The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Testing LAB Tolerance to Pepsin in Acidic pH
2.3. Testing LAB Tolerance to Bile Salts Presence
2.4. Testing the Inhibitory Activity of the LAB on Pathogenic Microorganisms by Disk-Diffusion Assay
2.5. Testing the LAB Hemolytic Activity
2.6. Antioxidant Assay
2.7. Antibiotic Susceptibility Test
2.8. Testing the Kombucha Selected LAB for NaCl and pH Tolerance
2.9. Testing the Behavior of Kombucha Selected LAB to the Lyophilization Procedure
2.10. Statistical Analysis
3. Results
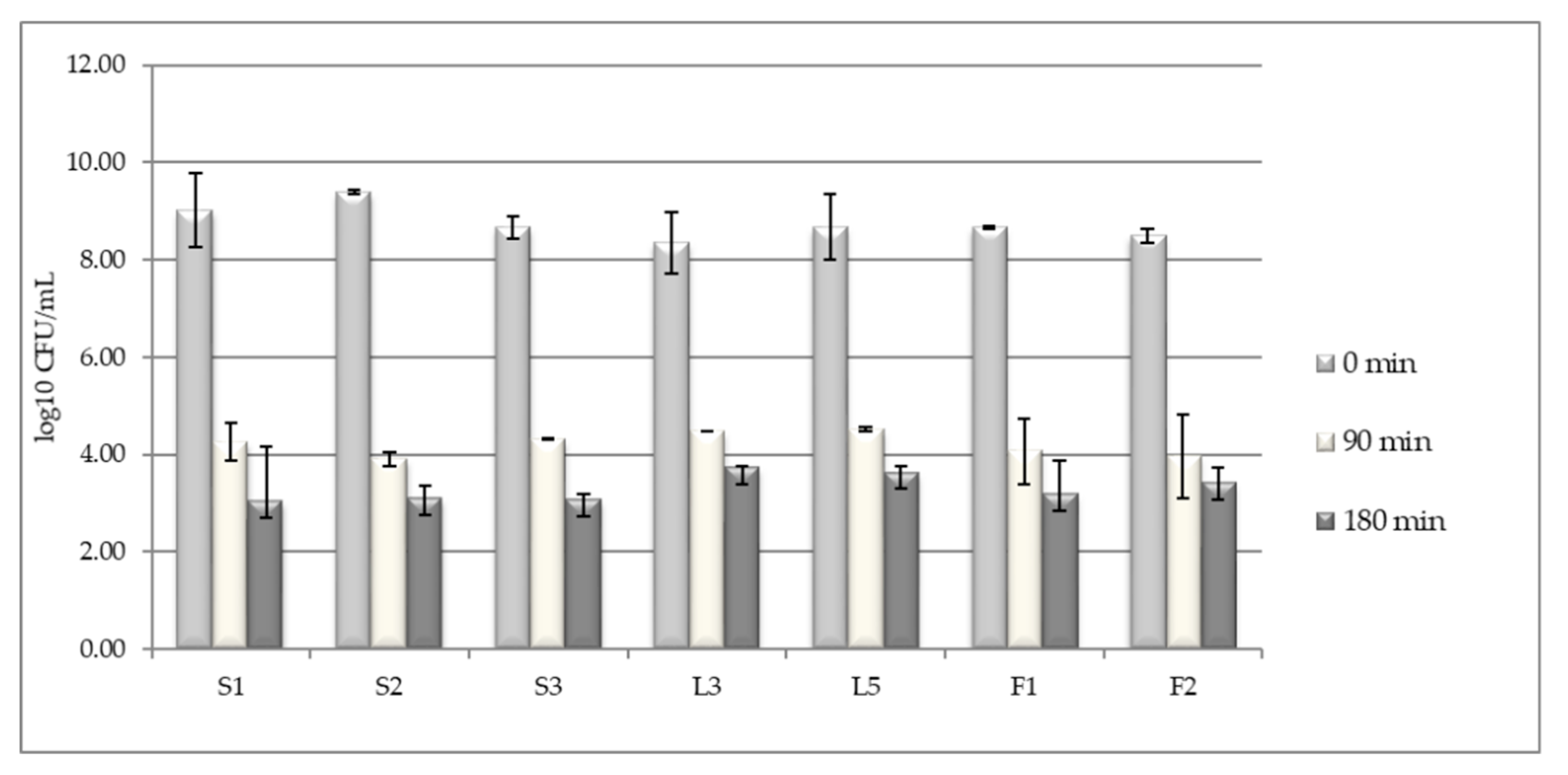
3.1. LAB Tolerance to Pepsin Presence in Acid pH
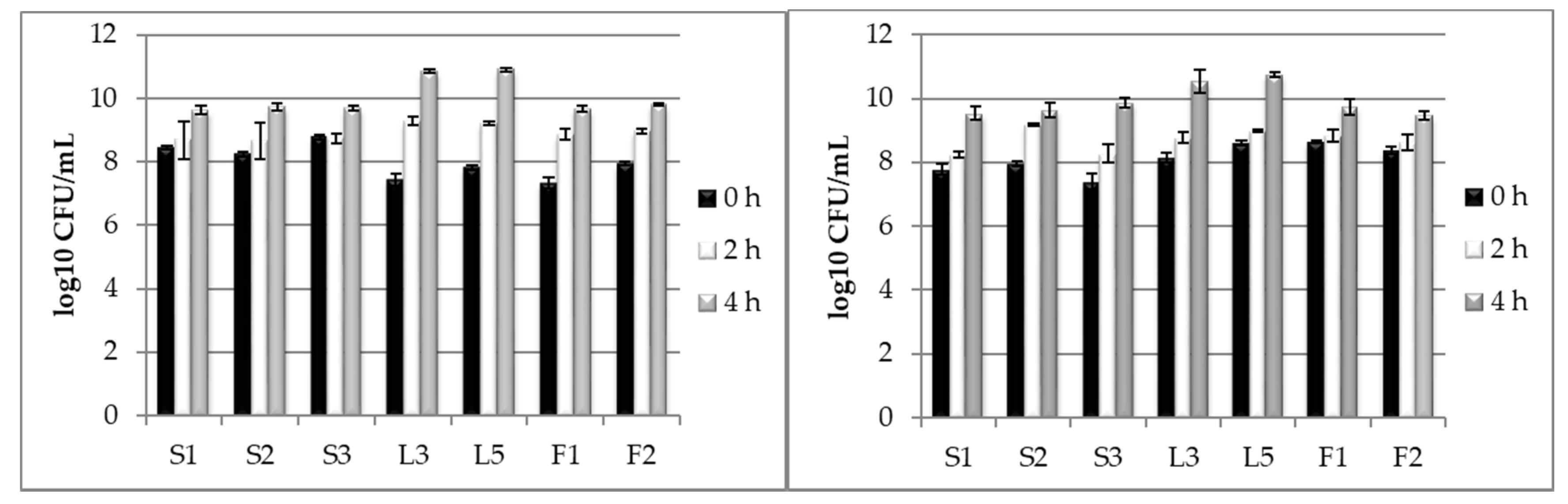
3.2. Testing LAB Tolerance to Bile Salts Presence
3.3. Inhibitory Activity of Kombucha LAB on Pathogenic Microorganisms
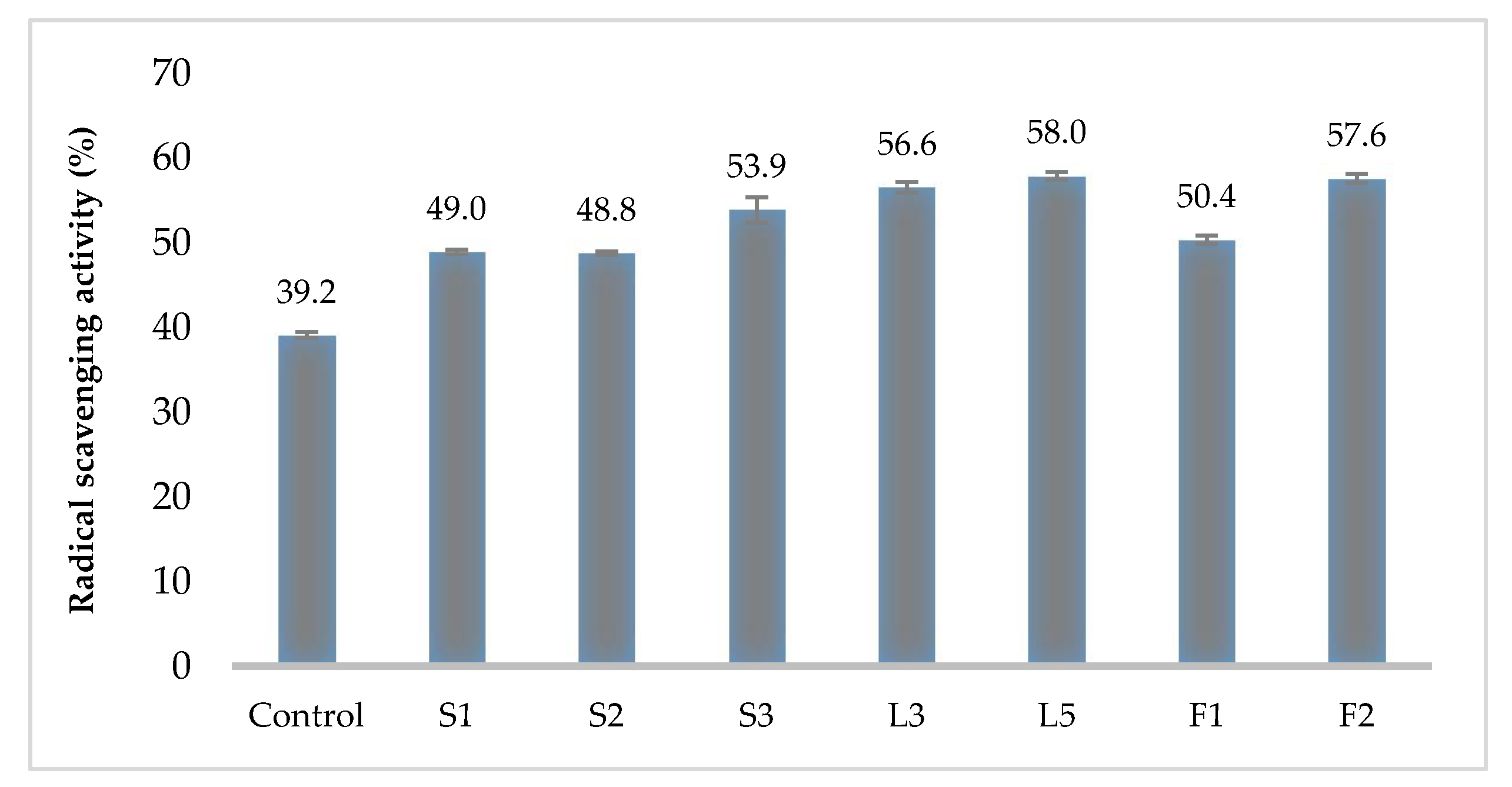
3.4. Hemolytic and Antioxidant Activity of Kombucha LAB Strains
3.5. Antibiotic Susceptibility of Selected LAB Isolates
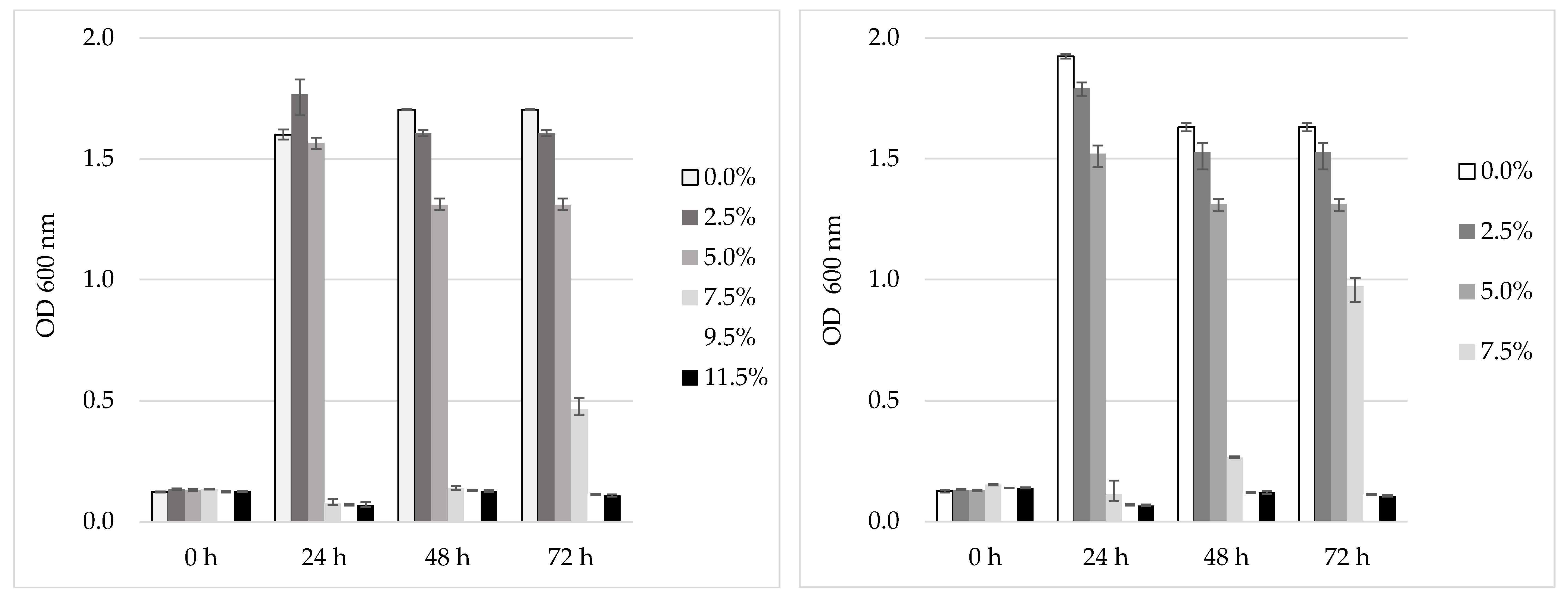
3.6. Tolerance of the Selected LAB to NaCl and pH
3.7. The LAB Behavior under Lyophilization Procedure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. FAO/WHO Joint Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food (30 April 2002 and 1 May 2002); Scientific Research Publishing: London, ON, Canada, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- De Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Dey, G. Non-dairy Probiotic Foods: Innovations and Market Trends. In Innovations in Technologies for Fermented Food and Beverage Industries. Food Microbiology and Food Safety; Panda, S., Shetty, P., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Sornplang, P.; Piyadeatsoontorn, S. Probiotic isolates from unconventional sources: A review. J. Anim. Sci. Technol. 2016, 58, 1–11. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Pramateftaki, P.; Argyri, A.A.; Nychas, G.-J.E.; Tassou, C.C.; Panagou, E.Z. Molecular characterization of lactic acid bacteria isolated from industrially fermented Greek table olives. LWT 2013, 50, 353–356. [Google Scholar] [CrossRef]
