Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ODF Preparation and Micronization
2.3. Preparation of ODF-SPI Conjugates via a Maillard Reaction
2.4. Characterization of ODF-SPI Conjugates
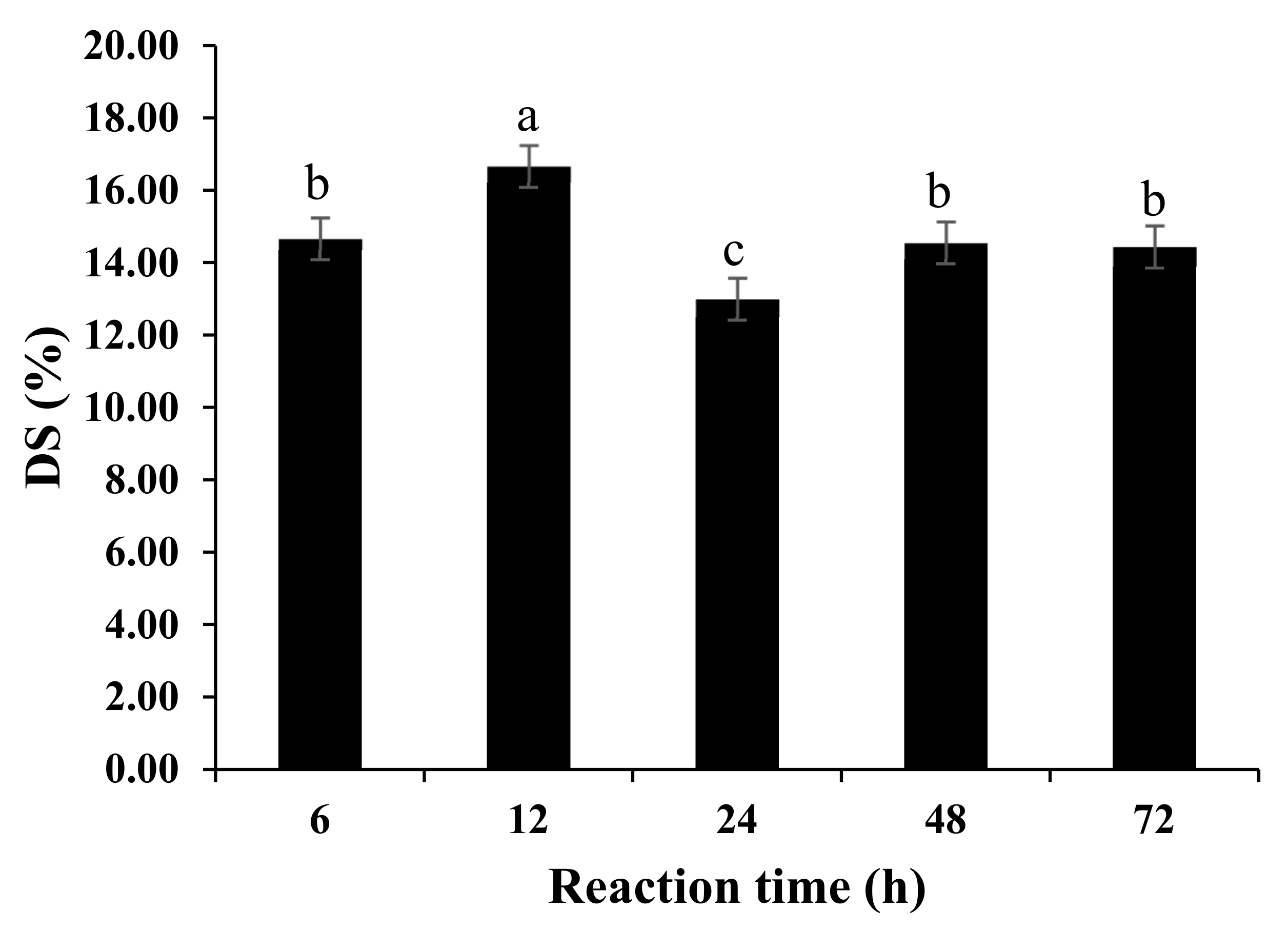
2.4.1. Degree of Substitution
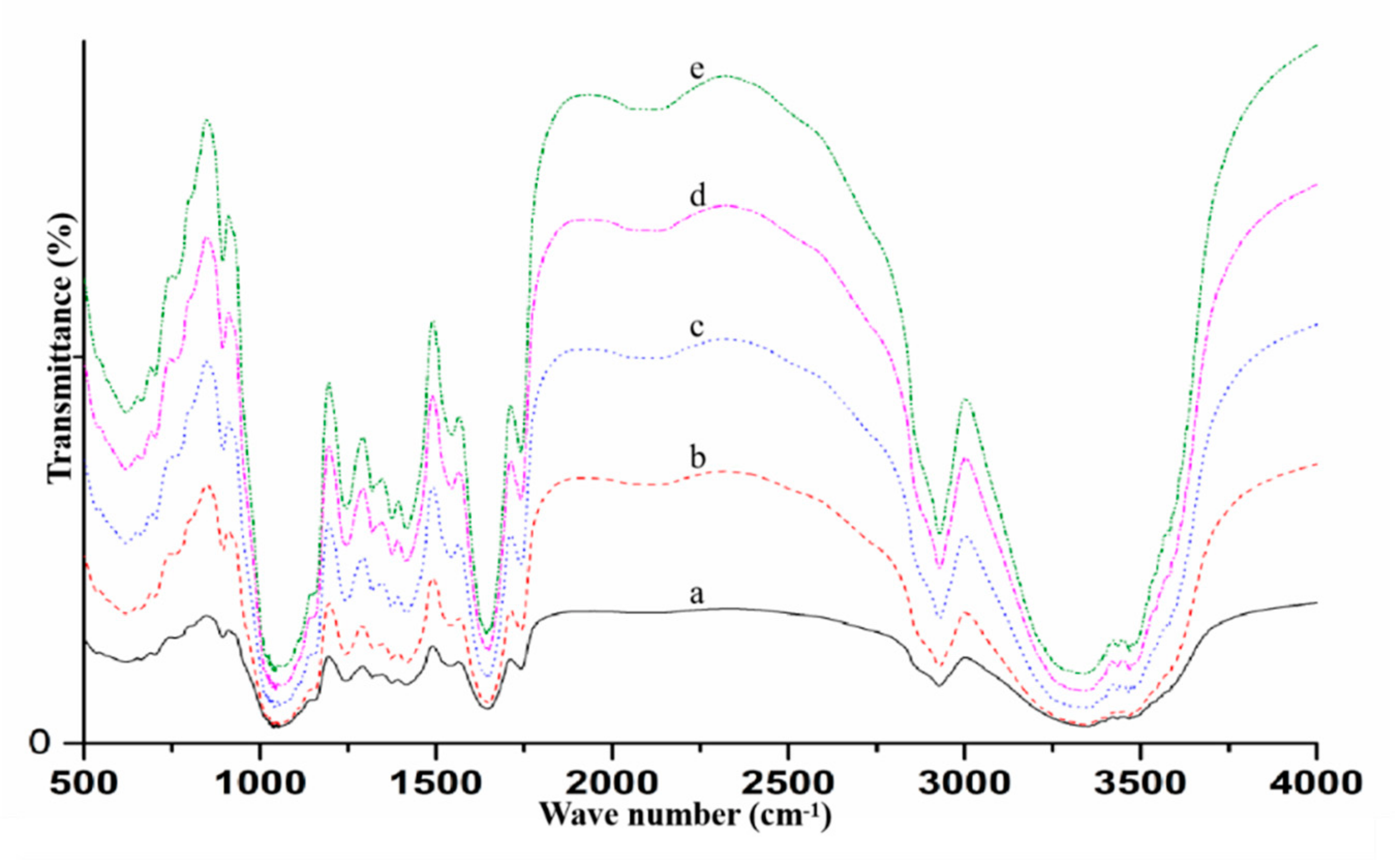
2.4.2. Fourier Transform Infrared (FT-IR) Spectroscopy
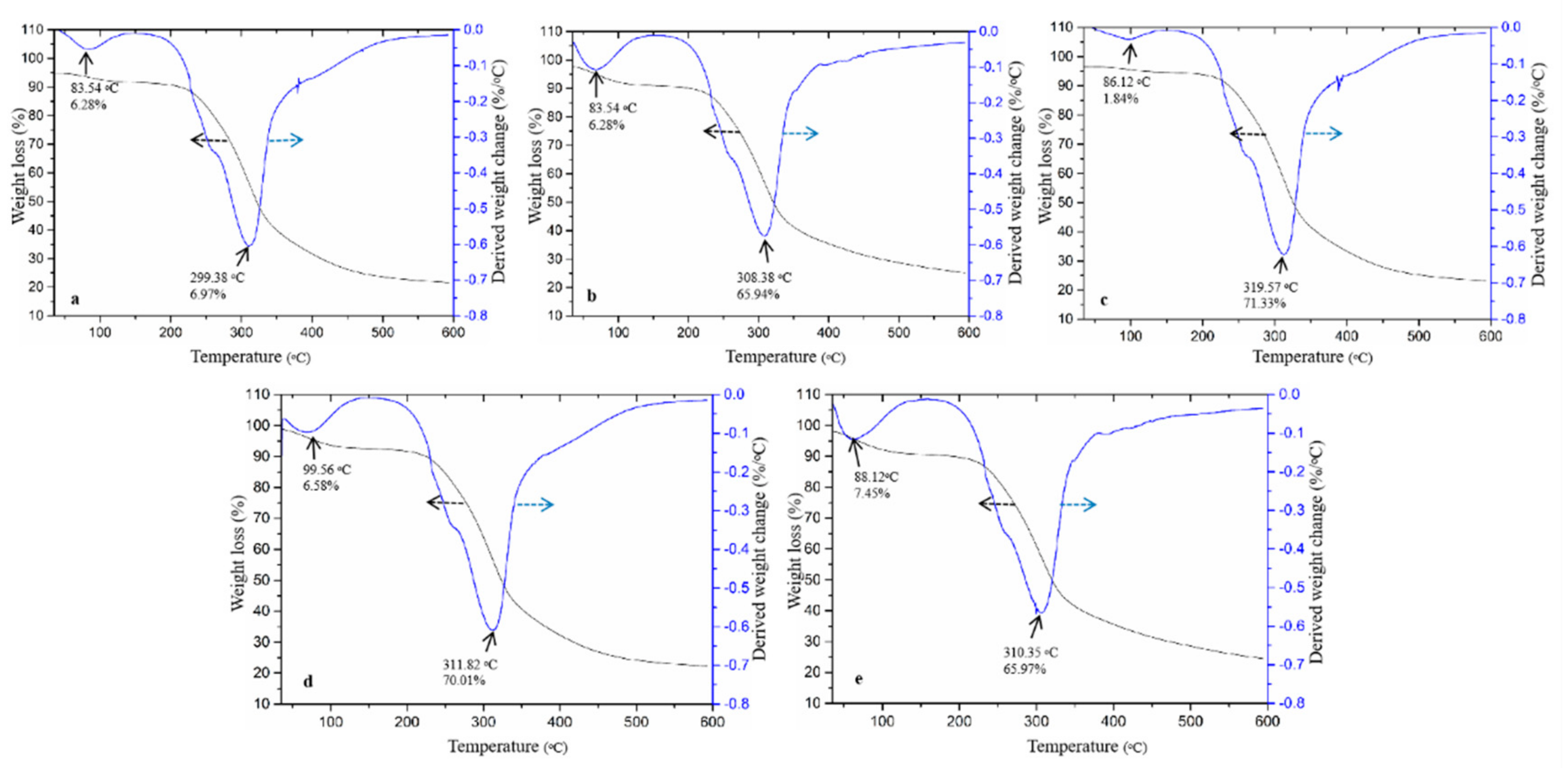
2.4.3. Thermogravimetric Analysis (TGA)
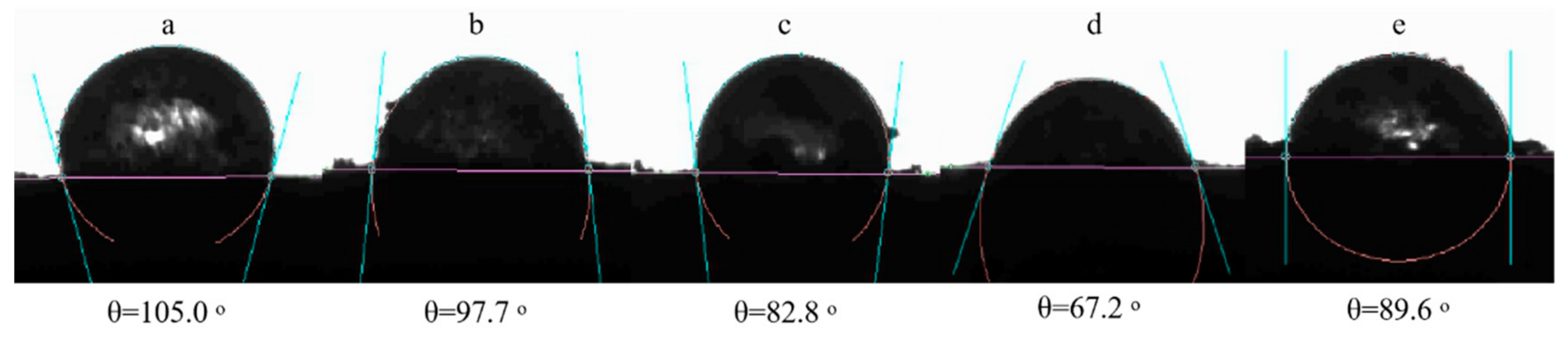
2.4.4. Contact Angle Measurement
2.4.5. Amino Acid Composition
2.5. Determination of Emulsifying Properties
2.5.1. Preparation of Pickering Emulsions
2.5.2. Emulsifying Activity Index (EAI) and Emulsion Stability Index (ESI)
2.5.3. Colour Analysis
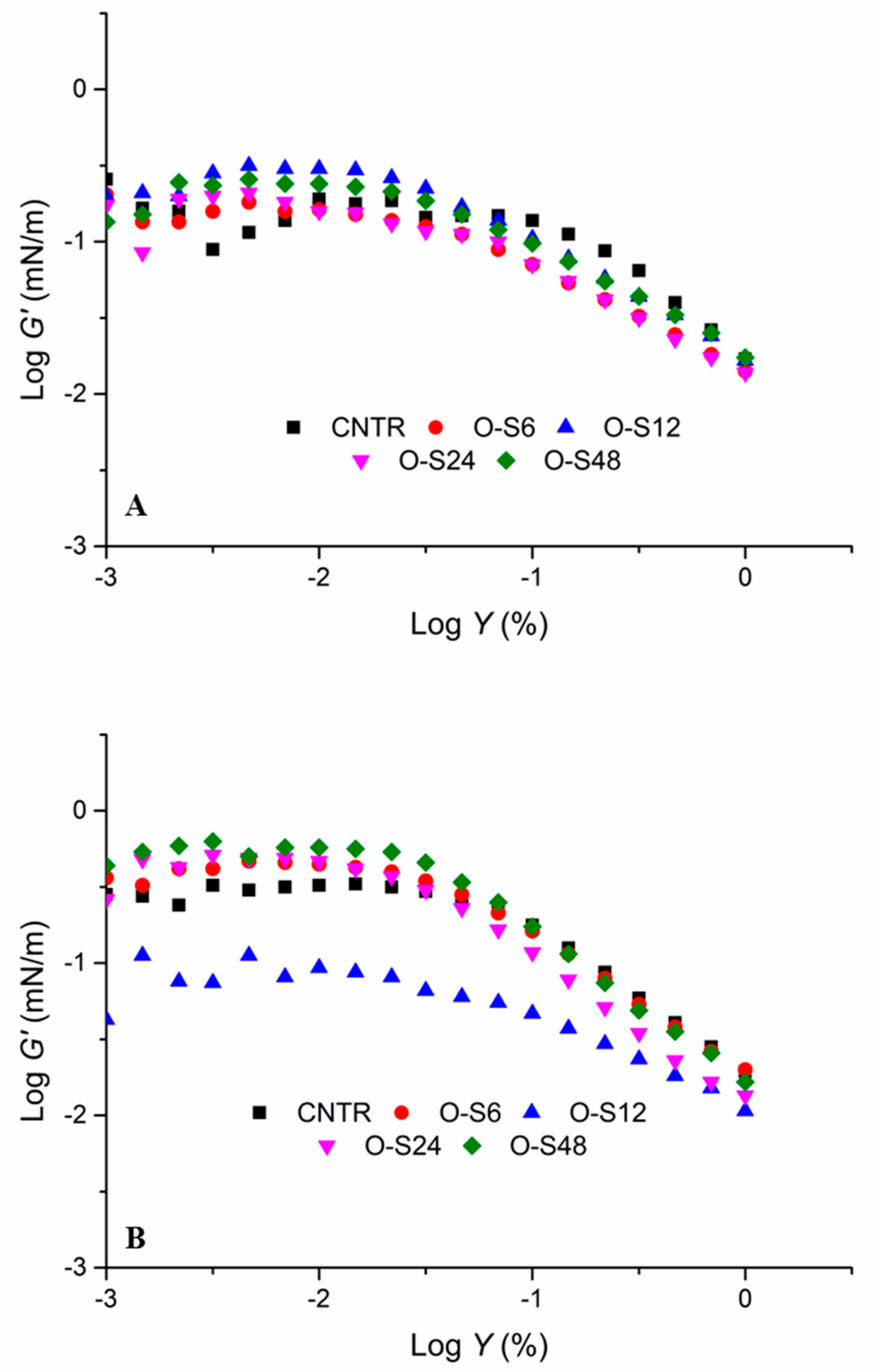
2.5.4. Interfacial Rheology Measurements
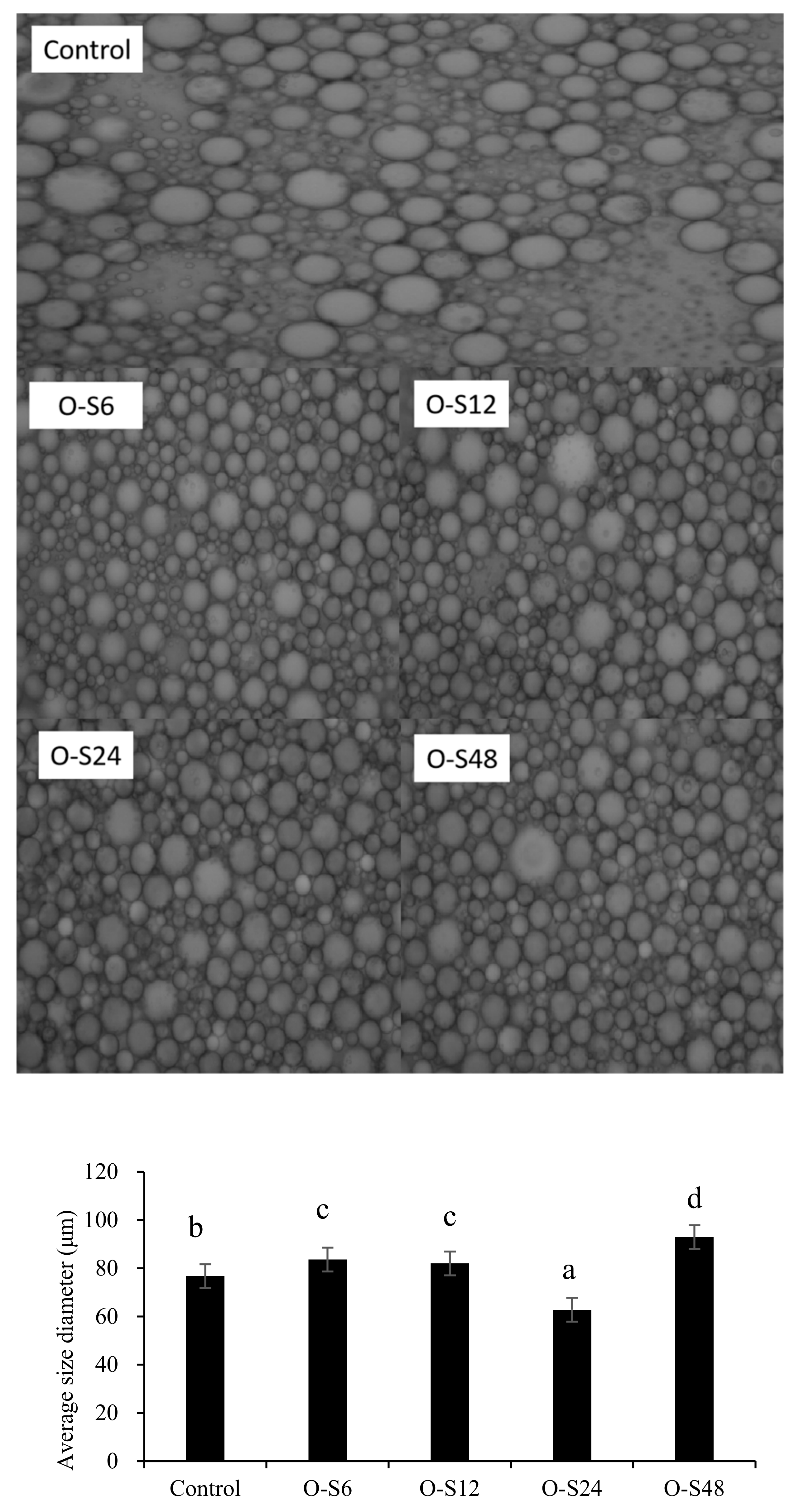
2.5.5. Microstructure Analysis and Droplet Size Measurement
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of ODF-SPI Conjugates
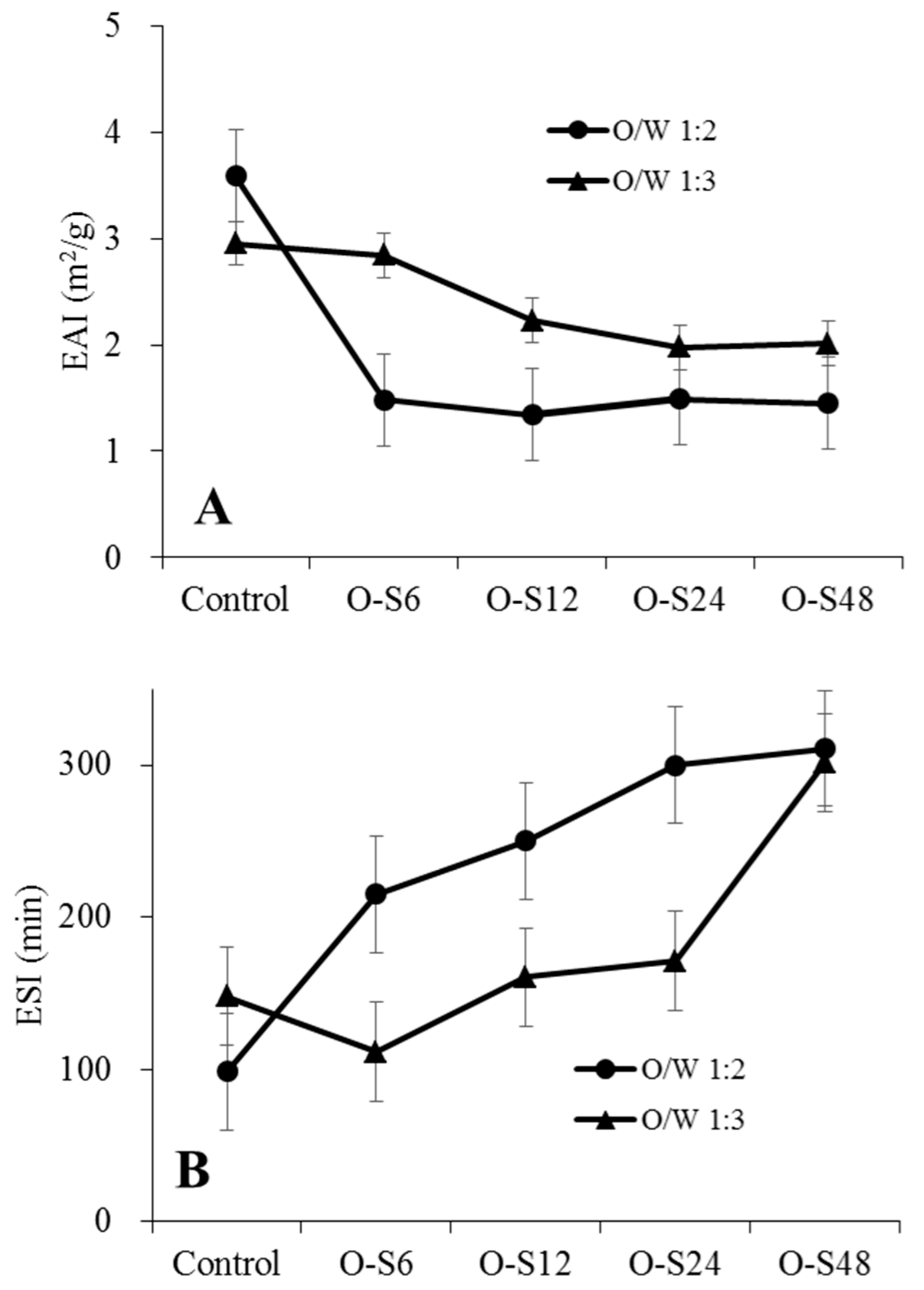
3.2. Pickering Capacity of ODF-SPI Conjugates
3.3. Interfacial Rheological Behaviours of Pickering Emulsions
3.4. Colour Parameters of Pickering Emulsions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Cai, Y.; Liu, T.; Huang, L.; Deng, X.; Zhao, Q.; Zhao, M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physico-chemical treatments. Food Hydrocoll. 2019, 93, 167–175. [Google Scholar] [CrossRef]
