Effect of Barley Antifreeze Protein on Dough and Bread during Freezing and Freeze-Thaw Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dough Preparation and Storage
2.3. Dynamic Rheological Measurement of Dough Samples
2.4. Microstructure Measurement of Dough Samples
2.5. Fermentation Properties Determination of Dough Samples
2.6. Baking Properties Determination of Dough Samples
2.7. Hardness Analysis of Bread Crumb
2.8. Crumb Grain Features Observation of Bread
2.9. Statistical Analysis
3. Results and Discussion
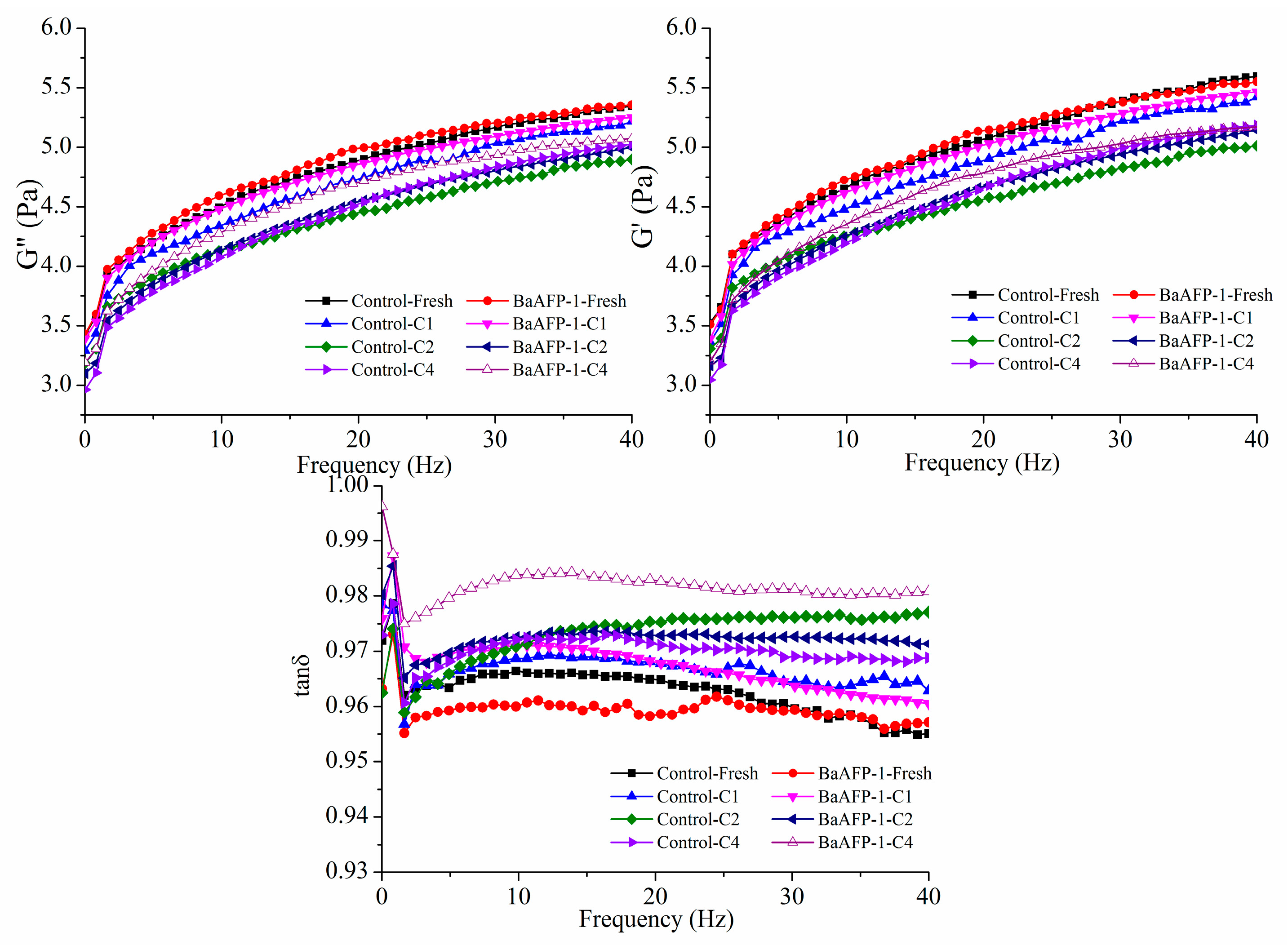
3.1. Effect of BaAFP-1 on the Dynamic Rheological Properties of Dough Samples
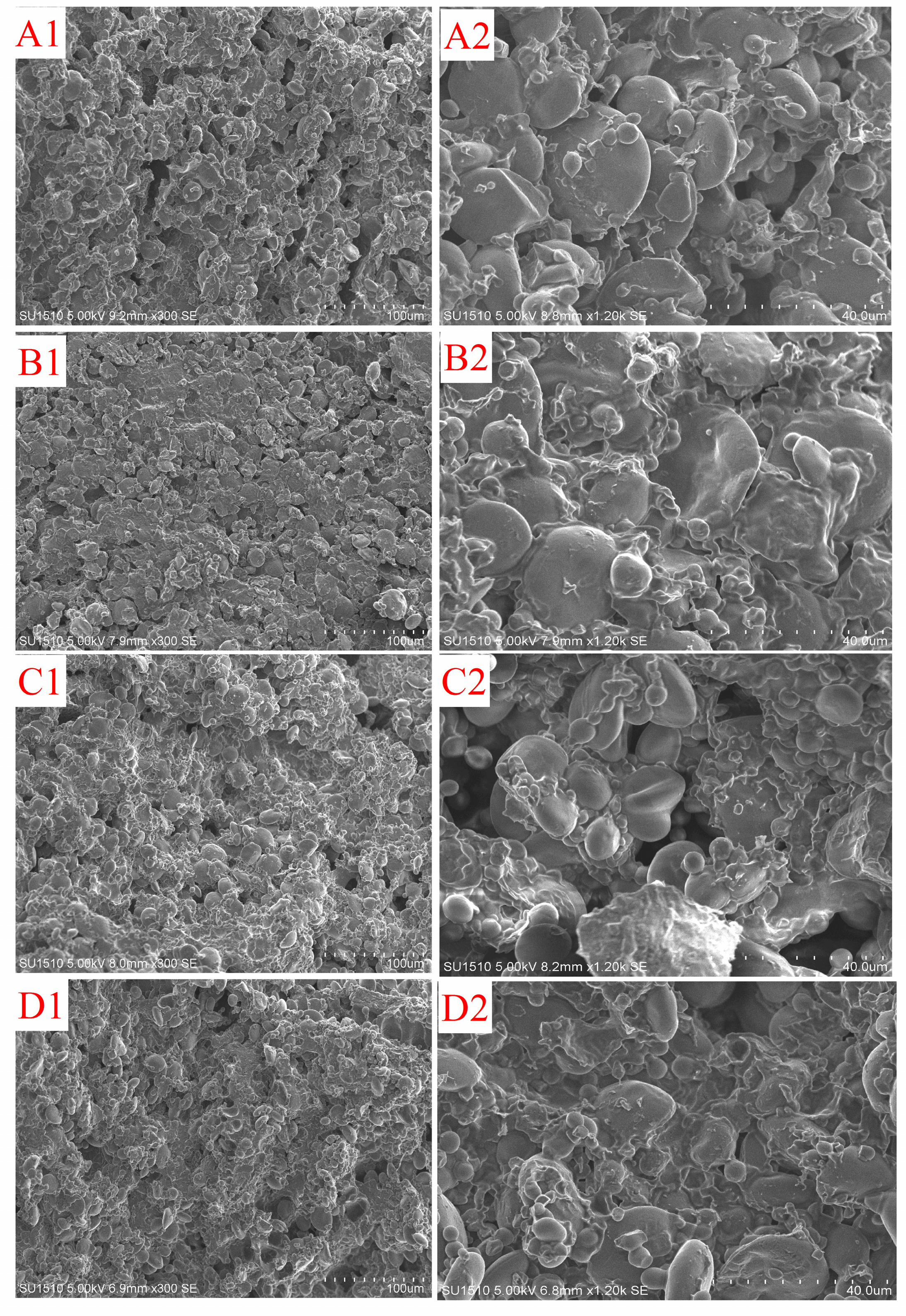
3.2. Effect of BaAFP-1 on the Microstructure of Dough Samples
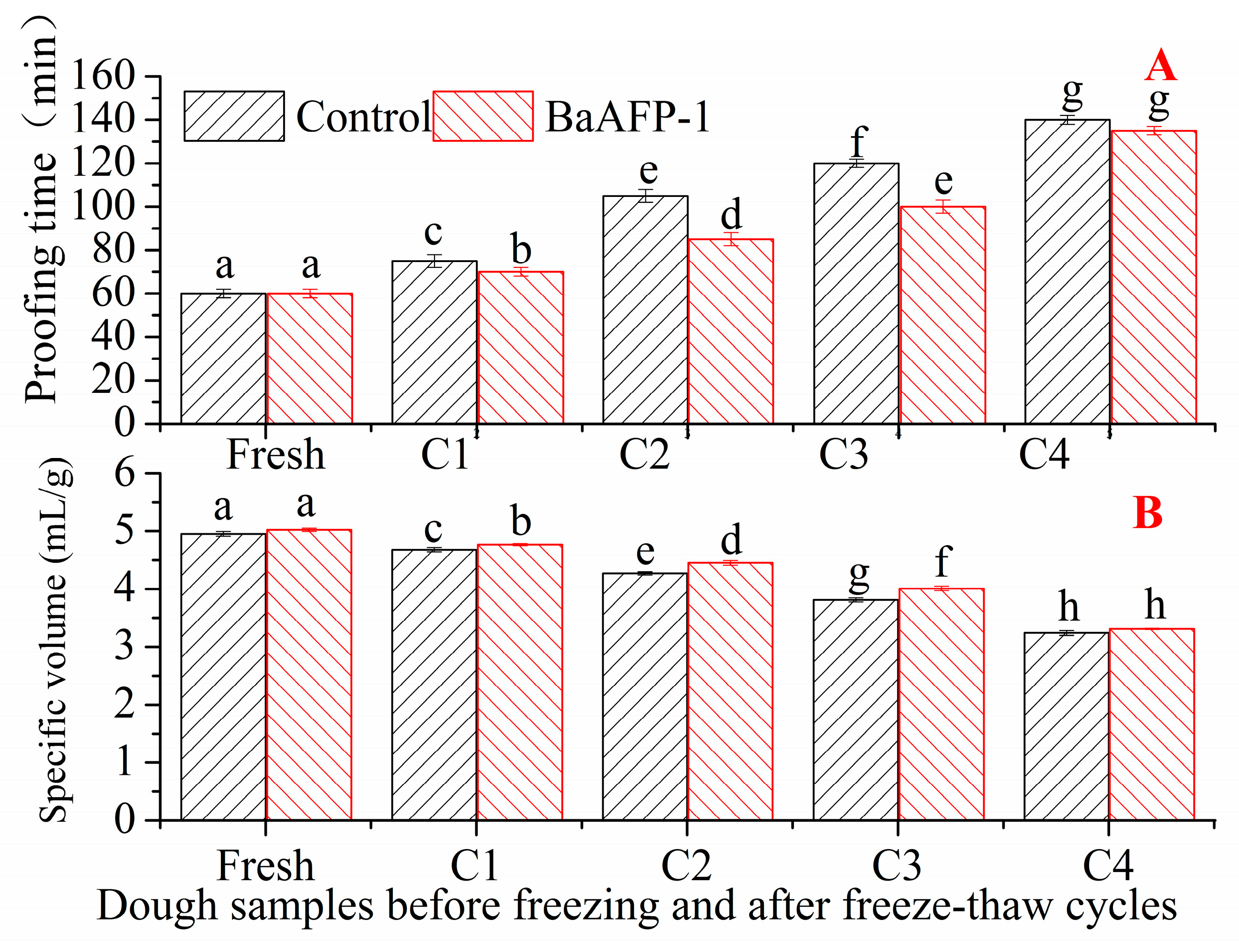