- Siripornadulsil, W.; Tasaku, S.; Buahorm, J.; Siripornadulsil, S. Probiotic properties of lactic acid bacteria isolated from fermented food. Intl. J. Biol. Food Vet Agric. Eng. 2014, 8, 364–366. [Google Scholar]
- Bacha, K.; Mehari, T.; Ashenafi, M. In-vitro probiotic potential of lactic acid bacteria isolated from ‘Wakalim’, a traditional Ethiopian fermented beef sausage. Ethiop. J. Health Sci. 2009, 19, 21–29. [Google Scholar]
- Krishnamoorthy, M.; Arjun, P. Probiotic and antimicrobial activity of bacteria from fermented toddy of Cocus nucifera. J. Acad. Indus. Res. 2012, 1, 127–131. [Google Scholar]
- Cardoso, R.R.; Neto, R.O.; D’Almeida, C.T.D.S.; Nascimento, T.P.D.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea. Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Matei, B.; Salzat, J.; Diguta, C.F.; Cornea, C.P.; Luta, G.; Utoiu, E.R.; Matei, F. Lactic acid bacteria isolated from Kombucha as potential probiotics. Rom. Biotech. Letters 2018, 23, 13592–13598. [Google Scholar] [CrossRef]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Vidhyasagar, V.; Jeevaratnam, K. Evaluation of Pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro. J. Funct. Foods 2013, 5, 235–243. [Google Scholar] [CrossRef]
- Oh, Y.J.; Jung, D.S. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented millet alcoholic beverage in Korea. LWT 2015, 63, 437–444. [Google Scholar] [CrossRef]
- Oluwajoba, S.O.; Akinyosoye, F.A.; Oyetayo, V.O. In Vitro Screening and Selection of Probiotic Lactic Acid Bacteria Isolated from Spontaneously Fermenting Kunu-Zaki. Adv. Microbiol. 2013, 3, 309–316. [Google Scholar] [CrossRef]
- Ventura, M.; Perozzi, G. Introduction to the special issue “Probiotic bacteria and human gut microbiota & rdquo. Genes Nutr. 2011, 6, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Chen, L.; Ren, X.; Ge, H.; Li, B.; Ma, G.; Ke, X.; Zhu, J.; Li, L.; et al. Potential probiotic characterization of Lactobacillus reuteri from traditional Chinese highland barley wine and application for room-temperature-storage drinkable yogurt. J. Dairy Sci. 2018, 101, 5780–5788. [Google Scholar] [CrossRef] [PubMed]