- Quintana, G.; Gerbino, E.; Gómez-Zavaglia, A. Valorization of okara oil for the encapsulation of Lactobacillus plantarum. Food Res. Int. 2018, 106, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Porfiri, M.C.; Vaccaro, J.; Stortz, C.A.; Navarro, D.A.; Wagner, J.R.; Cabezas, D.M. Insoluble soybean polysaccharides: Obtaining and evaluation of their O/W emulsifying properties. Food Hydrocoll. 2017, 73, 262–273. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.; Zhang, M. Structural characteristics and physicochemical properties of okarasoybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT-Food Sci. Technol. 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Karunasawat, K.; Anprung, P. Effect of depolymerized mango pulp as a stabilizer in, oil-in-water emulsion containing sodium caseinate. Food Bioprod. Process. 2010, 88, 202–208. [Google Scholar] [CrossRef]
- Saatchi, A.; Kiani, H.; Labbafi, M. A new functional protein-polysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. Int. J. Biol. Macromol. 2019, 122, 659–666. [Google Scholar] [CrossRef]
- Dong, X.; Du, S.; Deng, Q.; Tang, H.; Yang, C.; Wei, F.; Chen, H.; Quek, S.Y.; Zhou, A.; Liu, L. Study on the antioxidant activitiy and emulsifying properties of flaxseed gum-whey protein isolate conjugates prepared by Maillard reaction. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Matemu, A.O.; Kayahara, H.; Murasawa, H.; Nakamura, S. Importance of size and charge of carbohydrate chains in the preparation of functional glycoproteins with excellent emulsifying properties from tofu whey. Food Chem. 2009, 114, 1328–1334. [Google Scholar] [CrossRef]
- Guo, X.; Guo, X.; Meng, H.; Chen, X.; Zeng, Q.; Yu, S. Influences of different pectins on the emulsifying performance of conjugates formed between pectin and whey protein isolate. Int. J. Biol. Macromol. 2019, 123, 246–254. [Google Scholar] [CrossRef]
- Ullah, I.; Hu, Y.; You, J.; Yin, T.; Xiong, S.; Din, Z.U.; Huang, Q.; Liu, R. Influence of okara dietary fiber with varying particle sizes on gelling properties, water state and microstructure of tofu gel. Food Hydrocoll. 2019, 89, 512–522. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of Pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, T.X.; Li, X.T.; Tang, C.H. Novel nanoparticles from insoluble soybean polysaccharides of okara as unique Pickering stabilizers for oil-in-water emulsions. Food Hydrocoll. 2019, 94, 255–267. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zheng, J.; Xu, Y.T.; Yin, S.W.; Liu, F.; Tang, C.H. Surface modification improves fabrication of Pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2018, 75, 125–130. [Google Scholar] [CrossRef]