3.3. Effect of BaAFP-1 on the Fermentation Properties of Dough Samples
3.4. Effect of BaAFP-1 on the Baking Properties of Dough Samples
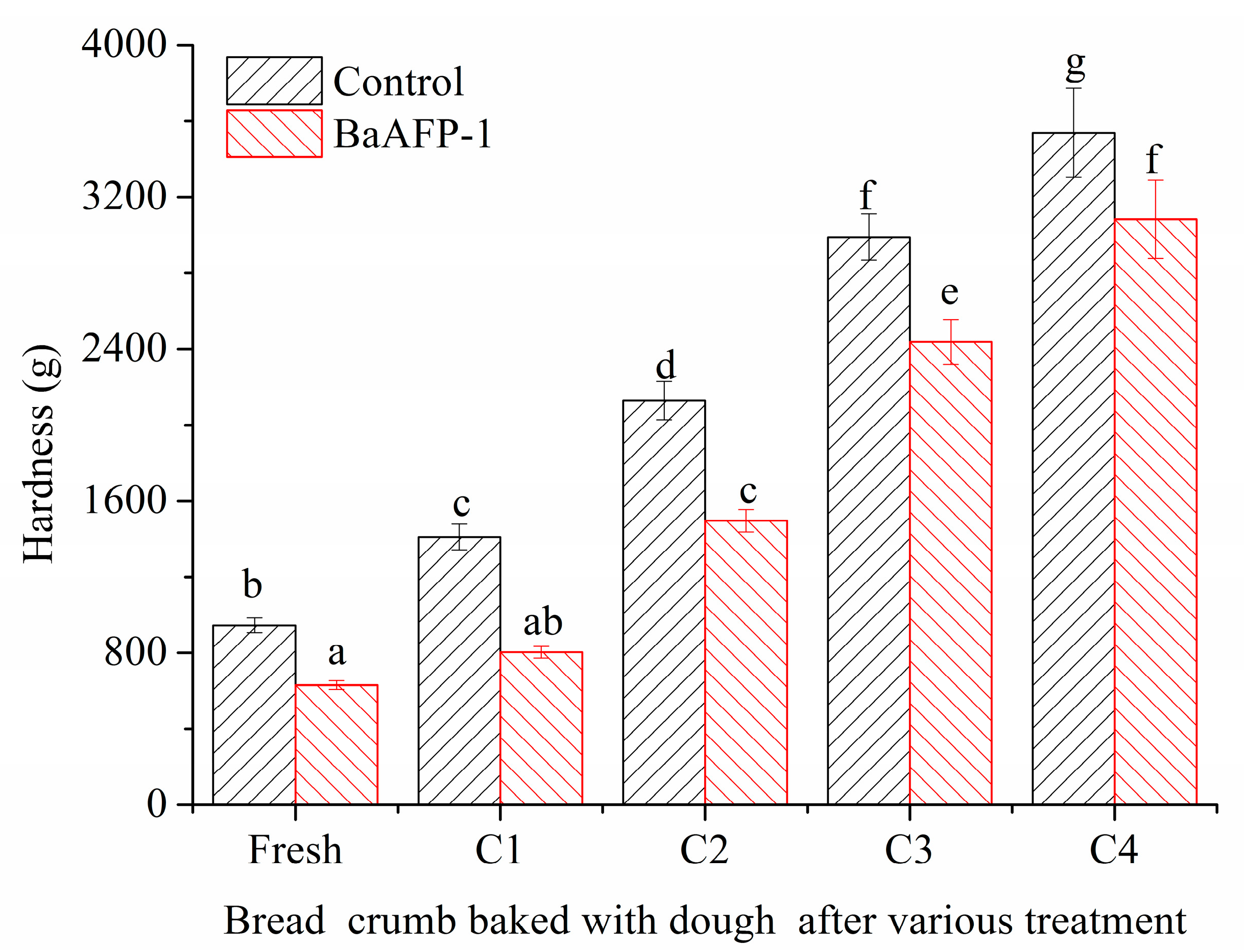
3.5. Effect of BaAFP-1 on Bread Crumb Hardness
3.6. Effect of BaAFP-1 on the Grain Features of Bread Crumbs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kontogiorgos, V.; Goff, H.D.; Kasapis, S. Effect of aging and ice structuring proteins on the morphology of frozen hydrated gluten networks. Biomacromolecules 2007, 8, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V.; Goff, H.D.; Kasapis, S. Effect of aging and ice-structuring proteins on the physical properties of frozen flour–water mixtures. Food Hydrocoll. 2008, 22, 1135–1147. [Google Scholar] [CrossRef]
