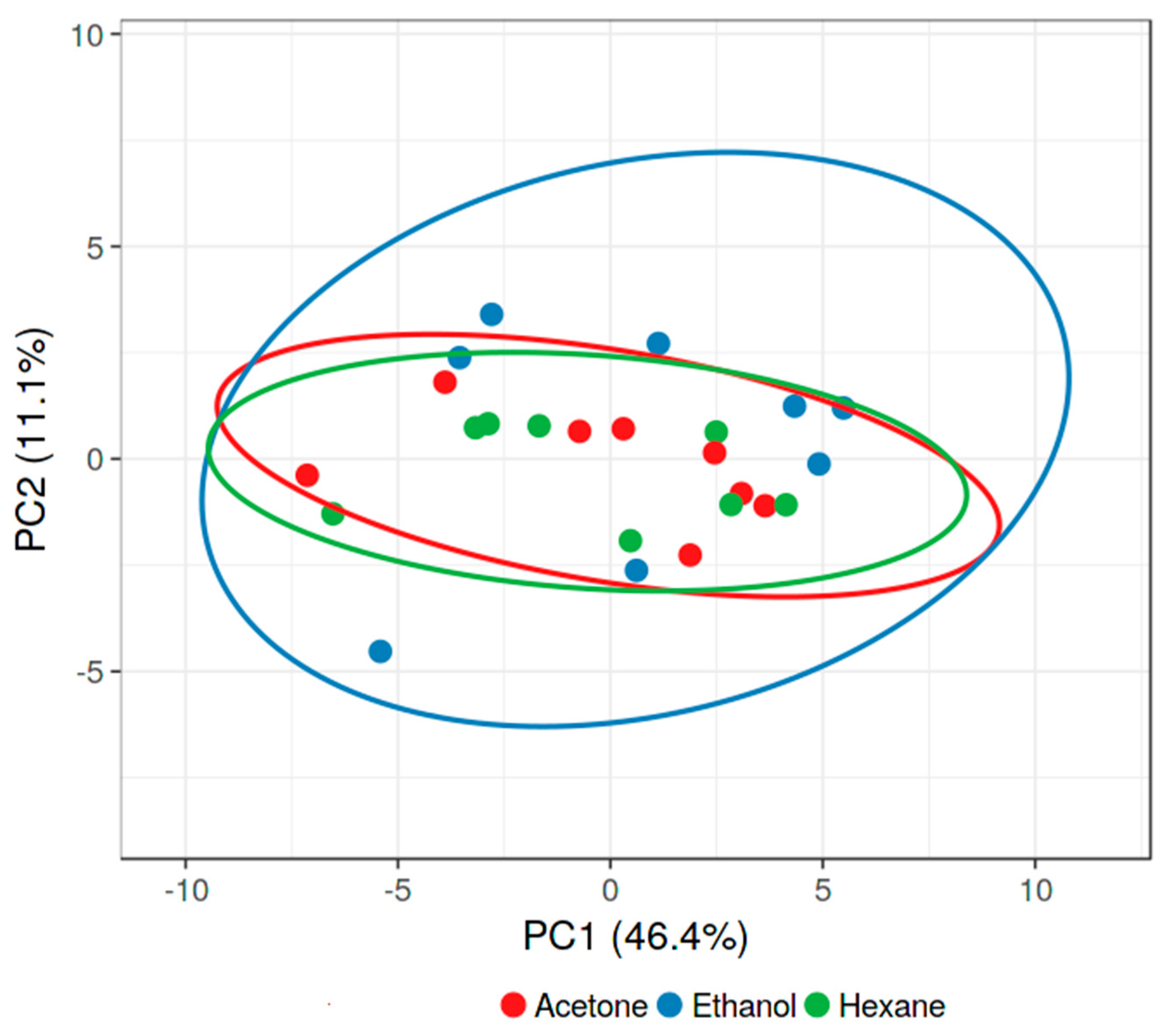
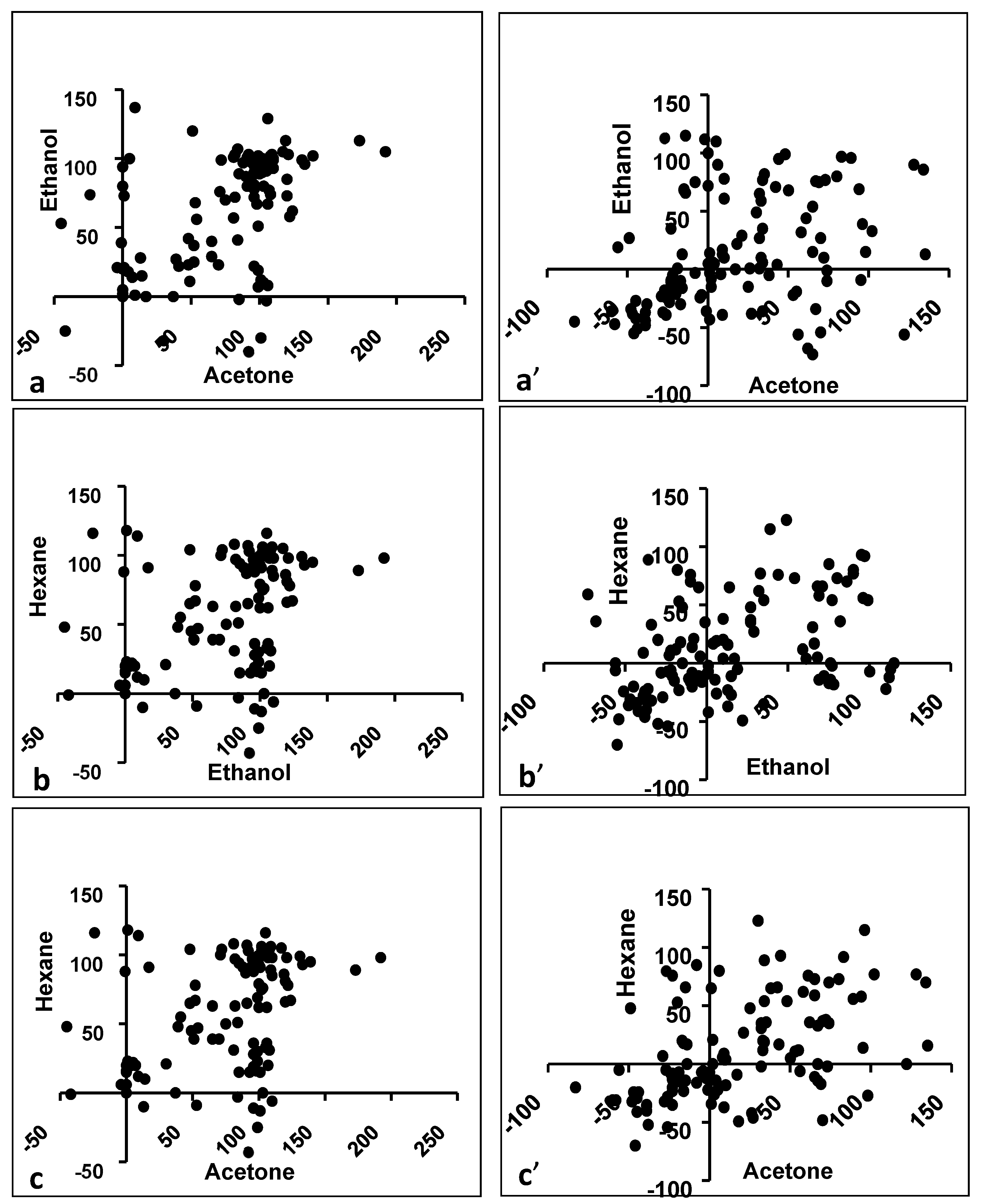
3.1. Effect of Solvent
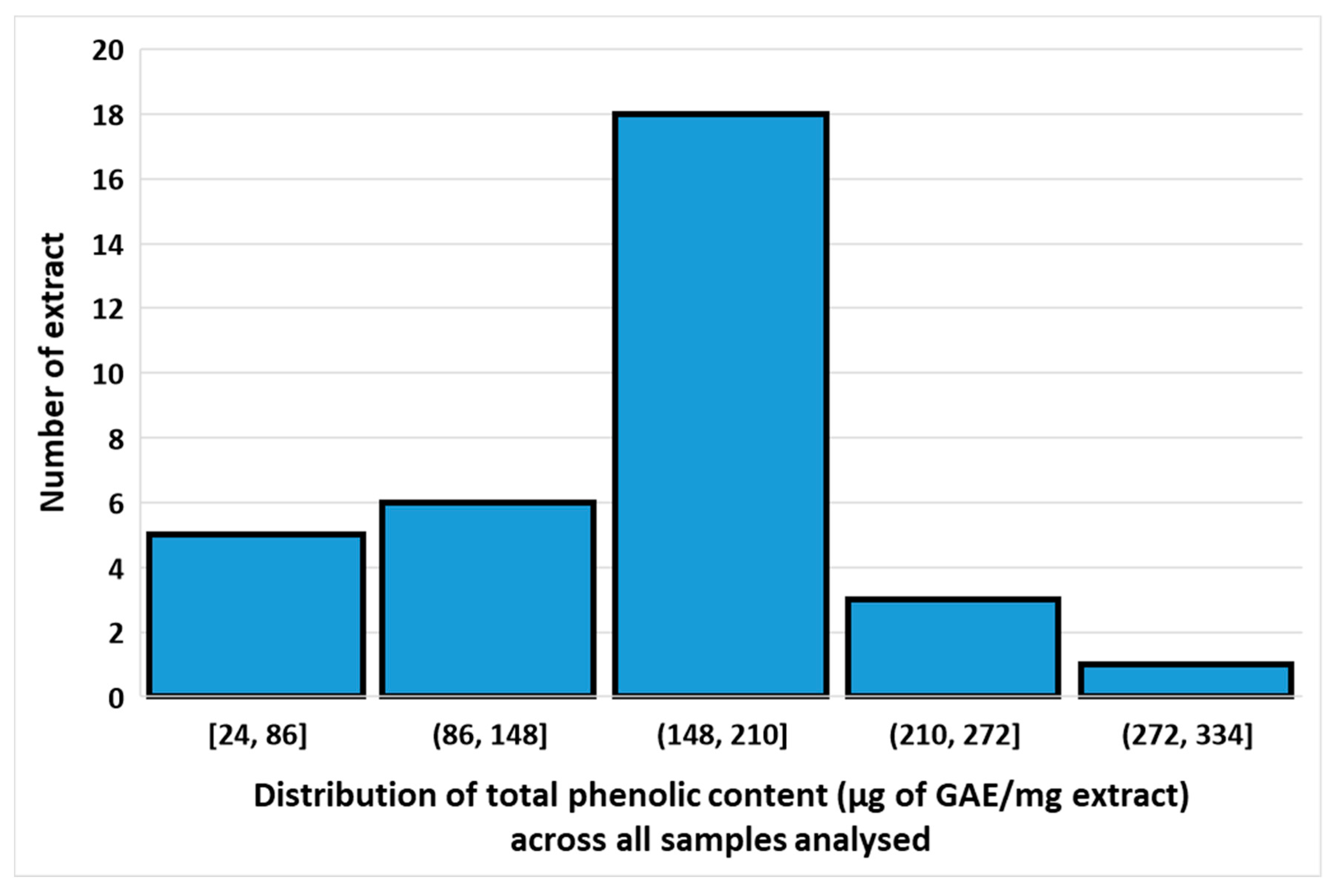
3.2. Total Phenolic Contents of Different Extracts
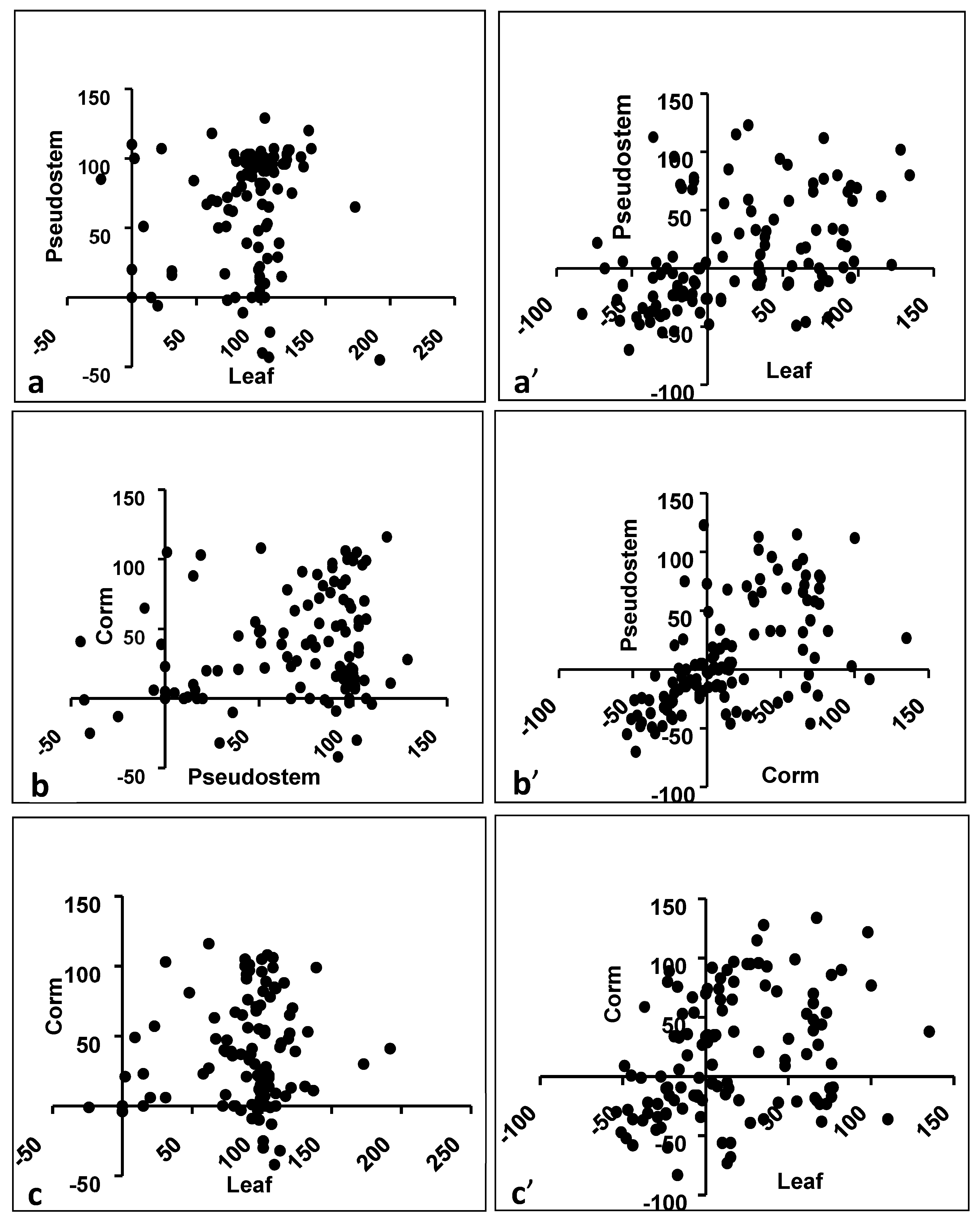
3.3. Influence of Plant Part for Gram-Positive Activity
3.4. Effect of Solvent for Activity Against Gram-Positives
3.5. Effect of Plant Parts for Activity Against Gram-Negatives
3.6. Effect of Solvent for Activity Against Gram-Negatives
3.7. Effect of Bacterial Strain
3.8. Study of Antibacterial IC50 Values
3.9. Antifungal Activity Against Candida Biofilm
4. Discussion
4.1. Microbial Strains Used
4.2. Effect of Solvent on Antibacterial Activity
4.3. Aqueous Extract Problems
4.4. Effect of Plant Part on Antibacterial Activity
4.5. Antimicrobial Activity Patterns and Their Implications for Bioactive Compounds
4.6. Effect of Cultivar on Antibacterial Activity
4.7. Role of Phenolic Compounds in Observed Biological Effects
4.8. Application of Banana in the Food Industry and Beyond
4.9. Strengths and Limitations of Our Study
4.10. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Corbo, M.R.; Speranza, B.; Filippone, A.; Granatiero, S.; Conte, A.; Sinigaglia, M.; Del Nobile, M.A. Study on the synergic effect of natural compounds on the microbial quality decay of packed fish hamburger. Int. J. Food Microbiol. 2008, 127, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste; Working paper; World Research Institute: Washington, DC, USA, 2013; pp. 1–40. [Google Scholar]
- Panda, S.K. Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 2014, 151, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China. Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Passo Tsamo, C.V.; Herent, M.-F.; Tomekpe, K.; Happi Emaga, T.; Quetin-Leclercq, J.; Rogez, H.; Larondelle, Y.; Andre, C.M. Effect of boiling on phenolic profiles determined using HPLC/ESI-LTQ-Orbitrap-MS, physico-chemical parameters of six plantain banana cultivars (Musa sp). J. Food Compos. Anal. 2015, 44, 158–169. [Google Scholar] [CrossRef]
- Pereira, A.; Maraschin, M. Banana (Musa spp) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Mathew, N.S.; Negi, P.S. Traditional uses, phytochemistry and pharmacology of wild banana (Musa acuminata Colla): A review. J. Ethnopharmacol. 2017, 196, 124–140. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Panda, S.K.; Patra, N.; Sahoo, G.; Bastia, A.K.; Dutta, S.K. Anti-diarrheal activities of medicinal plants of Similipal Biosphere Reserve, Odisha, India. Int. J. Med. Aromatic Plants 2012, 2, 123–134. [Google Scholar]