- Adetoye, A.; Pinloche, E.; Adeniyi, B.A.; Ayeni, F.A. Characterization and anti-salmonella activities of lactic acid bacteria isolated from cattle faeces. BMC Microbiol. 2018, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Puniya, A.K.; Shukla, P. Probiotic Properties of Lactobacillus plantarum RYPR1 from an Indigenous Fermented Beverage Raabadi. Front. Microbiol. 2016, 7, 1683. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- CLSI. CLSI Supplement M100S. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of antimicrobial properties and their substances against pathogenic bacteria in-vitro by probiotic Lactobacilli strains isolated from commercial yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef]
- Utoiu, E.; Oancea, A.; Stanciuc, A.M.; Stefan, M.L.; Toma, A.; Moraru, A.; Diguta, C.F.; Matei, F.; Cornea, C.P.; Oancea, F. Prebiotic content and probiotic effect of kombucha fermented pollen. AgroLife Sci. J. 2018, 7, 149–156. [Google Scholar]
- Kivanc, M.; Yilmaz, M.; Cakir, E. Isolation and identification of lactic acid bacteria from Boza, and their microbial activity against several reporter strains. Turk. J. Biol. 2011, 35, 313–324. [Google Scholar] [CrossRef]
- Shin, M.; Han, S.; Ryu, J.; Kim, K.; Wan-Kyu, L. Isolation and partial characterization of a bacteriocin produced by Pediococcus pentosaceusK23-2 isolated from Kimchi. J. Appl. Microbiol. 2008, 105, 331–339. [Google Scholar] [CrossRef]
- Cao, Z.; Pan, H.; Tong, H.; Gu, D.; Li, S.; Xu, Y.; Ge, C.; Lin, Q. In vitro evaluation of probiotic potential of Pediococcus pentosaceus L1 isolated from paocai—a Chinese fermented vegetable. Ann. Microbiol. 2015, 66, 963–971. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Regan, P.; Laksanalamai, P.; Healey, S.; Hu, Z. Prevalence and methodologies for detection, characterization and subtyping of Listeria monocytogenes and L. ivanovii in foods and environmental sources. Food Sci. Hum. Wellness 2017, 6, 97–120. [Google Scholar] [CrossRef]