- Liu, F.; Zheng, J.; Huang, C.H.; Tang, C.H.; Ou, S.Y. Pickering high internal phase emulsions stabilized by protein-covered cellulose nanocrystals. Food Hydrocoll. 2018, 82, 96–105. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Liu, W.S.; Kwok, K.C.; Kwok, F. Isolation and characterization of proteins from soymilk residue (okara)*. Food Res. Int. 1996, 29, 799–805. [Google Scholar] [CrossRef]
- Wang, L.; Wu, M.; Liu, H.M. Emulsifying and physicochemical properties of soy hull hemicelluloses-soy protein isolate conjugates. Carbohydr. Polym. 2017, 163, 181–190. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Wu, W.; Wang, Y. Biological activities and physicochemical properties of Maillard reaction products in sugar–bovine casein peptide model systems. Food Chem. 2013, 141, 3837–3845. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Pan, Q.; Hou, Z.; Yuan, F.; Gao, Y. Development of soy protein isolate-carrageenan conjugates through Maillard reaction for the microencapsulation of Bifidobacterium longum. Food Hydrocoll. 2018, 84, 489–497. [Google Scholar] [CrossRef]
- Rufian-Henares, J.A.; Guerra-Hernandez, E.; García-Villanova, B. Maillard reaction in enteral formula processing: Furosine, loss of o-phthaldialdehyde reactivity, and fluorescence. Food Res. Int. 2002, 35, 527–533. [Google Scholar] [CrossRef]