- Sampath Kumar, K.P.; Bhowmik, D.; Duraivel, S.; Umadevi, M. Traditional and medicinal uses of banana. J. Pharmacogn. Phytochem. 2012, 3, 51–63. [Google Scholar]
- Panda, S.K.; Brahma, S.; Dutta, S.K. Selective antifungal action of crude extracts of Cassia fistula L.: A preliminary study on Candida and Aspergillus species. Malays. J. Microbiol. 2010, 6, 62–68. [Google Scholar]
- Onyenekwe, P.C.; Okereke, O.E.; Owolewa, S.O. Phytochemical screening and effect of Musa paradisiaca stem extrude on rat haematological parameters. Curr. Res. J. Biol. Sci. 2013, 5, 26–29. [Google Scholar] [CrossRef]
- Gore, M.A.; Akolekar, D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burn. J. Int. Soc. Burn Inj. 2003, 29, 487–492. [Google Scholar] [CrossRef]
- Guenova, E.; Hoetzenecker, W.; Kisuze, G.; Teske, A.; Heeg, P.; Voykov, B.; Hoetzenecker, K.; Schippert, W.; Moehrle, M. Banana leaves as an alternative wound dressing. Dermatol. Surg. 2013, 39, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Asuquo, E.G.; Udobi, C.E. Antibacterial and toxicity studies of the ethanol extract of Musa paradisiaca leaf. Cogent Biol. 2016, 2, 1219248. [Google Scholar] [CrossRef]
- Hegde, S.; Nair, L.P.; Chandran, H.; Irshad, H. Traditional Indian way of eating—An overview. J. Ethn. Foods 2018, 5, 20–23. [Google Scholar] [CrossRef]
- Richter, E.R.; Vore, L.A. Antimicrobial activity of banana puree. Food Microbiol. 1989, 6, 179–187. [Google Scholar] [CrossRef]
- Ahmad, I.; Beg, A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Hashinaga, F. Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am. J. Biochem. Biotechnol. 2005, 1, 125–131. [Google Scholar] [CrossRef]
- Alisi, C.; Nwanyanwu, C.; Akujobi, C.; Ibegbulem, C. Inhibition of dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (Var Sapientum). Afr. J. Biotechnol. 2008, 7, 1821–1825. [Google Scholar] [CrossRef]
- Jahan, M.; Mk, W.; Khatoon, F. Concentration influence on antimicrobial activity of banana blossom extract-incorporated chitosan-polyethylene glycol (CS-PEG) blended film. J. Chem. Pharm. Res 2010, 2, 373–378. [Google Scholar]
