Nutritional Composition of Apis mellifera Drones from Korea and Denmark as a Potential Sustainable Alternative Food Source: Comparison Between Developmental Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Nutritional Composition of Drones of Italian Bees and Buckfast Bees
2.1.1. Sample Preparation
2.1.2. Amino Acid Analysis
2.1.3. Fatty Acid Analysis
2.1.4. Mineral Analysis
2.2. Functional Properties of Buckfast Drone Bee Ethanol Extracts
2.2.1. Sample Preparation
2.2.2. Total Polyphenol, Flavonoid, Reducing Sugar
2.2.3. Antioxidant Activity
2.2.4. Antimicrobial Activity
2.2.5. Haemolysis Activity
2.3. Statistical Analysis
3. Results and Discussion
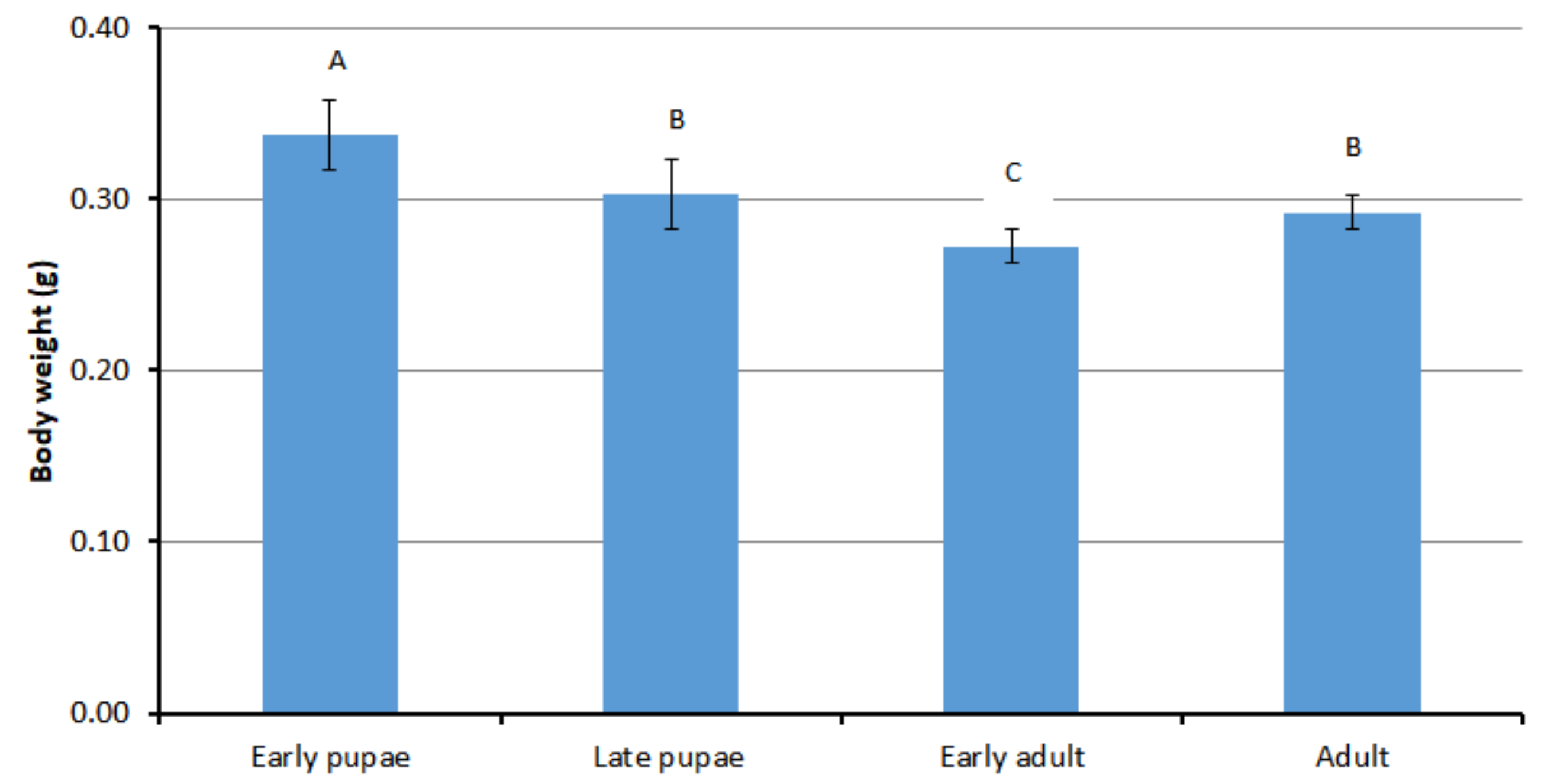
3.1. Body Weight of Different Developmental Stages of Drone
3.2. Nutritional Composition of Drone Bees
3.2.1. Amino Acid Composition
3.2.2. Fatty Acid Composition
3.2.3. Mineral Content
3.3. Functional Properties of Buckfast Honey Bee Drone Bee Ethanol Extract
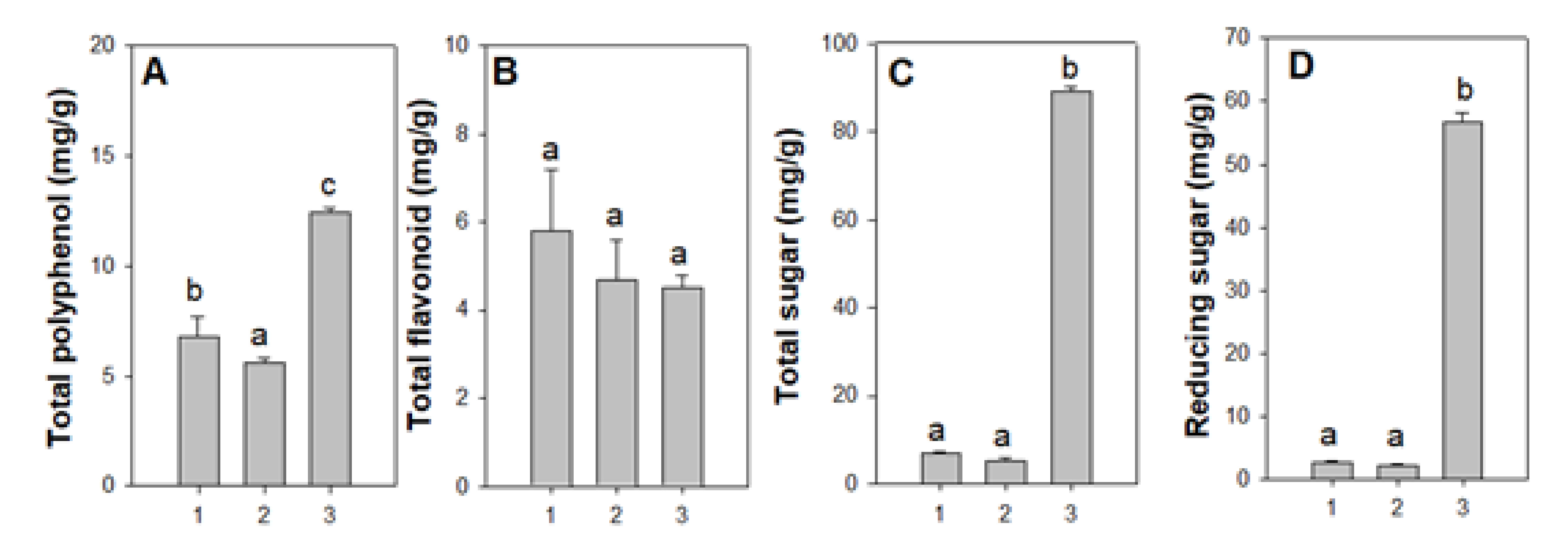
3.3.1. Total Polyphenol, Flavonoids, Reducing Sugar Content
3.3.2. Antioxidant and Antimicrobial Profiles
