Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Fruits and Sweeteners
2.3. Experimental Design
2.4. pH, Titratable Acidity, and Total Soluble Solids
2.5. Qualitative and Quantitative Analysis of Phenolic Compounds
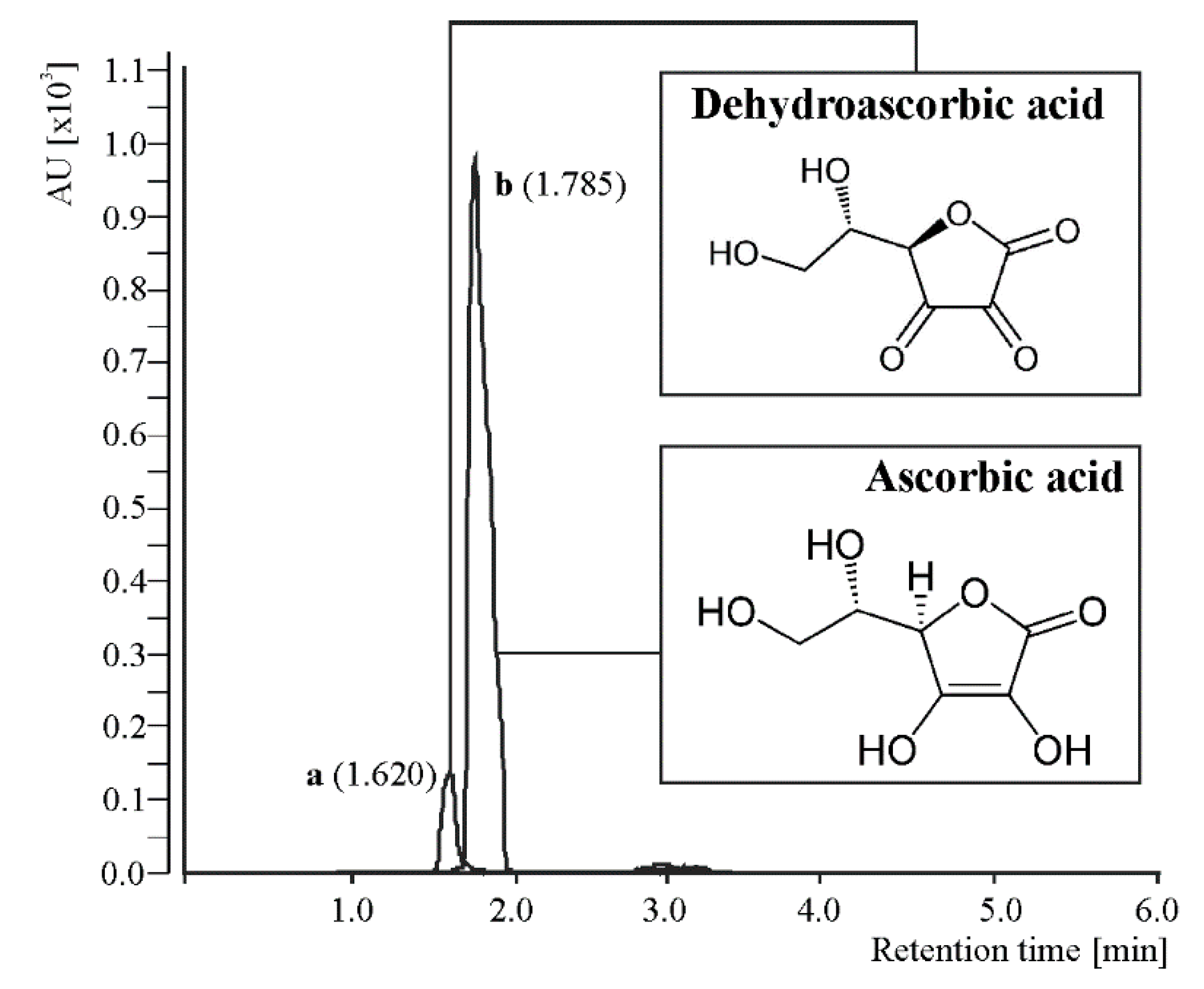
2.6. Extraction and Analysis of Vitamin C
2.7. Color Measurements
2.8. Statistical Analyses
3. Results and Discussion
3.1. Quality Parameters
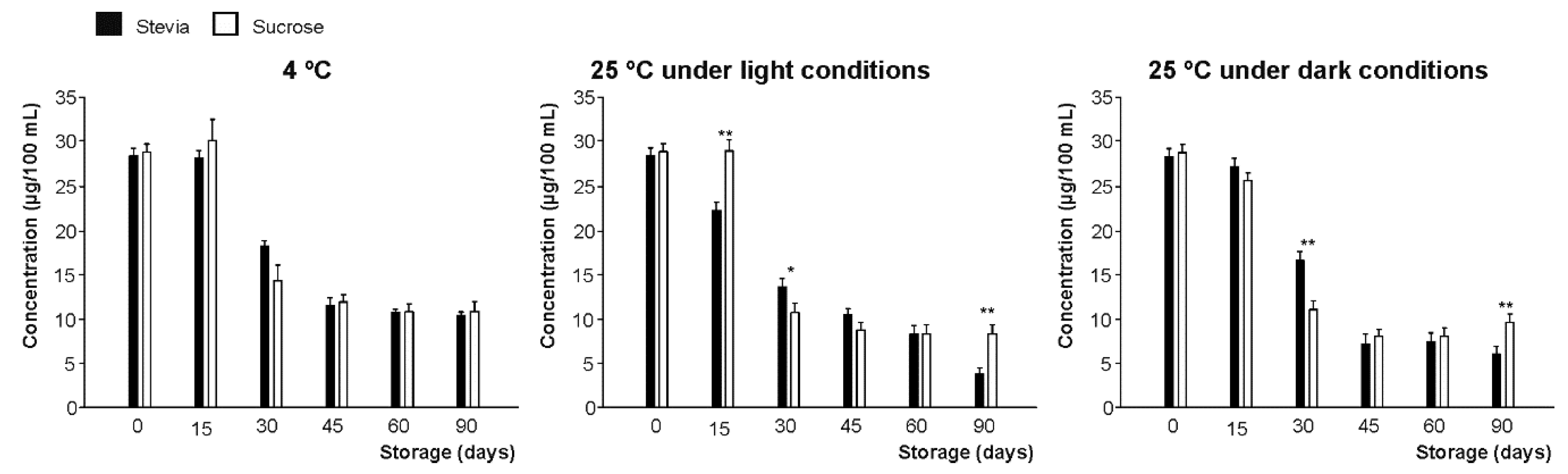
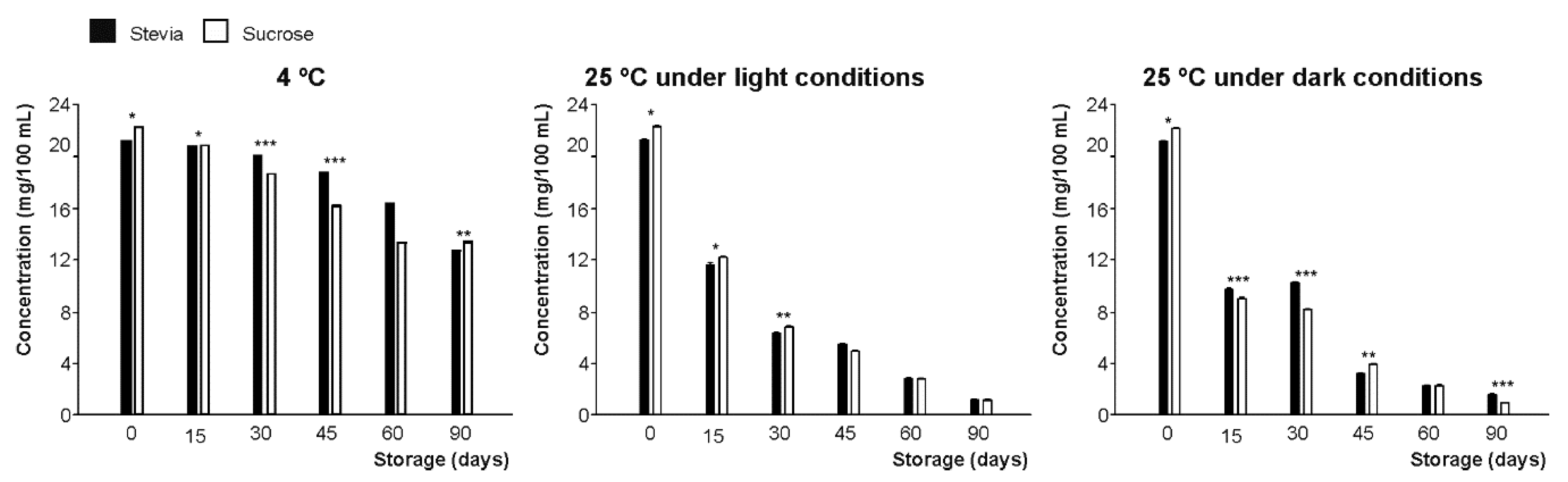
3.2. Vitamin C
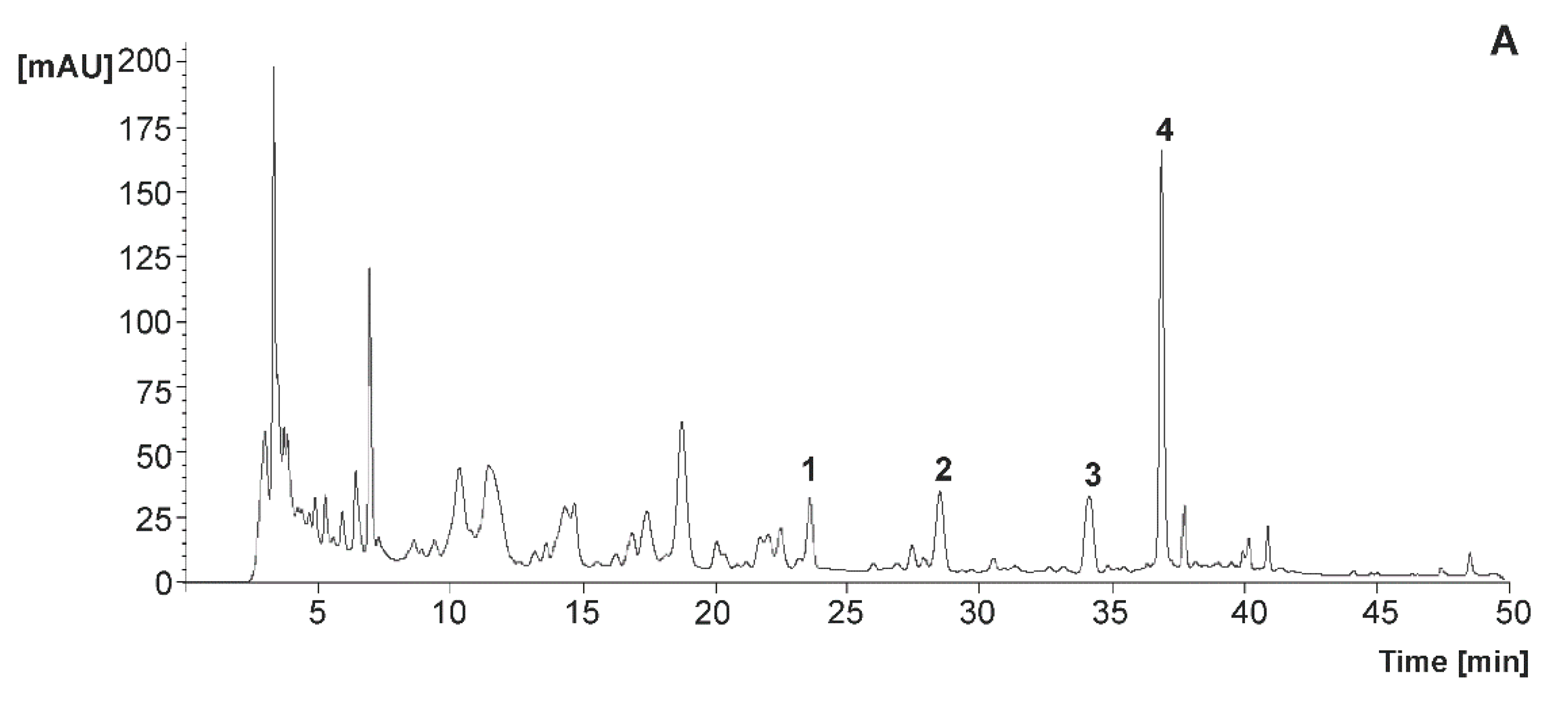
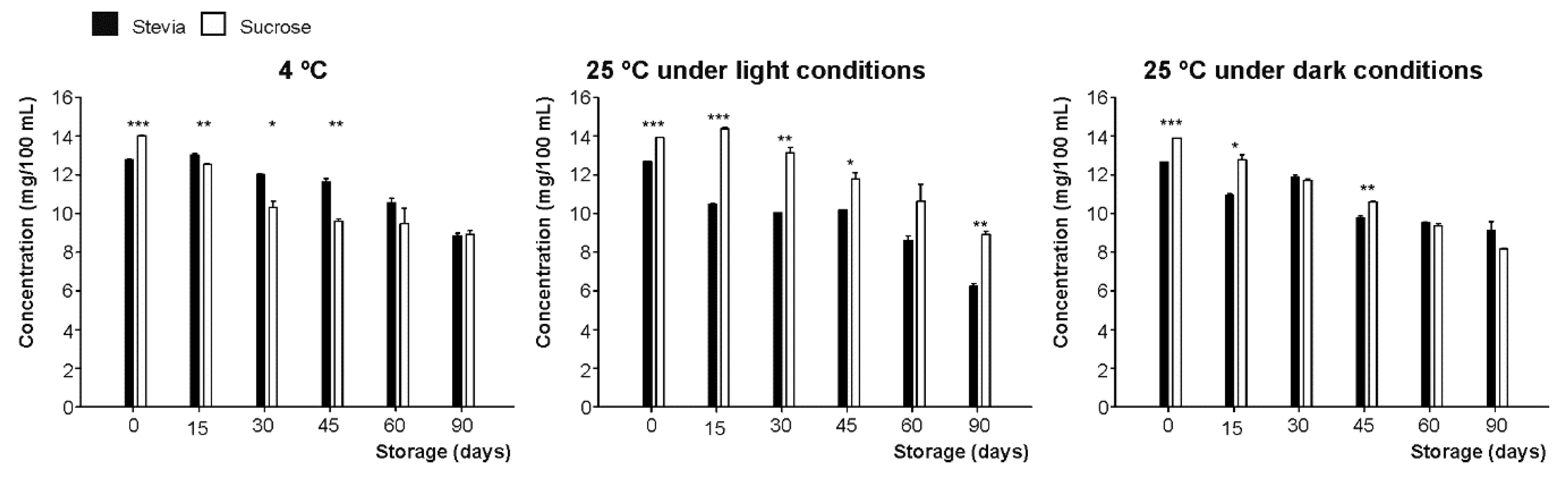
3.3. Phenolic Composition of Juices
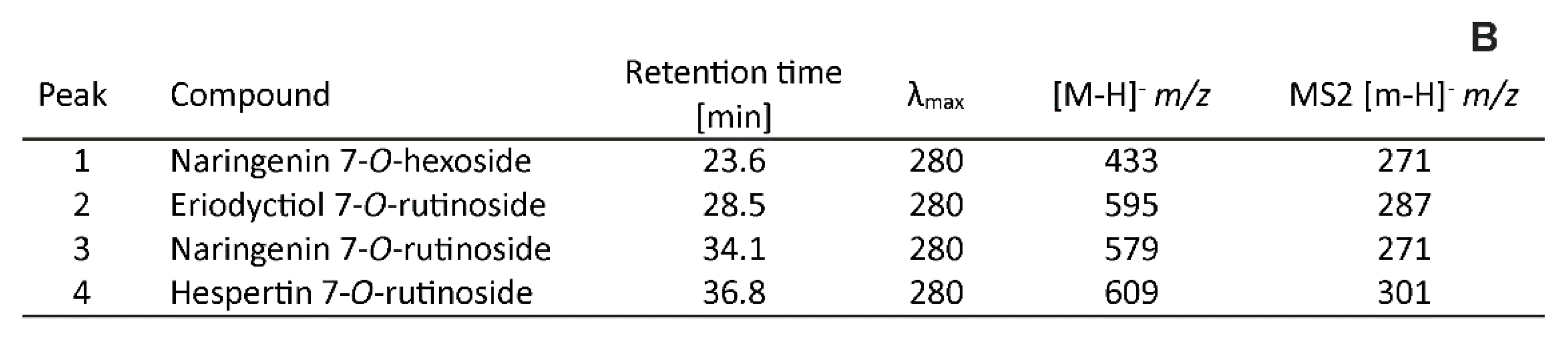
3.3.1. Flavanones
3.3.2. Caffeic and Ellagic Acids
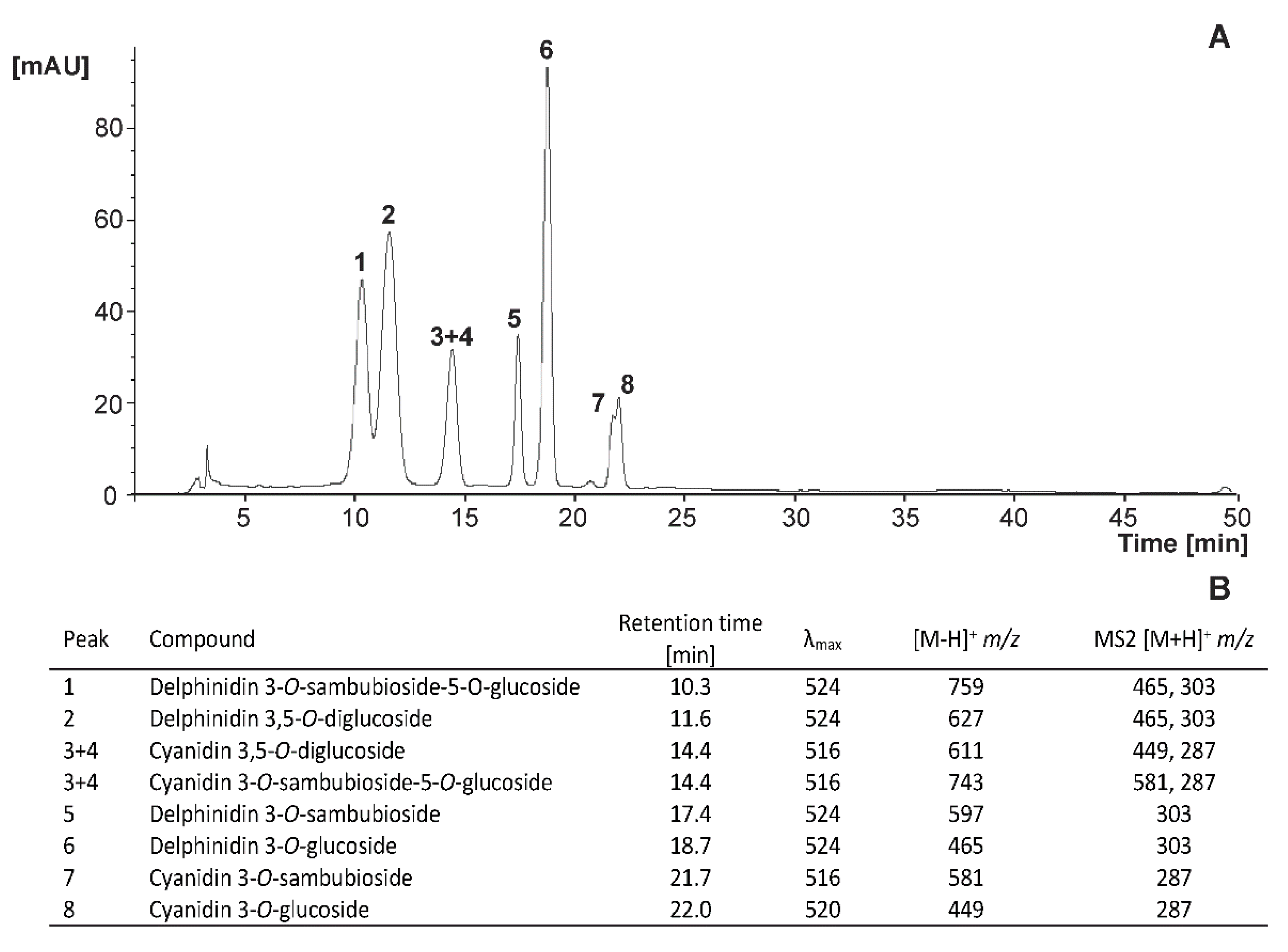
3.3.3. Anthocyanins
3.4. Color Changes during Storage
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Xiao, X.; Hu, M.; Zhang, X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018, 214, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Sweeteners and Risk of Obesity and Type 2 Diabetes: The Role of Sugar-Sweetened Beverages. Curr. Diabetes Rep. 2012, 12, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. Sugary beverages represent a threat to global health. Trends Endocrinol. Metab. 2012, 23, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutiérrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of Antioxidant Compounds and α-Glucosidase/α-Amylase Inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Salvador-Reyes, R.; Sotelo-Herrera, M.; Paucar-Menacho, L.M. Study of Stevia (Stevia rebaudiana Bertoni) as a natural sweetener and its use in benefit of the health. Sci. Agropecu. 2014, 5, 157–163. [Google Scholar] [CrossRef][Green Version]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S. Influence of Steviol Glycosides on the Stability of Vitamin C and Anthocyanins. J. Agric. Food Chem. 2014, 62, 11264–11269. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K.S. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Törrönen, R.; McDougall, G.J.; Dobson, G.; Stewart, D.; Hellström, J.; Mattila, P.; Pihlava, J.-M.; Koskela, A.; Karjalainen, R. Fortification of blackcurrant juice with crowberry: Impact on polyphenol composition, urinary phenolic metabolites, and postprandial glycemic response in healthy subjects. J. Funct. Foods 2012, 4, 746–756. [Google Scholar] [CrossRef]
- Girones-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; Garcia-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Sloan, A.E. Top 10 Functional Food Trends. Food Technol. 2018, 72, 26–43. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Boil. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, 67–90. [Google Scholar] [CrossRef]
- Araos, J.P. Aristotelia chilensis: A Possible Nutraceutical or Functional Food. Med. Chem. 2015, 5, 378–382. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, A.L.; Pérez-Alvarez, J.A.; Fernández-López, J.; A Muñoz, L.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A.; Grace, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef]
- Cespedes-Acuña, C.L.; El-Hafidi, M.; Pavón, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 820–829. [Google Scholar] [CrossRef]
- Fuentealba, J.; Dibarrart, A.; Saez-Orellana, F.; Fuentes-Fuentes, M.C.; Oyanedel, C.N.; Guzmán, L.; Perez, C.; Becerra, J.; Aguayo, L. Synaptic Silencing and Plasma Membrane Dyshomeostasis Induced by Amyloid-β Peptide are Prevented by Aristotelia chilensis Enriched Extract. J. Alzheimer’s Dis. 2012, 31, 879–889. [Google Scholar] [CrossRef]
- Watson, R.R.; Schönlau, F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: A review. Minerva Cardioangiol. 2015, 63, 1–12. [Google Scholar]
- Girones-Vilaplana, A.; Moreno, D.A.; Garcia-Viguera, C. Phytochemistry and biological activity of Spanish Citrus fruits. Food Funct. 2014, 5, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Girones-Vilaplana, A.; Mena, P.; Garcia-Viguera, C.; Moreno, D.A. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT 2012, 47, 279–286. [Google Scholar] [CrossRef]