- Chabuck, Z.A.G.; Al-Charrakh, A.H.; Hindi, N.K.K.; Hindi, S.K.K. Antimicrobial effect of aqueous banana peel extract. Iraq. Res. Gate Pharm. Sci. 2013, 1, 73–75. [Google Scholar]
- Kapadia, S.P.; Pudakalkatti, P.S.; Shivanaikar, S. Detection of antimicrobial activity of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2015, 6, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Das, R.; Leyssen, P.; Neyts, J.; Luyten, W. Assessing medicinal plants traditionally used in the Chirang Reserve Forest, Northeast India for antimicrobial activity. J. Ethnopharmacol. 2018, 225, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Padhi, L.; Leyssen, P.; Liu, M.; Neyts, J.; Luyten, W. Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the Similipal Biosphere Reserve, Odisha, India. Front. Pharmacol. 2017, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Luyten, W. Antimicrobial activity of select edible plants from Odisha, India against food-borne pathogens. LWT 2019, 113, 108246. [Google Scholar] [CrossRef]
- Kerkoub, N.; Panda, S.K.; Yang, M.-R.; Lu, J.-G.; Jiang, Z.-H.; Nasri, H.; Luyten, W. Bioassay-guided isolation of anti-candida biofilm compounds from methanol extracts of the aerial parts of Salvia officinalis (Annaba, Algeria). Front. Pharmacol. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Christelová, P.; De Langhe, E.; Hřibová, E.; Čížková, J.; Sardos, J.; Hušáková, M.; Van den houwe, I.; Sutanto, A.; Kepler, A.K.; Swennen, R.; et al. Molecular and cytological characterization of the global Musa germplasm collection provides insights into the treasure of banana diversity. Biodivers. Conserv. 2017, 26, 801–824. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Karthik, M.; Pushpakanth, P.; Krishnamoorthy, R.; Senthilkumar, M. Endophytic bacteria associated with banana cultivars and their inoculation effect on plant growth. J. Hortic. Sci. Biotechnol. 2017, 92, 568–576. [Google Scholar] [CrossRef]
- Karuppiah, P.; Mustaffa, M. Antibacterial and antioxidant activities of Musa sp. leaf extracts against multidrug resistant clinical pathogens causing nosocomial infection. Asian Pac. J. Trop. Biomed. 2013, 3, 737–742. [Google Scholar] [CrossRef]