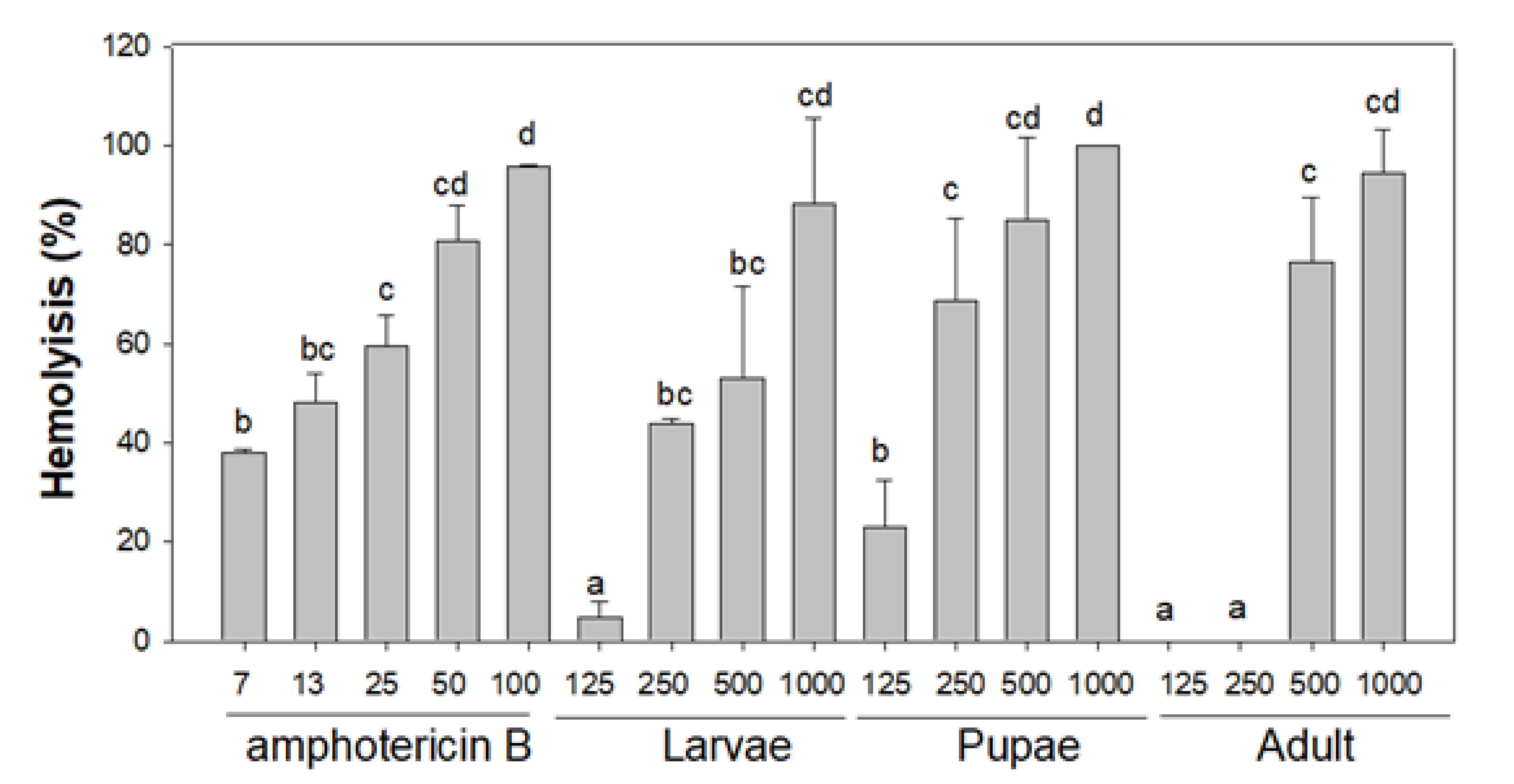
3.3.3. Haemolysis Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- Evans, J.; Alemu, M.H.; Flore, R.; Frøst, M.B.; Halloran, A.; Jensen, A.B.; Maciel-Vergara, G.; Meyer-Rochow, V.B.; Münke-Svendsen, C.; Olsen, S.B.; et al. ‘Entomophagy’: An evolving terminology in need of review. J. Insects Food Feed 2015, 1, 293–305. [Google Scholar] [CrossRef]
- Bequaert, J. Insects as food: How they have augmented the food supply of mankind in early and recent years. Nat. Hist. J. 1921, 21, 191–200. [Google Scholar]
- Bergier, E. Peuples Entomophages et Insectes Comestibles: Étude sur les Moeurs de L’homme et de L’insecte; Imprimérie Rullière Frères: Avignon, France, 1941. [Google Scholar]
- Bodenheimer, F.S. Insects as Human Food; W. Junk Publishers: The Hague, The Netherlands, 1951. [Google Scholar]
- DeFoliart, G.R. Insects as food: Why the western attitude is important. Ann. Rev. Entomol. 1999, 44, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Insects as food in Sub-Saharan Africa. Insect Sci. Appl. 2003, 23, 163–185. [Google Scholar] [CrossRef]
- Chakravorty, J.; Ghosh, S.; Meyer-Rochow, V.B. Comparative survey of entomophagy and entomotherapeutic practices in six tribes of eastern Arunachal Pradesh (India). J. Ethnobiol. Ethnomed. 2013, 9, 50. [Google Scholar] [CrossRef]
- Costa-Neto, E.M.; Dunkel, F.V. Insects as Food: History, culture, and modern use around the world. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–58. [Google Scholar] [CrossRef]
- Mitsuhashi, J. Edible Insects of the World; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Van Itterbeeck, J.; Andrianavalona, I.N.R.; Rajemison, F.I.; Rakotondrasoa, J.F.; Ralantoarinaivo, V.R.; Hugel, S.; Fisher, B.L. Diversity and use of edible grasshoppers, locusts, crickets, and katydids (Orthoptera) in Madagascar. Foods 2019, 8, 666. [Google Scholar] [CrossRef]
- Van Huis, A.; van Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible insects: Future prospects for food and feed security. In FAO Forestry Paper 171; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Jongema, Y. Worldwide List of Edible Insects 2017. Available online: https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (accessed on 15 January 2020).