- Agulló, V.; Villaño, D.; Garcia-Viguera, C.; Domínguez-Perles, R. Anthocyanin Metabolites in Human Urine after the Intake of New Functional Beverages. Molecules 2020, 25, 371. [Google Scholar] [CrossRef] [PubMed]
- Agulló, V.; Domínguez-Perles, R.; Moreno, D.A.; Zafrilla, P.; Garcia-Viguera, C. Alternative Sweeteners Modify the Urinary Excretion of Flavanones Metabolites Ingested through a New Maqui-Berry Beverage. Foods 2020, 9, 41. [Google Scholar] [CrossRef]
- Baenas, N.; Salar, F.J.; Domínguez-Perles, R.; Garcia-Viguera, C. New UHPLC-QqQ-MS/MS Method for the Rapid and Sensitive Analysis of Ascorbic and Dehydroascorbic Acids in Plant Foods. Molecules 2019, 24, 1632. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Al-Dabbas, J.M. Effect of partial replacement of sucrose with the artificial sweetener, sucralose on the physico-chemical, sensory, microbial characteristics, and final cost saving of orange nectar. Int. Food Res. J. 2012, 19, 679–683. [Google Scholar]
- Goraya, R.K.; Bajwa, U. The Sweetness Technology of Sugar Substituted Low-Calorie Beverages. Food Nutr. J. 2016, 2. [Google Scholar] [CrossRef]
- Martí, N.; Mena, P.; Cánovas, J.A.; Micol, V.; Saura, D. Vitamin C and the role of citrus juices as functional food. Nat. Prod. Commun. 2009, 4, 677–700. [Google Scholar] [CrossRef]
- Girones-Vilaplana, A.; Mena, P.; Moreno, D.A.; Garcia-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Sci. Food Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- González-Molina, E.; Moreno, D.A.; García-Viguera, C. Aronia-Enriched Lemon Juice: A New Highly Antioxidant Beverage. J. Agric. Food Chem. 2008, 56, 11327–11333. [Google Scholar] [CrossRef]
- Ahmed, M.; Eun, J.B. Flavonoids in fruits and vegetables after thermal and nonthermal processing: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 3159–3188. [Google Scholar] [CrossRef] [PubMed]