- Sahaa, R.K.; Acharyaa, S.; Shovon, S.S.H.; Royb, P. Medicinal activities of the leaves of Musa sapientum var. sylvesteris in vitro. Asian Pac. J. Trop. Biomed. 2013, 3, 476–482. [Google Scholar] [CrossRef]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.-H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Padhi, L.P.; Mohanty, G. Antibacterial activities and phytochemical analysis of Cassia fistula (Linn.) leaf. J. Adv. Pharm. Technol. Res. 2011, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Jain, R.; Kumar, K.; Mishra, R.; Chandy, A. Antibacterial activity and physicochemical evaluation of roots of Butea monosperma. Asian Pac. J. Trop. Biomed. 2012, 2, S881–S883. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Antibacterial and antioxidative activities of the various solvent extracts of banana (Musa paradisiaca cv. Mysore) Inflorescences. J. Ofbiological Sci. 2012, 12, 62–73. [Google Scholar]
- Joshi, A.P.K.; Rupasinghe, H.P.V.; Khanizadeh, S. Impact of drying processes on bioactive phenolics, vitamin c and antioxidant capacity of red-fleshed apple slices. J. Food Process. Preserv. 2011, 35, 453–457. [Google Scholar] [CrossRef]
- Koh, G.Y.; Chou, G.; Liu, Z. Purification of a Water Extract of Chinese Sweet Tea Plant (Rubus suavissimus S. Lee) by Alcohol Precipitation. J. Agric. Food Chem. 2009, 57, 5000–5006. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Effect of bioclimatic area on the essential oil composition and antibacterial activity of Rosmarinus officinalis L. Food Control 2013, 30, 463–468. [Google Scholar] [CrossRef]
- Venkatesh, K.V.; Girish, K.K.; Pradeepa, K.; Santosh, K.S.R. Antibacterial activity of ethanol extract of Musa paradisiaca cv. Puttabale and Musa acuminate cv. grand naine. Asian J. Pharm. Clin. Res. 2013, 6, 169–172. [Google Scholar]
- Siddique, S.; Nawaz, S.; Muhammad, F.; Akhtar, B.; Aslam, B. Phytochemical screening and in-vitro evaluation of pharmacological activities of peels of Musa sapientum and Carica papaya fruit. Nat. Prod. Res. 2018, 32, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Oliveira, L.; Vilela, C.; Domingues, R.M.; Freitas, N.; Cordeiro, N.; Freire, C.S.R.; Silvestre, A.J.D. High valuable compounds from the unripe peel of several Musa species cultivated in Madeira Island (Portugal). Ind. Crop. Prod. 2013, 42, 507–512. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of Beta-sitosterol-A Review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite 2018, 25, 10. [Google Scholar] [CrossRef]
- World Health Organization. Fruit and Vegetables for Health: Report of the Joint FAO; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Vasco, C. Phenolic Compounds in Ecuadorian Fruits; Tesis doctoral Swedish University of Agricultural Sciences: Uppsala, Sweden, 2009. [Google Scholar]