- Oonincx, D.G.A.B.; Itterbeck, J.V.; Heetkamp, M.J.W.; Brand, H.V.D.; Loon, J.J.A.V.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef]
- Cherry, R.H. Use of insects by Australian Aborigines. Am. Entomol. 1991, 37, 8–13. [Google Scholar] [CrossRef]
- Murray, S.S.; Schoeninger, M.J.; Bunn, H.T.; Pickering, T.R.; Marlett, J.A. Nutritional composition of some wild plant foods and honey used by Hazda foragers of Tanzania. J. Food Comp. Anal. 2001, 13, 3–13. [Google Scholar] [CrossRef]
- Wongsiri, S.; Lekprayoon, C.; Thapa, R.; Thirakupt, K.; Rinderer, T.E.; Sylvester, H.A.; Oldroyd, B.P.; Booncham, U. Comparative biology of Apis andreniformis and Apis florea in Thailand. Bee World 1997, 78, 23–35. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Moreno, J.M.P.; Prado, E.E.; Perez, M.A.; Otero, J.L.; de Guavara, O.L. Nutritional value of edible insects from the state of Oaxaca, Mexico. J. Food Comp. Anal. 1997, 10, 142–157. [Google Scholar] [CrossRef]
- Onore, G. A brief note on edible insects in Ecuador. Ecol. Food Nutr. 1997, 36, 277–285. [Google Scholar] [CrossRef]
- Mbata, K.J. Traditional use of arthropods in Zambia. I. The food insects. Food Insects Newsl. 1995, 8, 5–7. [Google Scholar]
- Gessain, M.; Kinzler, T. Miel et Insectes à miel chez les Bassari et D’autres Populations du Sénégal Oriental [Honey and Honey Making Insects in the Bassari and Other Populations of Eastern Senegal]. In L’homme et L’annimal; Pujol, R., Ed.; Premier Colloque d’Ethnozoologie: Paris, France, 1975; pp. 247–254. [Google Scholar]
- Jensen, A.B.; Evans, J.; Jonas-Levi, A.; Benjamin, O.; Martinez, I.; Dahle, B.; Roos, N.; Lecocq, A.; Foley, K. Standard methods for Apis mellifera brood as human food. J. Apic. Res. 2019, 58, 1–28. [Google Scholar] [CrossRef]
- Lou, Z.-Y. Insects as food in China. Ecol. Food Nutr. 1997, 36, 201–207. [Google Scholar] [CrossRef]
- Evans, J.; Müller, A.; Jensen, A.B.; Dahle, B.; Flore, R.; Eilenberg, J.; Frøst, M.B. A descriptive sensory analysis of honeybee drone brood from Denmark and Norway. J. Insects Food Feed 2016, 2, 277–283. [Google Scholar] [CrossRef]
- Finke, M.D. Nutrient composition of bee brood and its potential as human food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia-Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Ghosh, S.; Chuttong, B.; Burgett, M.; Meyer-Rochow, V.B.; Jung, C. Nutritional value of brood and adult workers of the Asia honeybee species Apis cerana and Apis dorsata. In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Mariod, A.A., Ed.; Springer: Basel, Switzerland, 2020; pp. 265–273. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Charrière, J.-D.; Imdorf, A.; Bachofen, B.; Tschan, A. The removal of capped drone brood: An effective means of reducing the infestation of Varroa in honey bee colonies. Bee World 2003, 84, 117–124. [Google Scholar] [CrossRef]
- Ambüehl, D. Beezza! The kingbee Cook Book. Skyfood; Selbstverlag: Unterterzen, Switzerland, 2016. [Google Scholar]
- Lecocq, A.; Foley, K.; Jensen, A.B. Drone brood production in Danish apiaries and its potential for human consumption. J. Apic. Res. 2018, 57, 331–336. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washinton, DC, USA, 1990. [Google Scholar]
- Korean Food Standard Codex; Ministry of Food and Drug Safety: Cheongju, Korea, 2010.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.H.; Aquiar, R.S.; Siika-aho, M.; Ramos, L.P. Assessment of the enzymatic hydrolysis profile of cellulose substrates based on reducing sugar release. Bioresur. Technolol. 2014, 151, 392–396. [Google Scholar] [CrossRef]
- Taipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Jumina, J.; Mutmainah, M.; Purwono, B.; Kurniawan, Y.S.; Syah, Y.M. Antibacterial and antifungal activity of three monosaccharide monomyristate derivatives. Molecules 2019, 24, 3692. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Woo, E.; Lee, D.G. (-)-Nortrachelogenin from Partrinia scabiosaefolia elicits an apoptotoc response in Candida albicans. FEMS Yeast Res. 2016, 16, fow013. [Google Scholar] [CrossRef]
- Rowell, G.A.; Taylor, O.R., Jr.; Locke, S.J. Variation in drone mating flight times among commercial honey bee stockes. Apidologie 1986, 17, 137–158. [Google Scholar] [CrossRef]
- Kim, S.G.; Woo, S.O.; Bang, K.W.; Jang, H.R.; Han, S.M. Chemical composition of drone pupa of Apis mellifera and its nutritional evaluation. J. Apic. 2018, 33, 17–23. [Google Scholar] [CrossRef]
- Kent, N.L.; Evers, A.D. Technology of Cereals, 4th ed.; Pergamon Press: Oxford, UK, 1994. [Google Scholar]
- WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; WHO Technical Report Series 935; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Metzler, D.E. Biochmeistry: TheChemical Reactions of Living Cells, Vol. 1 and 2, 2nd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Pickering, M.V.; Newton, P. Amino acid hydrolysis: Old problems, new solutions. Lc Gc 1990, 8, 778–781. [Google Scholar]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. 2011, 3, 1192–1200. [Google Scholar] [CrossRef]