- Dalié, D.; Deschamps, A.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control. 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Mandal, V.; Sen, S.K.; Mandal, N.C. Detection, Isolation and Partial Characterization of Antifungal Compound(s) Produced by Pediococcus acidilactici LAB 5. Nat. Prod. Commun. 2007, 2, 671–674. [Google Scholar] [CrossRef]
- Rouse, S.; Harnett, D.; Vaughan, A.; Van Sinderen, D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008, 104, 915–923. [Google Scholar] [CrossRef]
- Belkacem-Hanfi, N.; Fhoula, I.; Semmar, N.; Guesmi, A.; Perraud-Gaime, I.; Ouzari, H.-I.; Boudabous, A.; Roussos, S. Lactic acid bacteria against post-harvest moulds and ochratoxin A isolated from stored wheat. Biol. Control. 2014, 76, 52–59. [Google Scholar] [CrossRef]
- Matei, B.; Matei, F.; Diguta, C.; Popa, O. Potential use of Kombucha crude extract in postharvest grape moulds control. Sci. Bull. Ser. F Biotechnol. 2017, XXI, 77–80. [Google Scholar]
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.V.; Sreenivasa, M.Y. Probiotic Properties of Lactic Acid Bacteria Isolated from Neera: A Naturally Fermenting Coconut Palm Nectar. Front. Microbiol. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Cryoprotection of probiotic bacteria with poly-γ-glutamic acid produced by Bacillus subtilis and Bacillus licheniformis. J. Genet. Eng. Biotechnol. 2016, 14, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seo, H.; Al Mahmud, H.; Islam, I.; Sultana, O.F.; Lee, Y.; Kim, M.; Song, M.K.A.H.-Y. Melanin Bleaching and Melanogenesis Inhibition Effects of Pediococcus acidilactici PMC48 Isolated from Korean Perilla Leaf Kimchi. J. Microbiol. Biotechnol. 2020, 30, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Kim, A.R.; Lee, K.-S.; Xuan, S.H.; Kang, H.C.; Lee, D.H.; Cha, M.Y.; Kim, H.J.; An, M.; Park, S.N. Anti-Aging Activity of Lavandula angustifolia Extract Fermented with Pediococcus pentosaceus DK1 Isolated from Diospyros kaki Fruit in UVB-Irradiated Human Skin Fibroblasts and Analysis of Principal Components. J. Microbiol. Biotechnol. 2019, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