- Borrero, A.E.; Santacruz, S. Phenolic compounds from the peel of Musa cavendish, Musa acuminata and Musa cavandanaish. Rev. Politécnica 2017, 38, 69–74. [Google Scholar]
- Tsamo, C.V.P.; Herent, M.F.; Tomekpe, K.; Emaga, T.H.; Quetin-Leclercq, J.; Rogez, H.; Larondelle, Y.; Andre, C. Phenolic profiling in the pulp and peel of nine plantain cultivars (Musa sp.). Food Chem. 2015, 167, 197–204. [Google Scholar] [CrossRef]
- Aquino, C.F.; Salomão, L.C.C.; Ribeiro, S.; Rocha, M.; Siqueira, D.L.D.; Cecon, P.R. Carbohydrates, phenolic compounds and antioxidant activity in pulp and peel of 15 banana cultivars. Rev. Bras. Frutic. 2016, 38, e-090. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2014, 51, 3527–3545. [Google Scholar] [CrossRef] [PubMed]
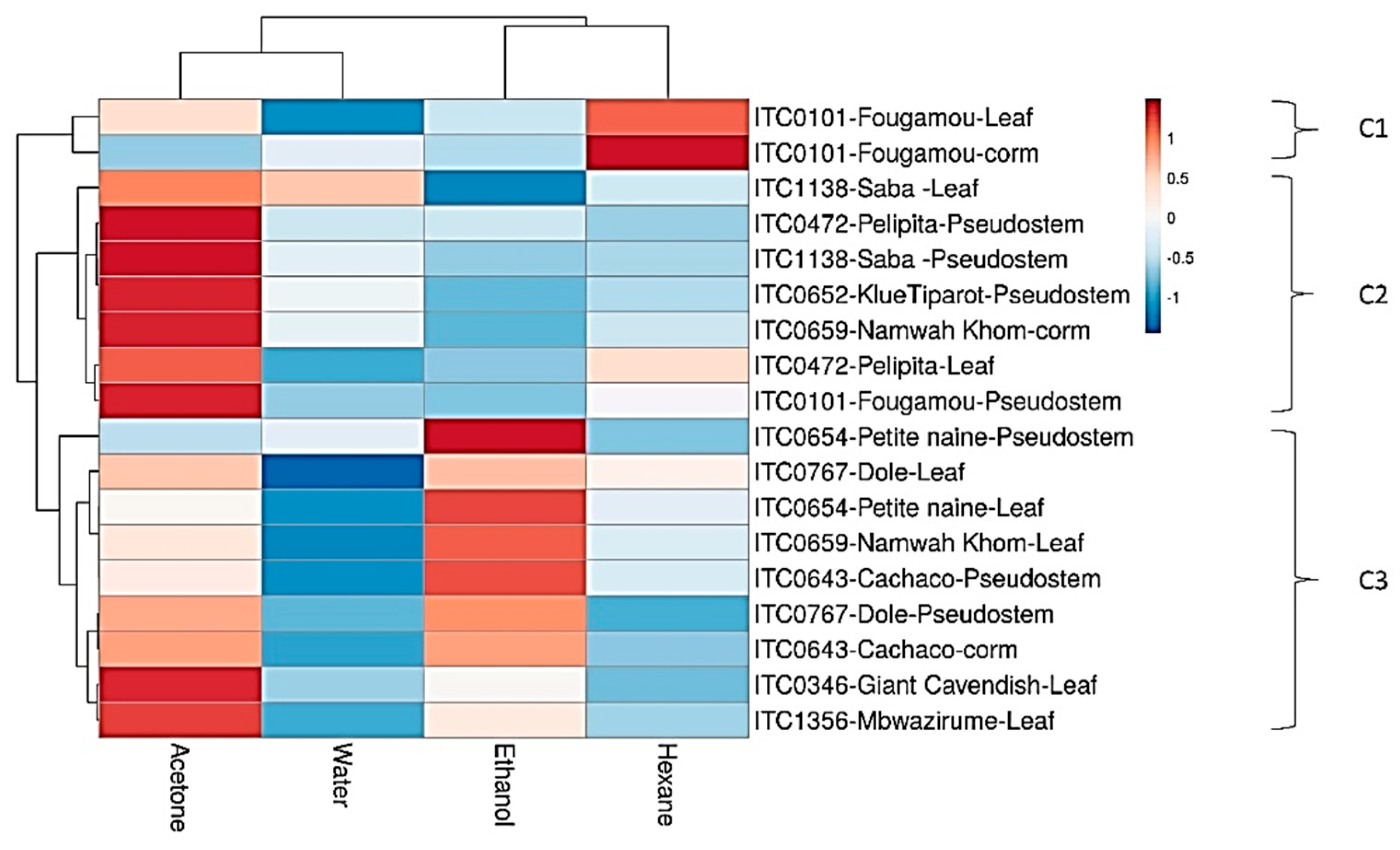
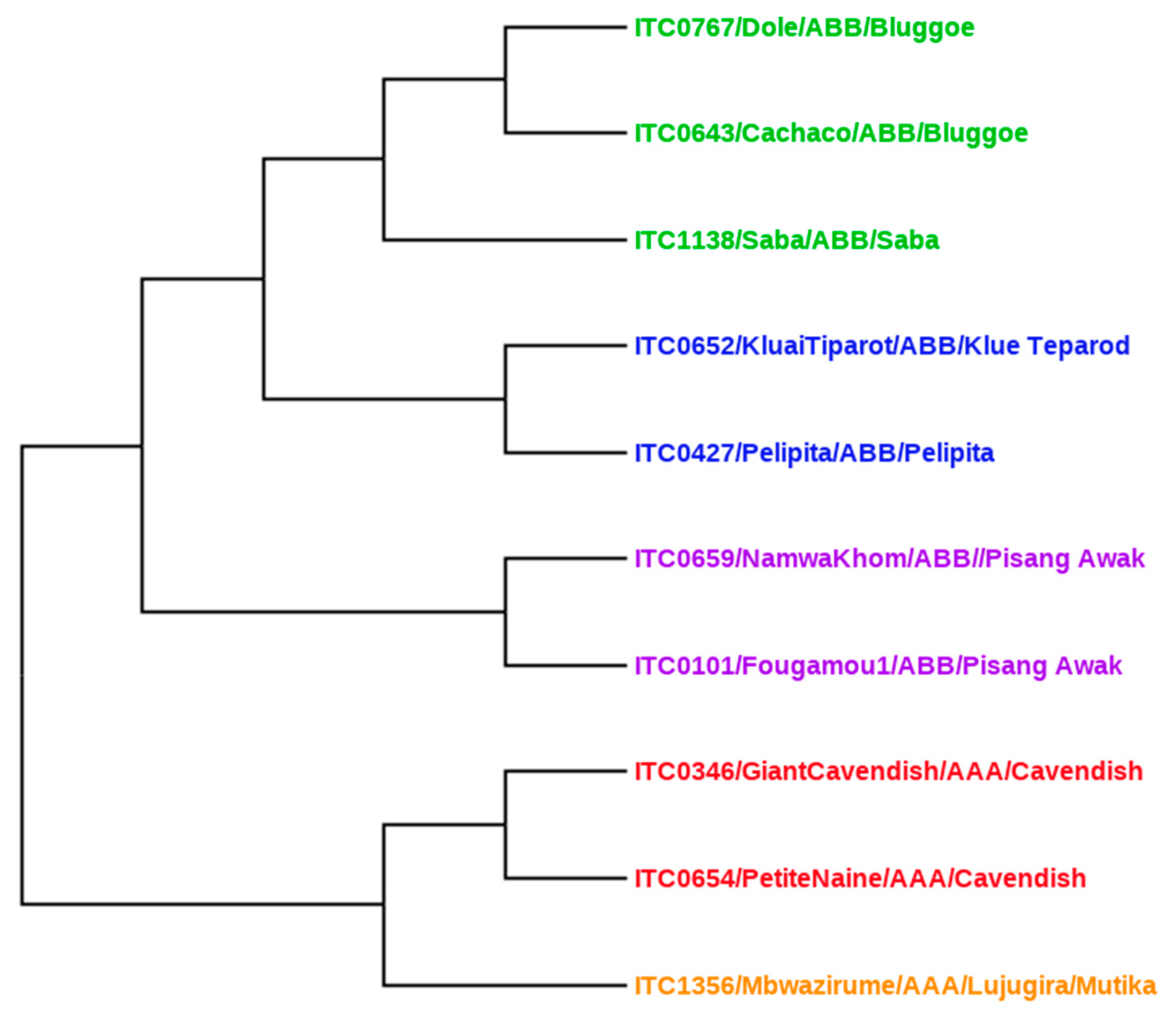
| ITC * | Cultivar | Genome | Subgroup |
|---|---|---|---|
| ITC0767 | Dole | ABB | Bluggoe |
| ITC0643 | Cachaco | ABB | Bluggoe |
| ITC1138 | Saba | ABB | Saba |
| ITC0652 | Kluai Tiparot | ABB | unknown |
| ITC0472 | Pelipita | ABB | unknown |
| ITC0659 | Namwah Khom | ABB | Pisang Awak |
| ITC0101 | Fougamou | ABB | Pisang Awak |
| ITC0654 | Petite Naine | AAA | Cavendish |
| ITC0346 | Giant Cavendish | AAA | Cavendish |
| ITC1356 | Mbwazirume | AAA | Mutika/Lujugira |
| ITC/Cultivar Name/Plant Part | B. cereus | M. luteus | S. aureus | S. faecalis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | E | H | A | E | H | A | E | H | A | E | H | |
| ITC0346-Giant Cavendish-leaf | 101 | 99 | 99 | 105 | 112 | 98 | 80 | 0 | 0 | 96 | 133 | 93 |
| ITC1356-Mbwazirume-leaf | 99 | 131 | 99 | 113 | 113 | 89 | 101 | 109 | 106 | 100 | 102 | 106 |
| ITC0659-Namwah Khom-leaf | 103 | 121 | 98 | 89 | 100 | 62 | 98 | 93 | 15 | 90 | 102 | 75 |
| ITC1138-Saba-leaf | 97 | 100 | 79 | 85 | 120 | 81 | 101 | 81 | 31 | 90 | 96 | 88 |
| ITC0472-Pelipita-leaf | 102 | 139 | 95 | 73 | 120 | 66 | 103 | 92 | 103 | 99 | 101 | 91 |
| ITC0652-Kluai Tiparot-leaf | 99 | 110 | 98 | 62 | 124 | 67 | 100 | 110 | 98 | 93 | 110 | 85 |
| ITC0643-Cachaco-leaf | 105 | 117 | 105 | 74 | −24 | 116 | 99 | 72 | 104 | 74 | −24 | 116 |
| ITC0767-Dole-leaf | 97 | 88 | 91 | 58 | 122 | 78 | 101 | 91 | 107 | 58 | 122 | 78 |