- Igual, M.; García-Martínez, E.; Camacho, M.D.M.; Martínez-Navarrete, N. Changes in flavonoid content of grapefruit juice caused by thermal treatment and storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 153–162. [Google Scholar] [CrossRef]
- González-Molina, E.; Moreno, D.A.; Garcia-Viguera, C. A new drink rich in healthy bioactives combining lemon and pomegranate juices. Food Chem. 2009, 115, 1364–1372. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Serrano, P.; Abellan, P.; Garcia-Viguera, C. Influence of packaging material on pomegranate juice colour and bioactive compounds, during storage. J. Sci. Food Agric. 2004, 84, 639–644. [Google Scholar] [CrossRef]
- Girones-Vilaplana, A.; Villaño, D.; Moreno, D.A.; Garcia-Viguera, C. New isotonic drinks with antioxidant and biological capacities from berries (maqui, açaí and blackthorn) and lemon juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906. [Google Scholar] [CrossRef]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Effect of sweeteners on anthocyanin stability and colour properties of sour cherry and strawberry nectars during storage. J. Food Sci. Technol. 2018, 55, 4346–4355. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A. The high ascorbic acid content is the main cause of the low stability of anthocyanin extracts from acerola. Food Chem. 2007, 103, 935–943. [Google Scholar] [CrossRef]
- Poei-Langston, M.S.; Wrolstad, R.E. Color Degradation in an Ascorbic Acid-Anthocyanin-Flavanol Model System. J. Food Sci. 1981, 46, 1218–1236. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Bridle, P. Influence of structure on colour stability of anthocyanins and flavylium salts with ascorbic acid. Food Chem. 1999, 64, 21–26. [Google Scholar] [CrossRef]
- Hernández-Brenes, C.; Del Pozo-Insfran, D.; Talcott, S.T. Stability of Copigmented Anthocyanins and Ascorbic Acid in a Grape Juice Model System. J. Agric. Food Chem. 2005, 53, 49–56. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Color and stability of anthocyanins in strawberry nectars containing various co-pigment sources and sweeteners. Food Chem. 2019, 310, 125856. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.H. Effect of Colorant on Cytogenetic, Biochemical and Histochemical Parameters and Quality Changes during Storage of some Commercial Fruit Drinks. J. Nutr. Food Sci. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and Instrumental Color Evaluation in Red Wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Türkyılmaz, M.; Hamzaoğlu, F.; Özkan, M. Effects of sucrose and copigment sources on the major anthocyanins isolated from sour cherries. Food Chem. 2019, 281, 242–250. [Google Scholar] [CrossRef]
| Code | Beverage and Storage Condition |
|---|---|
| ST4 | Beverage with stevia stored at 4 °C under darkness conditions |
| ST25L | Beverage with stevia stored at 25 °C under light conditions |
| ST25O | Beverage with stevia stored at 25 °C under darkness conditions |
| SA4 | Beverage with sucrose stored at 4 °C under darkness conditions |
| SA25L | Beverage with sucrose stored at 25 °C under light conditions |
| SA25O | Beverage with sucrose stored at 25 °C under darkness conditions |
| Condition Z | pH | TA (g CA/100 mL) | TSS (°Brix) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | p-Value | Initial | Final | p-Value | Initial | Final | p-Value | |
| ST4 | 3.26 | 3.16 | ** X | 4.28 | 4.16 | * | 7.6 | 7.7 | ** |
| SA4 | 3.28 | 3.27 | N.s. | 3.36 | 3.34 | N.s. | 13.7 | 13.6 | N.s. |
| p-Value | N.s. | ** | *** | ** | *** | *** | |||
| ST25L | 3.26 | 3.21 | * | 4.28 | 3.86 | ** | 7.6 | 7.7 | ** |
| SA25L | 3.28 | 3.22 | * | 3.36 | 3.61 | ** | 13.7 | 14.3 | * |
| p-Value | N.s. | N.s. | *** | * | *** | *** | |||
| ST25O | 3.26 | 3.25 | N.s. | 4.28 | 3.54 | *** | 7.6 | 7.6 | N.s. |
| SA25O | 3.28 | 3.23 | * | 3.36 | 3.66 | *** | 13.7 | 14.3 | * |
| p-Value | N.s. | N.s. | *** | ** | *** | *** | |||
| Parameter | Days | Stevia | Sucrose | p-Value |
|---|---|---|---|---|
| CIEL* | 0 | 17.35 ± 0.02d Z | 22.37 ± 0.01c | *** X |
| 15 | 15.27 ± 0.11b | 20.95 ± 0.01a | *** | |