- Muppalla, S.R.; Sonavale, R.; Chawla, S.P.; Sharma, A. Functional properties of nisin–carbohydrate conjugates formed by radiation induced Maillard reaction. Radiat. Phys. Chem. 2012, 81, 1917–1922. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B 2016, 138, 70–77. [Google Scholar] [CrossRef]
- Li, Y.; Lu, F.; Luo, C.; Chen, Z.; Mao, J.; Shoemaker, C.; Zhong, F. Functional properties of the Maillard reaction products of rice protein with sugar. Food Chem. 2009, 117, 69–74. [Google Scholar] [CrossRef]
- Ames, J.M. The Maillard reaction. In Biochemistry of Food Proteins; Springer: Boston, MA, USA, 1992; pp. 99–153. [Google Scholar]
- Liu, H.; Li, C.; Sun, X.S. Improved water resistance in undecylenic acid (UA)-modified soy protein isolate (SPI)-based adhesives. Ind. Crop. Prod. 2015, 74, 577–584. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Wnorowski, A.; Yaylayan, V.A. Monitoring carbonyl-amine reaction between pyruvic acid and α-amino alcohols by FTIR spectroscopy a possible route to Amadori products. J. Agric. Food Chem. 2003, 51, 6537–6543. [Google Scholar] [CrossRef]
- Tomoglu, S.; Caner, G.; Arabaci, A.; Mutlu, I. Production and sulfonation of bioactive polyetheretherketone foam for bone substitute applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 1167–1176. [Google Scholar] [CrossRef]