| Strain | Origin | Special Characteristics |
|---|---|---|
| Bacteria | ||
| Escherichia coli ATCC 8739 | ATCC® | - |
| Salmonella enterica Typhimurium ATCC 14028 | ATCC® | - |
| Staphylococcus epidermidis ATCC 12228 | ATCC® | vancomycin sensitive |
| Staphylococcus epidermidis ATCC 51625 | ATCC® | Methicillin resistant |
| Staphylococcus aureus ATCC 25923 | ATCC® | methicillin sensitive |
| Staphylococcus aureus ATCC 6538 | ATCC® | methicillin sensitive |
| Staphylococcus aureus ATCC 43300 | ATCC® | methicillin and oxacilin -resistant |
| Staphylococcus aureus ATCC 33592 | ATCC® | methicillin and gentamicin -resistant |
| Listeria ivanovii ATCC 19119 | ATCC® | resistant in acid medium |
| Listeria monocytogenes ATCC 7644 | ATCC® | serogroup 1/2c |
| Proteus hauseri (vulgaris) ATCC 13315 | ATCC® | - |
| Streptococcus pyogenes ATCC 19615 | ATCC® | β-hemolytic |
| Bacillus cereus CP1 | UASVM Bucharest | - |
| Yeast | ||
| Candida albicans ATCC 10231 | ATCC® | serotype A |
| Candida parapsilosis ATCC 20019 | ATCC® | - |
| Candida guilermondii MI 40 | UASVM Bucharest | - |
| Candida krusei MI 41 | UASVM Bucharest | - |
| Molds | ||
| Aspergillus niger M4 | UASVM Bucharest | |
| Aspergillus carbonarius MI 15 | UASVM Bucharest | |
| Aspergillus flavus MI 24 | UASVM Bucharest | |
| Penicillium digitatum MI 22 | UASVM Bucharest | |
| Penicillium expansum MI BB Huși | UASVM Bucharest |
| Pathogenic Microorganisms | S1 | S2 | S3 | L3 | L5 | F1 | F2 |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Escherichia coli ATCC 8739 | + | + | + | + | + | + | + |
| Salmonella enterica Typhimurium ATCC 14028 | + + + | + ++ | + + + | + + + | + + | + + + | + + + |
| Staphylococcus epidermidis ATCC 12228 | + + | + + | + + | + + | + + | + + | + + |