| 30 | 15.14 ± 0.01b | 21.87 ± 0.01b | *** | |
| 45 | 14.76 ± 0.01a | 23.23 ± 0.04d | *** | |
| 60 | 15.79 ± 0.04c | 25.55 ± 0.01e | *** | |
| 90 | 33.67 ± 0.01e | 35.93 ± 0.01f | *** | |
| CIEa* | 0 | 47.61 ± 0.14d | 48.64 ± 0.03d | *** |
| 15 | 42.90 ± 0.19c | 47.97 ± 0.02c | *** | |
| 30 | 42.32 ± 0.05b | 47.74 ± 0.01b | *** | |
| 45 | 41.70 ± 0.01a | 44.57 ± 0.03a | *** | |
| 60 | 42.76 ± 0.01c | 50.57 ± 0.04e | *** | |
| 90 | 57.32 ± 0.01e | 60.09 ± 0.01f | *** | |
| CIEb* | 0 | 29.31 ± 0.01e | 36.82 ± 0.07c | *** |
| 15 | 26.31 ± 0.16c | 34.90 ± 0.02a | *** | |
| 30 | 25.96 ± 0.04b | 36.12 ± 0.05b | *** | |
| 45 | 25.25 ± 0.01a | 37.89 ± 0.01d | *** | |
| 60 | 26.90 ± 0.08d | 40.66 ± 0.04f | *** | |
| 90 | 45.05 ± 0.01f | 38.93 ± 0.01e | *** | |
| Chroma | 0 | 52.55 ± 0.01d | 61.01 ± 0.06d | *** |
| 15 | 50.32 ± 0.07c | 59.32 ± 0.02b | *** | |
| 30 | 49.65 ± 0.06b | 59.86 ± 0.02c | *** | |
| 45 | 48.76 ± 0.01a | 58.50 ± 0.01a | *** | |
| 60 | 50.52 ± 0.09c | 64.89 ± 0.01e | *** | |
| 90 | 72.90 ± 0.01e | 71.60 ± 0.01f | *** | |
| Hue angle | 0 | 33.91 ± 0.01c | 37.13 ± 2.41c | *** |
| 15 | 31.52 ± 0.27a | 36.04 ± 0.01b | ** | |
| 30 | 31.53 ± 0.01a | 37.11 ± 0.04c | *** | |
| 45 | 31.20 ± 0.02a | 40.37 ± 0.03e | *** | |
| 60 | 32.17 ± 0.04b | 38.80 ± 0.04d | *** | |
| 90 | 38.17 ± 0.01d | 32.94 ± 0.01a | *** | |
| ΔE | 0 | 0.00a | 0.00a | N.s. |
| 15 | 5.96 ± 0.03c | 2.48 ± 0.01c | *** | |
| 30 | 6.64 ± 0.06d | 1.25 ± 0.03b | *** | |
| 45 | 7.62 ± 0.01e | 4.35 ± 0.09d | *** | |
| 60 | 5.64 ± 0.08b | 5.34 ± 0.01e | * | |
| 90 | 24.66 ± 0.01f | 17.88 ± 0.01f | *** |
| Parameter | Days | Stevia | Sucrose | p-Value |
|---|---|---|---|---|
| CIEL* | 0 | 17.35 ± 0.02a Z | 22.37 ± 0.01b | *** X |
| 15 | 21.51 ± 1.09b | 21.60 ± 0.01a | N.s. | |
| 30 | 21.84 ± 0.03bc | 24.55 ± 0.01c | *** | |
| 45 | 20.90 ± 0.01b | 25.82 ± 0.01d | *** | |
| 60 | 23.48 ± 0.01c | 30.56 ± 0.01e | N.s. | |
| 90 | 32.70 ± 0.01d | 33.02 ± 0.01f | *** | |
| CIEa* | 0 | 47.61 ± 0.01b | 48.64 ± 0.03e | *** |