- Sosa, N.; Schebor, C.; Pérez, O.E. Encapsulation of citral in formulations containing sucrose or trehalose: Emulsions properties and stability. Food Bioprod. Process. 2014, 92, 266–274. [Google Scholar] [CrossRef]
- Yoshii, H.; Furuta, T.; Maeda, H.; Mori, H. Hydrolysis kinetics of okara and characterization of its water-soluble polysaccharides. Biosci. Biotechnol. Biochem. 1996, 60, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability, and bioaccessibility of β-carotene emulsions. Food Hydrocoll. 2016, 61, 578–588. [Google Scholar] [CrossRef]
- Pugnaloni, L.A.; Dickinson, E.; Ettelaie, R.; Mackie, A.R.; Wilde, P.J. Competitive adsorption of proteins and low-molecular-weight surfactants: Computer simulation and microscopic imaging. Adv. Colloid Interface Sci. 2004, 107, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Mixed biopolymers at interfaces: Competitive adsorption and multilayer structures. Food Hydrocoll. 2011, 25, 1966–1983. [Google Scholar] [CrossRef]
- Wang, L.; Xie, H.; Qiao, X.; Goffin, A.; Hodgkinson, T.; Yuan, X.; Sun, K.; Fuller, G.G. Interfacial rheology of natural silk fibroin at air/water and oil/water interfaces. Langmuir 2011, 28, 459–467. [Google Scholar] [CrossRef]
| Amino Acid (%, Dry Weight Basis) | SPI | ODF-SPI Mixture | O-S6 | O-S12 | O-S24 | O-S48 |