| Staphylococcus epidermidis ATCC 51625 | + + | + + | + + | + + | + + | + + | + + |
| Staphylococcus aureus ATCC 25923 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Staphylococcus aureus ATCC 6538 | + + | + + + | + + + | + + + | + + + | + + + | + + |
| Staphylococcus aureus ATCC 43300 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Staphylococcus aureus ATCC 33592 | + + | + + | + + | + + + | + + + | + + | + + + |
| Listeria ivanovii ATCC 19119 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Listeria monocytogenes ATCC 7644 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Proteus hauseri (vulgaris) ATCC 13315 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Streptococcus pyogenes ATCC 19615 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Bacillus cereus CP1 | + + + | + + + | + + + | + + + | + + + | + + + | + + + |
| Yeast | |||||||
| Candida albicans ATCC 10231 | + | ++ | + | ++ | ++ | + | + |
| Candida parapsilosis ATCC 20019 | + | + | + | ++ | ++ | + | + |
| Candida guilermondii MI 40 | + | + | + | ++ | ++ | + | + |
| Candida krusei MI 41 | - | - | - | - | - | - | - |
| Molds | |||||||
| Aspergillus niger M4 | + | + | - | + | + | - | + |
| Aspergillus carbonarius MI 15 | + | + | + | ++ | ++ | ++ | + |
| Aspergillus flavus MI 24 | + | + | - | + | + | - | - |
| Penicillium digitatum MI 22 | +++ | ++ | ++ | +++ | +++ | +++ | +++ |
| Penicillium expansum MI BB Huși | +++ | +++ | ++ | ++ | +++ | +++ | +++ |
| Antibiotic Classes/Antibiotic | LAB Isolates | |
|---|---|---|
| L3 P. pentosaceus | L5 P.acidilactici | |
| Penicillins | ||
| Ampicillin 10 µg/disc | MS | S |
| Penicillin 2 µg/disc | R | S |
| Amoxicillin/Clavulanic acid 20/10 µg/disc | R | R |
| Cephalosporins | ||
| Cephalexin 30 µg/disc | R | R |
| Cefuroxime 30 µg/disc | R | R |
| Ceftriaxone 30 µg/disc | MS | MS |
| Fluoroquinolones | ||
| Ciprofloxacin 1 µg/disc | R | R |
| Norfloxacin 30 µg/disc | R | R |
| Nalidixic acid 30 µg/disc | R | R |
| Aminoglycosides | ||
| Amikacin 10 µg/disc | R | R |