| 15 | 46.65 ± 0.91c | 46.46 ± 0.01d | N.s. | |
| 30 | 42.92 ± 0.06b | 45.65 ± 0.01c | *** | |
| 45 | 42.63 ± 0.01b | 49.26 ± 0.03f | *** | |
| 60 | 39.28 ± 0.01a | 43.13 ± 0.01b | *** | |
| 90 | 37.86 ± 0.01a | 38.26 ± 0.01a | ** | |
| CIEb* | 0 | 29.32 ± 0.01a | 36.82 ± 0.01b | *** |
| 15 | 35.32 ± 1.31b | 35.49 ± 0.01a | N.s. | |
| 30 | 35.87± 0.08b | 39.43 ± 0.01c | *** | |
| 45 | 34.58 ± 0.06b | 39.88 ± 0.04d | *** | |
| 60 | 38.49 ± 0.05c | 45.96 ± 0.01e | *** | |
| 90 | 49.72 ± 0.01d | 50.30 ± 0.01f | *** | |
| Chroma | 0 | 52.55 ± 0.01a | 61.01 ± 0.06c | *** |
| 15 | 58.51 ± 1.52c | 58.46 ± 0.01a | N.s. | |
| 30 | 55.93 ± 0.09b | 60.32 ± 0.01b | *** | |
| 45 | 54.89 ± 0.04ab | 63.38 ± 0.01f | *** | |
| 60 | 55.00 ± 0.02ab | 63.02 ± 0.01d | *** | |
| 90 | 62.49 ± 0.02d | 63.19 ± 0.01e | *** | |
| Hue angle | 0 | 33.91 ± 0.01a | 37.13 ± 0.03a | *** |
| 15 | 37.13 ± 0.48b | 37.38 ± 0.01b | N.s. | |
| 30 | 39.89 ± 0.04d | 40.82 ± 0.01d | *** | |
| 45 | 39.05 ± 0.05c | 39.00 ± 0.05c | N.s. | |
| 60 | 44.42 ± 0.04e | 46.82 ± 0.01e | *** | |
| 90 | 52.71 ± 0.01f | 52.74 ± 0.01f | N.s. | |
| ΔE | 0 | 0.00a | 0.00a | N.s. |
| 15 | 7.81 ± 0.06b | 2.67 ± 0.01d | *** | |
| 30 | 9.23 ± 0.02d | 4.53 ± 0.01c | *** | |
| 45 | 8.08 ± 0.04c | 4.66 ± 0.04b | *** | |
| 60 | 13.83 ± 0.04e | 13.46 ± 0.01e | ** | |
| 90 | 27.34 ± 0.01f | 20.10 ± 0.01f | *** |
| Parameter | Days | Stevia | Sucrose | p-Value |
|---|---|---|---|---|
| CIEL* | 0 | 17.35 ± 0.02a Z | 22.37 ± 0.01a | *** X |
| 15 | 20.83 ± 0.27c | 24.60 ± 0.01d | ** | |
| 30 | 19.94 ± 0.02b | 23.70 ± 0.01c | *** | |
| 45 | 23.68 ± 0.01d | 22.62 ± 0.03b | *** | |
| 60 | 22.25 ± 0.01e | 29.64 ± 0.01e | *** | |
| 90 | 35.14 ± 0.01f | 34.60 ± 0.01f | *** | |
| CIEa* | 0 | 47.61 ± 0.01c | 48.64 ± 0.03f | *** |
| 15 | 46.45 ± 0.18e | 47.47 ± 0.03e | * | |
| 30 | 44.66 ± 0.04d | 45.93 ± 0.02c | *** | |
| 45 | 39.85 ± 0.01a | 46.99 ± 0.06d | *** | |
| 60 | 40.08 ± 0.01a | 40.27 ± 0.01a | *** | |
| 90 | 41.36 ± 0.01b | 40.90 ± 0.02b | ** | |