|---|---|---|---|---|---|---|
| Aspartic acid (Asp) | 0.86 | 0.71 | 0.66 | 0.58 | 0.64 | 0.59 |
| Threonine (Thr) | 0.25 | 0.19 | 0.52 | 0.47 | 0.51 | 0.40 |
| Serine (Ser) | 0.30 | 0.23 | 0.89 | 0.81 | 0.89 | 0.72 |
| Glutamate (Glu) | 1.78 | 1.48 | 2.41 | 2.12 | 2.35 | 1.97 |
| Glycine (Gly) | 0.60 | 0.43 | 5.42 | 4.80 | 5.92 | 4.70 |
| Alanine (Ala) | 0.55 | 0.71 | 0.79 | 0.67 | 0.73 | 1.05 |
| Cysteine (Cys) | 0.09 | 0.10 | 23.19 | 23.95 | 25.06 | 22.45 |
| Valine (Val) | 0.05 | 0.08 | 18.81 | 18.66 | 12.81 | 19.05 |
| Methionine (Met) | 2.70 | 2.23 | 2.89 | 2.95 | 3.23 | 2.64 |
| Isoleucine (Ile) | 1.01 | 3.03 | 1.88 | 1.80 | 1.71 | 2.02 |
| Leucine (Leu) | 12.10 | 24.25 | 6.82 | 6.51 | 7.31 | 9.07 |
| Tyrosine (Tyr) | 7.95 | 7.30 | 3.54 | 3.56 | 3.94 | 2.89 |
| Phenylalanine (Phe) | 11.77 | 10.03 | 5.17 | 5.08 | 5.67 | 6.01 |
| Lysine (Lys) | 11.26 | 8.54 | 4.88 | 4.83 | 5.36 | 4.53 |
| Tryptophan (Trp) | 28.57 | 22.13 | 13.31 | 14.31 | 14.05 | 14.35 |
| Histidine (His) | 5.08 | 4.13 | 2.19 | 2.17 | 2.41 | 1.94 |
| Arginine (Arg) | 14.99 | 14.06 | 6.44 | 6.40 | 7.09 | 5.13 |
| Proline (Pro) | 0.10 | 0.15 | 0.21 | 0.33 | 0.31 | 0.50 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample | L * | a * | b * | ∆E | ||||
|---|---|---|---|---|---|---|---|---|
| 1:2 | 1:3 | 1:2 | 1:3 | 1:2 | 1:3 | 1:2 | 1:3 | |