| Gentamicin 10 µg/disc | MS | MS |
| Streptomycin 10 µg/disc | R | R |
| Kanamycin 30 µg/disc | R | R |
| Macrolides | ||
| Erythromycin 10 µg/disc | S | S |
| Lincosamide | ||
| Lincomycin 10 µg/disc | S | S |
| Sulfonamides | ||
| Sulphamethoxazole 25 µg/disc | R | R |
| Glycopeptides | ||
| Vancomycin 10 µg/disc | R | R |
| Tetracyclines | ||
| Tetracycline 30 µg/disc | R | MS |
| Oxytetracycline 30 µg/disc | MS | MS |
| Other | ||
| Chloramphenicol 30 µg/disc | R | MS |
| Colistin 10 µg/disc | R | R |
| Bacitracin 10 U | R | MS |
| Fluconazole 10 µg/disc | R | R |
| Nitrofurantoin 300 µg/disc | R | MS |
| Pathogenic Microorganisms | L3 | L5 |
|---|---|---|
| Listeria ivanovii ATCC 19119 | + + + | + + + |
| Listeria monocytogenes ATCC 7644 | + + + | + + + |
| Salmonella enetrica Typhimurium ATCC 14028 | + + + | + + |
| Staphylococcus aureus ATCC 25923 | + + + | + + + |
| Staphylococcus aureus ATCC 6538 | + + + | + + + |
| Staphylococcus aureus ATCC 43300 | + + + | + + + |
| Staphylococcus aureus ATCC 33592 | + + + | + + + |
| Bacillus cereus CP1 | + + + | + + + |
| Candida albicans ATCC 10231 | ++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diguță, C.F.; Nițoi, G.D.; Matei, F.; Luță, G.; Cornea, C.P. The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods 2020, 9, 1780. https://doi.org/10.3390/foods9121780
Diguță CF, Nițoi GD, Matei F, Luță G, Cornea CP. The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods. 2020; 9(12):1780. https://doi.org/10.3390/foods9121780
Chicago/Turabian StyleDiguță, Camelia Filofteia, George Daniel Nițoi, Florentina Matei, Gabriela Luță, and Călina Petruța Cornea. 2020. "The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium" Foods 9, no. 12: 1780. https://doi.org/10.3390/foods9121780
APA StyleDiguță, C. F., Nițoi, G. D., Matei, F., Luță, G., & Cornea, C. P. (2020). The Biotechnological Potential of Pediococcus spp. Isolated from Kombucha Microbial Consortium. Foods, 9(12), 1780. https://doi.org/10.3390/foods9121780