- Schneider, E.; Rolli-Derkinderen, M.; Arock, M.; Dy, M. Trends in histamine research: New functions during immune responses and hematopoiesis. Trends Immunol. 2002, 23, 255–263. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 53, 802–823. [Google Scholar] [CrossRef]
- Chakravorty, J.; Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of Chondacris rosea and Brachutrupes orientalis: Two common insects used as food by tribes of Arunachal Pradesh, India. J. Asia-Pac. Entomol. 2014, 17, 407–415. [Google Scholar] [CrossRef]
- Chakravorty, J.; Ghosh, S.; Megu, K.; Jung, C.; Meyer-Rochow, V.B. Nutritional and anti-nutritional composition of Oecophylla smaragdina (Hymenoptera: Formicidae) and Odototermes sp. (Isoptera: Termitidae): Two preferred edible insects of Arunachal Pradesh, India. J. Asia-Pac. Entomol. 2016, 19, 711–720. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Choi, K.; Choi, H.Y.; Kim, H.W.; Kim, S. Body fatty and amino acid composition of a native bumblebee, Bombus ignitus relative to B. terrestris of foreign origin in Korea. J. Apic. 2017, 32, 111–117. [Google Scholar] [CrossRef]
- Ghosh, S.; Debelo, D.G.; Lee, W.; Meyer-Rochow, V.B.; Jung, C.; Dekebo, A. Termites in human diet: An investigation into their nutritional profile. In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Mariod, A.A., Ed.; Springer: Basel, Switzerland, 2020; pp. 293–306. [Google Scholar] [CrossRef]
- Berger, B.; Crailsheim, K.; Leonhard, B. Proline, leucine and phynylalanine metabolism in adult honeybee drones (Apis mellifera carnica Pollm.). Insect Biochem. Mol. Biol. 1997, 27, 587–593. [Google Scholar] [CrossRef]
- Grundy, S.M. What is the desirable ratio of saturated, polyunsaturated, and monounsaturated fatty acids in the diet? Am. J. Clin. Nutr. 1997, 66, 988S–990S. [Google Scholar] [CrossRef] [PubMed]
- De Renobales, M.; Cripps, C.; Stanley-Samuelson, D.W.; Jurenka, R.A.; Blomquist, G.B. Biosynthesis of linoleic acid in insects. TIBS 1987, 12, 364–366. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Hang, X.; Wang, H.; Yang, W.; Xu, B. Nutritional effect of alpha-linolenic acid on honey bee colony development (Apis mellifera L.). J. Apic. Sci. 2015, 59, 63–72. [Google Scholar] [CrossRef]
- Arien, Y.; Dag, A.; Zarchin, S.; Masci, T.; Shafir, S. Omega-3 deficeincy impairs honey bee learning. Proc. Natl. Acad. Sci. USA 2015, 112, 15761–15766. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of monounsaturated fatty acids versus complex carbohydrates on high-density lipoproteins in healthy men and women. Lancet 1987, 329, 122–125. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. AHA Science Advisory: Monounsaturated fatty acids and risk of cardiovascular disease. J. Nutr. 1999, 129, 2280–2284. [Google Scholar]
- Treasure, J.; Ploth, D. Role of potassium in the treatment of hypertension. Hypertension 1983, 5, 864–872. [Google Scholar] [CrossRef]
- Houston, M.C. The importance of potassium in managing hypertension. Curr. Hypertens. Rep. 2011, 13, 309–317. [Google Scholar] [CrossRef]
- Milman, N. Anemia-still a major health problem in many parts of the world! Ann. Hematol. 2011, 90, 369–377. [Google Scholar] [CrossRef]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef]
- Wu, F.Y.-H.; Wu, C.-W. Zinc in DNA replication and transcription. Ann. Rev. Nutr. 1987, 7, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Adeduntan, S.A. Nutritional and antinutritional characteristics of some insects foraging in Akure reserve Ondo state, Nigeria. J. Food Technol. 2005, 3, 563–567. [Google Scholar]
- Pyo, S.-J.; Kang, D.-G.; Jung, C.; Sohn, H.-Y. Anti-thrombotic, antioxidant and haemolysis activities of six edible insect species. Commun. Foods 2020. Accepted. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G. Oxidative stress, aging and disease. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Zhou, L.; Elias, R.J. Chapter 9—Understanding antioxidant and prooxidant mechanisms of phenolics in food lipids. In Lipid Oxidation Challenges in Food Systems; Logan, A., Nienaber, U., Pan, X., Eds.; Elsevier; Academic Press; AOCS Press: Amsterdam, The Netherlands, 2013; pp. 297–321. [Google Scholar]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Morazzoni, P.; Roveri, A.; Pifferi, G. A novel antioxidant flavonoid (IdB 1031) affecting molecular mechanisms of cellular activation. Free Radic. Biol. Med. 1994, 16, 547–553. [Google Scholar] [CrossRef]
- WHO-EM/NUT/270/E. In Summary Report on the Technical Consultation on Reducing Sugar Intake in the Eastern Mediterranean Region; World Health Organization Regional Office for the Eastern Mediterranean: Amman, Jordan, 2015.