| O-S | 67.80 ± 1.58 d | 62.49 ± 4.91 d | 2.01 ± 0.10 b | 1.97 ± 0.62 a | 15.66 ± 1.20 a | 17.63 ± 1.00 b | - | - |
| O-S6 | 58.16 ± 3.12 b | 57.62 ± 0.20 c | 2.18 ± 0.20 c | 2.21 ± 0.09 c | 17.72 ± 0.22 b | 18.70 ± 0.24 d | 9.86 ± 0.51 b | 4.98 ± 0.11 a |
| O-S12 | 57.61 ± 0.11 a | 53.19 ± 3.33 b | 2.01 ± 0.50 a | 2.38 ± 0.49 c | 18.00 ± 1.40 c | 18.20 ± 1.00 d | 10.46 ± 0.20 d | 9.32 ± 0.78 c |
| O-S24 | 58.08 ± 3.80 b | 54.62 ± 5.00 b | 1.79 ± 0.13 a | 1.99 ± 0.52 b | 18.37 ± 0.33 c | 17.89 ± 0.00 b | 10.09 ± 0.73 c | 7.87 ± 0.45 b |
| O-S48 | 59.87 ± 1.21 | 51.33 ± 3.12 a | 2.23 ± 0.31 d | 2.83 ± 0.11 d | 18.48 ± 0.33 c | 16.88 ± 0.51 a | 8.42 ± 0.44 a | 11.22 ± 0.88 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashaolu, T.J.; Zhao, G. Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction. Foods 2020, 9, 143. https://doi.org/10.3390/foods9020143
Ashaolu TJ, Zhao G. Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction. Foods. 2020; 9(2):143. https://doi.org/10.3390/foods9020143
Chicago/Turabian StyleAshaolu, Tolulope Joshua, and Guohua Zhao. 2020. "Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction" Foods 9, no. 2: 143. https://doi.org/10.3390/foods9020143
APA StyleAshaolu, T. J., & Zhao, G. (2020). Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction. Foods, 9(2), 143. https://doi.org/10.3390/foods9020143