| CIEb* | 0 | 29.32 ± 0.01a | 36.82 ± 0.07a | *** |
| 15 | 34.59 ± 0.35c | 39.69 ± 0.03c | ** | |
| 30 | 34.43 ± 0.09b | 38.21 ± 0.01b | *** | |
| 45 | 38.67 ± 0.01e | 37.05 ± 0.13a | ** | |
| 60 | 36.80 ± 0.07d | 46.18 ± 0.06d | *** | |
| 90 | 50.13 ± 0.14f | 49.24 ± 0.04e | ** | |
| Chroma | 0 | 52.55 ± 0.01a | 61.01 ± 0.06b | *** |
| 15 | 57.92 ± 0.35d | 61.88 ± 0.04c | ** | |
| 30 | 55.79 ± 0.09c | 59.74 ± 0.03a | *** | |
| 45 | 55.53 ± 0.01c | 59.84 ± 0.13a | *** | |
| 60 | 54.42 ± 0.05b | 61.26 ± 0.06b | *** | |
| 90 | 64.99 ± 0.11e | 64.01 ± 0.04d | ** | |
| Hue angle | 0 | 33.91 ± 0.01a | 37.13 ± 0.04a | *** |
| 15 | 36.67 ± 0.17b | 39.90 ± 0.01d | ** | |
| 30 | 36.81 ± 0.04b | 39.76 ± 0.01c | *** | |
| 45 | 44.14 ± 0.01d | 38.26 ± 0.06b | *** | |
| 60 | 42.56 ± 0.06c | 48.92 ± 0.04e | *** | |
| 90 | 50.48 ± 0.06e | 50.29 ± 0.01f | N.s. | |
| ΔE | 0 | 0.00a | 0.00a | N.s |
| 15 | 6.44 ± 0.39c | 3.82 ± 0.01d | * | |
| 30 | 5.69 ± 0.04b | 3.33 ± 0.01c | *** | |
| 45 | 13.71 ± 0.01e | 2.21 ± 0.01b | *** | |
| 60 | 11.70 ± 0.05d | 14.51 ± 0.03e | *** | |
| 90 | 28.09 ± 0.09f | 19.07 ± 0.01f | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salar, F.J.; Agulló, V.; García-Viguera, C.; Domínguez-Perles, R. Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study. Foods 2020, 9, 219. https://doi.org/10.3390/foods9020219
Salar FJ, Agulló V, García-Viguera C, Domínguez-Perles R. Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study. Foods. 2020; 9(2):219. https://doi.org/10.3390/foods9020219
Chicago/Turabian StyleSalar, Francisco J., Vicente Agulló, Cristina García-Viguera, and Raúl Domínguez-Perles. 2020. "Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study" Foods 9, no. 2: 219. https://doi.org/10.3390/foods9020219
APA StyleSalar, F. J., Agulló, V., García-Viguera, C., & Domínguez-Perles, R. (2020). Stevia vs. Sucrose: Influence on the Phytochemical Content of a Citrus–Maqui Beverage—A Shelf Life Study. Foods, 9(2), 219. https://doi.org/10.3390/foods9020219