| ITC1356-Mbwazirume-pseudostem | 99 | 101 | 15 | 29 | 65 | 39 | −40 | 92 | −43 | 80 | 91 | 65 |
| ITC0101-Fougamou-pseudostem | 101 | 100 | 93 | 78 | 96 | −11 | 100 | 110 | −6 | 81 | 96 | 89 |
| ITC0659-Namwah Khom-pseudostem | 129 | 106 | 36 | 73 | 1 | 118 | 94 | 0 | 0 | 103 | 82 | 63 |
| ITC1138-Saba-pseudostem | 99 | 105 | 103 | 80 | 103 | 76 | 67 | 98 | 16 | 88 | 95 | 97 |
| ITC0472-Pelipita-pseudostem | 95 | 107 | 98 | 51 | 99 | 69 | 10 | 103 | 0 | 82 | 94 | 89 |
| ITC0652-Kluai Tiparot-pseudostem | 12 | 101 | 99 | 70 | 75 | 50 | 77 | 107 | 20 | 87 | 90 | 87 |
| ITC0643-Cachaco-pseudostem | 95 | 96 | 28 | −2 | 85 | 15 | 0 | 17 | 91 | −2 | 85 | 15 |
| ITC0767-Dole-pseudostem | 98 | 102 | 103 | 67 | 106 | 62 | 102 | 99 | −25 | 67 | 106 | 62 |
| ITC0767-Dole-corm | −3 | 105 | 33 | 23 | 70 | 39 | −30 | 101 | −13 | 23 | 70 | 39 |
| Parts/Solvents | Parameters | Gram-Positive | Gram-Negative |
|---|---|---|---|
| Leaf vs. Pseudostem | Spearman r | 0.2345 | 0.4936 |
| 95% confidence interval | 0.0523 to 0.4016 | 0.3401 to 0.6215 | |
| p value (two-tailed) | 0.0099 | <0.0001 | |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes | |
| Pseudostem vs. Corm | Spearman r | 0.2439 | 0.6321 |
| 95% confidence interval | 0.0623 to 0.4100 | 0.5068 to 0.7313 | |
| p value (two-tailed) | 0.0073 | <0.0001 | |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes | |
| Leaf vs. Corm | Spearman r | 0.1172 | 0.3396 |
| 95% confidence interval | −0.0687 to 0.2953 | 0.1655 to 0.4931 | |
| p value (two-tailed) | 0.2022 | 0.0001 | |
| Is the correlation significant? (alpha = 0.05) | No | Yes | |
| Acetone vs. Ethanol | Spearman r | 0,5105 | 0.4070 |
| 95% confidence interval | 0.3599 to 0.6351 | 0.2406 to 0.5502 | |
| p value (two-tailed) | <0.0001 | <0.0001 | |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes | |
| Ethanol vs. Hexane | Spearman r | 0.3523 | 0.4316 |
| 95% confidence interval | 0.1795 to 0.5040 | 0.2685 to 0.5706 | |
| p value (two-tailed) | <0.0001 | <0.0001 | |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes | |
| Acetone vs. Hexane | Spearman r | 0.5198 | 0.5326 |
| 95% confidence interval | 0.3709 to 0.6427 | 0.3861 to 0.6529 | |
| p value (two-tailed) | <0.0001 | <0.0001 | |
| Is the correlation significant? (alpha = 0.05) | Yes | Yes |
| ITC/Cultivar Name/Plant Part | A. hydrophila | E. coli | S. enterica | S. sonnei | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | E | H | A | E | H | A | E | H | A | E | H | |