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Kim, S.W.; Liu, G.; Wu, X.; et al. Dietary supplementation with L-glutamate and L-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 2016, 48, 53–64. [Google Scholar] [CrossRef]
- Anraku, M.; Shintomo, R.; Taguchi, K.; Kragh-Hansen, U.; Kai, T.; Maruyama, T.; Otagiri, M. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sci. 2015, 134, 36–41. [Google Scholar] [CrossRef]
- Kumar, G.; Karthik, L.; Rao, K.V.B. Haemolytic activity of Indian medicinal plants toward human erythrocytes: An in vitro study. Appl. Bot. 2011, 40, 5534–5537. [Google Scholar]
- Zohra, M.; Fawzia, A. Hemolytic activity of different herbal extracts used in Algeria. Int. J. Pharm. Sci. Res. 2014, 5, 495–500. [Google Scholar]
- Hossaini, A.A. Hemolytic and hemagglutinating activities of 222 plants. Vox Sang. 1968, 15, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lalanne, G.; Hernández-Áivarez, A.-J.; Salinas-Castro, A. Edible insects processing: Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1190. [Google Scholar] [CrossRef]
- Ulmer, M.; Smetana, S.; Heinz, V. Utilizing honeybee drone brood as a protein source for food products: Life cycle assessment of apiculture in Germany. Resour. Conserv. Recycl. 2020, 154, 104576. [Google Scholar] [CrossRef]
- Bolatovna, K.S.; Rustenov, A.; Eleuqalieva, N.; Omirzak, T.; Akhanov, U.K. Improving reproductive qualities of pigs using drone brood homogenate. Biol. Med. 2015, 7, 2. [Google Scholar]
| Amino Acid | D-Larvae | D-Late Pupae | D-Adult | K-Early Pupae | K-Late Pupae | K-Early Adult | K-Adult |
|---|---|---|---|---|---|---|---|
| Valine * | 2.87 ± 0.099 | 2.97 ± 0.198 | 3.79 ± 0.480 | 2.56 ± 0.016 | 2.97 ± 0.030 | 4.07 ± 0.043 | 4.22 ± 0.022 |
| Isoleucine * | 2.43 ± 0.021 | 2.56 ± 0.014 | 3.28 ± 0.340 | 2.13 ± 0.004 | 2.44 ± 0.014 | 3.16 ± 0.025 | 3.27 ± 0.007 |
| Leucine * | 3.96 ± 0.014 | 4.26 ± 0.092 | 5.51 ± 0.062 | 3.54 ± 0.004 | 4.14 ± 0.036 | 5.53 ± 0.043 | 5.65 ± 0.010 |
| Lysine * | 3.52 ± 0.035 | 3.68 ± 0.021 | 4.35 ± 0.521 | 3.00 ± 0.007 | 3.51 ± 0.023 | 4.43 ± 0.004 | 4.56 ± 0.009 |
| Tyrosine ** | 2.55 ± 0.042 | 2.76 ± 0.007 | 2.77 ± 0.219 | 2.20 ± 0.003 | 2.77 ± 0.038 | 3.04 ± 0.019 | 2.87 ± 0.053 |
| Threonine * | 1.86 ± 0.035 | 1.57 ± 0.134 | 1.95 ± 0.084 | 1.89 ± 0.303 | 1.93 ± 0.005 | 3.23 ± 0.006 | 2.66 ± 0.001 |
| Phenylalanine * | 2.08 ± 0.028 | 2.15 ± 0.049 | 2.35 ± 0.189 | 1.83 ± 0.009 | 2.00 ± 0.003 | 2.29 ± 0.009 | 2.38 ± 0.006 |
| Histidine * | 1.21 ± 0.042 | 1.27 ± 0.000 | 1.55 ± 0.439 | 0.94 ± 0.001 | 1.12 ± 0.016 | 1.42 ± 0.000 | 1.41 ± 0.000 |
| Methionine * | 1.15 ± 0.007 | 1.16 ± 0.000 | 1.44 ± 0.004 | 0.17 ± 0.031 | 0.44 ± 0.047 | 1.91 ± 0.581 | 2.28 ± 0.076 |
| Arginine *** | 2.18 ± 0.099 | 2.45 ± 0.042 | 3.67 ± 0.063 | 2.20 ± 0.018 | 2.55 ± 0.016 | 3.35 ± 0.001 | 3.55 ± 0.000 |