| ITC0346-Giant Cavendish-leaf | −21 | −20 | −15 | 34 | 77 | 54 | 134 | 86 | 70 | 34 | 35 | 54 |
| ITC1356-Mbwazirume-leaf | −17 | −10 | −16 | −83 | −45 | −20 | 48 | 99 | 54 | 67 | −34 | 33 |
| ITC0654-Petite naine-leaf | 95 | −9 | 14 | −38 | −30 | −52 | 38 | −5 | 65 | 74 | −1 | 35 |
| ITC0101-Fougamou-leaf | −23 | −9 | −8 | −56 | 19 | −5 | 128 | 90 | 77 | 56 | −56 | −6 |
| ITC1138-Saba-leaf | −23 | −9 | −20 | −28 | −37 | −32 | 72 | 10 | 38 | 122 | −56 | 0 |
| ITC0472-Pelipita-leaf | −23 | −10 | 76 | −1 | −36 | −22 | 32 | 27 | 35 | 6 | 90 | 82 |
| ITC0652-KlueTiparot-leaf | −20 | −17 | 53 | 1 | −43 | −34 | 83 | 97 | 92 | 65 | −73 | 59 |
| ITC0643-Cachaco-leaf | −16 | −16 | 18 | 9 | −39 | 9 | 96 | 39 | 115 | 34 | −36 | 89 |
| ITC0767-Dole-leaf | −27 | −18 | 81 | −58 | −47 | −31 | 44 | 95 | 93 | 62 | −68 | 36 |
| ITC0346-Giant Cavendish-pseudostem | −23 | −17 | −23 | −2 | 112 | −12 | 81 | 81 | 73 | 1 | −3 | −13 |
| ITC1356-Mbwazirume-pseudostem | −24 | −28 | −8 | −39 | −48 | −36 | 94 | 69 | 58 | 4 | 5 | −14 |
| ITC0654-Petite naine-pseudostem | −8 | 75 | 85 | −46 | −55 | −70 | 27 | −38 | −46 | 0 | 5 | −14 |
| ITC0101-Fougamou-pseudostem | −23 | −11 | −13 | −14 | 115 | 0 | 102 | 33 | 77 | 2 | −15 | 0 |
| ITC0659-Namwah Khom-pseudostem | −15 | 68 | 66 | −27 | 113 | −5 | 30 | 49 | 123 | 89 | 96 | 56 |
| ITC0346-Giant Cavendish-corm | 55 | −19 | 12 | 0 | 100 | −7 | 67 | 76 | 0 | −19 | 1 | −7 |
| ITC0654-Petite naine-corm | 25 | −15 | 48 | 70 | −54 | −48 | 135 | 13 | 16 | 8 | −4 | 6 |
| ITC1138-Saba corm | 73 | 77 | −2 | −47 | −38 | −24 | 43 | 4 | 17 | 98 | 15 | −27 |
| ITC0643-Cachaco-corm | −22 | −5 | −11 | −49 | 27 | 48 | 32 | 65 | 31 | 5 | 110 | −22 |
| Cultivar, Part, Solvent | Bacteria (IC50 Concentration in μg/mL) |
|---|---|
| ITC1356-Mbwazirume-ethanol | SA (511), BC (130), ML (88), SF (571) |
| ITC1356-Mbwazirume-acetone | BC (53), ML (33), SF (83), SS (152) |
| ITC0101-Fougamou-leaf -acetone | BC (315), ML (511), SF (271), SS (99) |
| ITC0659-Namwah Khom-acetone | SA (1104), BC (190), ML (45), SF (31) |
| ITC1138-Saba-acetone | SA (373), BC (99), ML (56), SF (37) |
| ITC0472-Pelipita-acetone | SA (442), BC (116), ML (52), SF (58) |
| ITC0652-KlueTiparot-ethanol | SA (407) |
| ITC0652-KlueTiparot-pseudostem-ethanol | AH (1354) |
| ITC0652-KlueTiparot-acetone | SA (433), BC (190), ML (31), SF (28) |
| ITC0643-Cachaco-leaf-ethanol | SA (1117), BC (159), ML (287), SF (704), EC (1787) |
| ITC0767-Dole-leaf-acetone | SA (580), BC (61), ML (144), SF (53), SS (107), AH (1973) |
| ITC0767-Dole pseudostem-acetone | SA (1239), BC (330), ML (121), SF (319) |
| ITC Code, Cultivar, Plant Part | Acetone | Ethanol | Hexane | Water |
|---|---|---|---|---|
| ITC0346-Giant Cavendish-Leaf | 51 | 35 | 25 | 27 |
| ITC1356-Mbwazirume-Leaf | 60 | 37 | 18 | 11 |
| ITC0654-Petite naine-Leaf | 36 | 56 | 31 | 16 |
| ITC0101-Fougamou-Leaf | 57 | 37 | 73 (220) | 22 |
| ITC0659-Namwah Khom-Leaf | 55 | 85 (31) | 34 | 3 |
| ITC1138-Saba -Leaf | 51 | 17 | 29 | 45 |
| ITC0472-Pelipita-Leaf | 72 | 22 | 52 | 16 |
| ITC0767-Dole-Leaf | 90 (71) | 92 (51) | 74 | 23 |
| ITC0654-Petite naine-Pseudostem | 24 | 76 (44) | 19 | 32 |
| ITC0101-Fougamou-Pseudostem | 66 | 22 | 36 | 23 |
| ITC1138-Saba -Pseudostem | 83 (177) | 23 | 25 | 36 |
| ITC0472-Pelipita-Pseudostem | 79 | 30 | 25 | 30 |
| ITC0652-KlueTiparot-Pseudostem | 82 (183) | 24 | 31 | 43 |
| ITC0643-Cachaco-Pseudostem | 41 | 60 | 31 | 16 |
| ITC0767-Dole-Pseudostem | 76 | 81 | 18 | 20 |
| ITC0101-Fougamou-corm | 12 | 15 | 61 | 22 |
| ITC0659-Namwah Khom-corm | 59 | 8 | 17 | 24 |
| ITC0643-Cachaco-corm | 50 | 50 | 24 | 19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouneghani, R.S.; Castro, A.H.F.; Panda, S.K.; Swennen, R.; Luyten, W. Antimicrobial Activity of Selected Banana Cultivars Against Important Human Pathogens, Including Candida Biofilm. Foods 2020, 9, 435. https://doi.org/10.3390/foods9040435
Jouneghani RS, Castro AHF, Panda SK, Swennen R, Luyten W. Antimicrobial Activity of Selected Banana Cultivars Against Important Human Pathogens, Including Candida Biofilm. Foods. 2020; 9(4):435. https://doi.org/10.3390/foods9040435
Chicago/Turabian StyleJouneghani, Ramin Saleh, Ana Hortência Fonsêca Castro, Sujogya Kumar Panda, Rony Swennen, and Walter Luyten. 2020. "Antimicrobial Activity of Selected Banana Cultivars Against Important Human Pathogens, Including Candida Biofilm" Foods 9, no. 4: 435. https://doi.org/10.3390/foods9040435
APA StyleJouneghani, R. S., Castro, A. H. F., Panda, S. K., Swennen, R., & Luyten, W. (2020). Antimicrobial Activity of Selected Banana Cultivars Against Important Human Pathogens, Including Candida Biofilm. Foods, 9(4), 435. https://doi.org/10.3390/foods9040435