| Aspartic acid | 3.23 ± 0.028 | 3.22 ± 0.028 | 3.68 ± 0.180 | 2.50 ± 0.012 | 2.72 ± 0.020 | 3.16 ± 0.007 | 3.40 ± 0.005 |
| Glutamic acid | 7.94 ± 0.262 | 8.78 ± 0.014 | 8.74 ± 0.863 | 10.01 ± 0.044 | 10.55 ± 0.036 | 12.16 ± 0.065 | 12.39 ± 0.050 |
| Serine | 2.03 ± 0.092 | 2.40 ± 0.141 | 2.91 ± 0.112 | 1.75 ± 0.111 | 2.09 ± 0.006 | 3.19 ± 0.021 | 2.93 ± 0.023 |
| Glycine | 2.29 ± 0.014 | 2.65 ± 0.007 | 4.19 ± 0.832 | 2.10 ± 0.004 | 2.84 ± 0.039 | 4.58 ± 0.042 | 4.40 ± 0.003 |
| Alanine | 2.36 ± 0.014 | 2.87 ± 0.000 | 5.28 ± 0.055 | 2.56 ± 0.009 | 3.44 ± 0.027 | 5.82 ± 0.069 | 5.97 ± 0.001 |
| Cysteine | 0.25 ± 0.014 | 0.35 ± 0.014 | 1.93 ± 0.957 | 0.19 ± 0.001 | 0.28 ± 0.032 | 0.39 ± 0.077 | 0.38 ± 0.003 |
| Proline | 1.58 ± 0.000 | 1.52 ± 0.028 | 2.33 ± 0.124 | 2.99 ± 0.026 | 3.60 ± 0.035 | 4.61 ± 0.044 | 4.70 ± 0.010 |
| Total | 43.49 | 46.62 | 59.72 | 42.56 | 49.39 | 66.34 | 67.02 |
| Fatty Acid | D-Larvae | D-Late Pupae | D-Adult | K-Early Pupae | K-Late Pupae | K-Early Adult | K-Adult |
|---|---|---|---|---|---|---|---|
| Lauric acid (C12:0) | 25.95 | 31.37 | 4.08 | 32.48 | 33.41 | 14.17 | 6.14 |
| Myristic acid (C14:0) | 359.51 | 365.50 | 15.97 | 333.07 | 258.05 | 48.35 | 18.31 |
| Palmitic acid (C16:0) | 4809.97 | 4879.12 | 294.67 | 4517.45 | 3570.83 | 802.94 | 384.12 |
| Stearic acid (C18:0) | 1110.26 | 1302.45 | 257.09 | 1356.94 | 1267.04 | 592.54 | 341.51 |
| Arachidic acid (C20:0) | ND | 56.17 | 35.92 | 120.62 | 145.82 | 157.05 | 104.24 |
| Behenic acid (C22:0) | ND | ND | 62.86 | 14.38 | 23.34 | 51.35 | 46.46 |
| Lignoceric acid (C24:0) | ND | ND | ND | 39.17 | 42.64 | 39.99 | 34.95 |
| Subtotal (SFA) | 6305.69 | 6634.61 | 670.59 | 6414.11 | 5341.13 | 1706.39 | 935.73 |
| Palmitoleic acid (C16:1) | 56.35 | 51.92 | 166.58 | 47.65 | 48.33 | 74.29 | 92.91 |
| Elaidic acid (C18:1n9t) | ND | ND | ND | 6.75 | 0.00 | 0.00 | 0.00 |
| Oleic acid (C18:1n9c) | 4720.25 | 5104.52 | 1783.36 | 4902.83 | 4412.01 | 2545.19 | 1900.32 |
| cis11-Eicosenic acid (C20:1n9) | ND | ND | 127.09 | 8.69 | 10.38 | 14.01 | 9.57 |
| Subtotal (MUFA) | 4776.60 | 5156.44 | 2077.03 | 4965.92 | 4470.72 | 2633.49 | 2002.80 |
| Linoleic acid (C18:2n6c) | ND | 67.87 | 61.8 | 22.76 | 30.69 | 37.43 | 35.92 |
| Linolenic acid (C18:3n3) | ND | ND | ND | 61.24 | 83.23 | 108.50 | 104.85 |
| cis-13,16-Docosadienoic acid (C22:2) | ND | ND | ND | 15.20 | 17.23 | 21.54 | 17.56 |
| Subtotal (PUFA) | ND | 67.87 | 61.8 | 99.20 | 131.15 | 167.47 | 158.33 |
| Total | 11,082.29 | 11,858.92 | 2809.42 | 11,479.23 | 9943.00 | 4507.35 | 3096.86 |
| Minerals | D-Larvae | D-Late Pupae | D-Adult | K-Early Pupae | K-Late Pupae | K-Adult |
|---|---|---|---|---|---|---|
| Calcium | 34.21 | 38.7 | 60.72 | 43.72 | 49.29 | 66.19 |
| Magnesium | 68.06 | 81.86 | 121.45 | 82.89 | 95.03 | 123.18 |
| Sodium | 30.08 | 38.02 | 79.45 | 7.29 | 8.52 | 11.33 |
| Potassium | 891.08 | 1101.98 | 1465.23 | 544.55 | 643.06 | 784.04 |
| Phosphorus | 686.88 | 802.61 | 1166.06 | 774.03 | 892.41 | 1132.35 |
| Iron | 5.62 | 5.99 | 12.23 | 4.86 | 5.67 | 10.58 |
| Zinc | 5.10 | 6.04 | 15.86 | 5.25 | 5.88 | 8.40 |
| Copper | 0.11 | 0.37 | 1.39 | 1.82 | 1.94 | 2.59 |
| Manganese | 0.87 | ND | 1.71 | 0.28 | 0.29 | 0.52 |
| Extract (mg/mL) | Antioxidant Activity (%) | Reducing Power (700 nm) | ||
|---|---|---|---|---|
| DPPH | ABTS | Nitrite | ||
| Larvae (0.5) | 0.3 ± 0.4 a | 10.4 ± 0.2 a | 25.6 ± 4.5 a | 0.018 ± 0.001 b |
| Late pupae (0.5) | 1.3 ± 0.4 a | 10.5 ± 0.5 a | 20.9 ± 4.1 a | 0.008 ± 0.002 a |
| Adult (0.5) | 18.5 ± 1.4 b | 40.1 ± 2.3 b | 40.4 ± 6.3 b | 0.230 ± 0.001 c |
| Vitamin C (0.1) | 92.5 ± 0.6 c | 95.2 ± 0.3 c | 85.6 ± 2.6 c | 1.545 ± 0.064 d |
| Extract | Antimicrobial Activity (Clear Zone: mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram Positive Bacteria | Gram Negative Bacteria | Fungi | ||||||||
| LM | SE | SA | BS | EC | PA | ST | PV | CA | SC | |
| Larvae | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Late pupae | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Adult | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Ampicillin | 13 ± 0.1 | 21 ± 0.2 | 15 ± 0.1 | 12 ± 0.2 | 6 ± 0.1 | 8 ± 0.2 | 11 ± 0.1 | 18 ± 0.2 | -- | -- |
| Miconazole | -- | -- | -- | -- | -- | -- | -- | -- | 8 ± 0.1 | 13 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Sohn, H.-Y.; Pyo, S.-J.; Jensen, A.B.; Meyer-Rochow, V.B.; Jung, C. Nutritional Composition of Apis mellifera Drones from Korea and Denmark as a Potential Sustainable Alternative Food Source: Comparison Between Developmental Stages. Foods 2020, 9, 389. https://doi.org/10.3390/foods9040389
Ghosh S, Sohn H-Y, Pyo S-J, Jensen AB, Meyer-Rochow VB, Jung C. Nutritional Composition of Apis mellifera Drones from Korea and Denmark as a Potential Sustainable Alternative Food Source: Comparison Between Developmental Stages. Foods. 2020; 9(4):389. https://doi.org/10.3390/foods9040389
Chicago/Turabian StyleGhosh, Sampat, Ho-Yong Sohn, Su-Jin Pyo, Annette Bruun Jensen, Victor Benno Meyer-Rochow, and Chuleui Jung. 2020. "Nutritional Composition of Apis mellifera Drones from Korea and Denmark as a Potential Sustainable Alternative Food Source: Comparison Between Developmental Stages" Foods 9, no. 4: 389. https://doi.org/10.3390/foods9040389
APA StyleGhosh, S., Sohn, H.-Y., Pyo, S.-J., Jensen, A. B., Meyer-Rochow, V. B., & Jung, C. (2020). Nutritional Composition of Apis mellifera Drones from Korea and Denmark as a Potential Sustainable Alternative Food Source: Comparison Between Developmental Stages. Foods, 9(4), 389. https://doi.org/10.3390/foods9040389