- Tao, H.; Wang, P.; Ali, B.; Wu, F.; Jin, Z.; Xu, X. Structural and functional properties of wheat starch affected by multiple freezing/thawing cycles. Starch Starke 2015, 67, 683–691. [Google Scholar] [CrossRef]
- Izawa, S.; Ikeda, K.; Takahashi, N.; Inoue, Y. Improvement of tolerance to freeze-thaw stress of baker’s yeast by cultivation with soy peptides. Appl. Microbiol. Biotechnol. 2007, 75, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Selomulyo, V.; Zhou, W. Frozen bread dough: Effects of freezing storage and dough improvers. J. Cereal Sci. 2007, 45, 1–17. [Google Scholar] [CrossRef]
- Ribotta, P.D.; León, A.E.; Añón, M.C. Effect of freezing and frozen storage of doughs on bread quality. J. Agric. Food Chem. 2001, 49, 913–918. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Langstaff, T.M.; Berzonsky, W.A. Effect of frozen storage and freeze–thaw cycles on the rheological and baking properties of frozen doughs. Food Res. Int. 2003, 36, 365–372. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Huang, W.; Liu, J.G.; Tilley, M.; Yao, Y. Rheology, microstructure, and baking characteristics of frozen dough containing Rhizopus chinensis lipase and transglutaminase. Cereal Chem. 2011, 88, 596–601. [Google Scholar] [CrossRef]
- Ribotta, P.; Perez, G.; Leon, A.; Anon, M. Effect of emulsifier and guar gum on micro structural, rheological and baking performance of frozen bread dough. Food Hydrocoll. 2004, 18, 305–313. [Google Scholar] [CrossRef]
- Kim, Y.S.; Huang, W.; Du, G.; Pan, Z.; Chung, O. Effects of trehalose, transglutaminase, and gum on rheological, fermentation, and baking properties of frozen dough. Food Res. Int. 2008, 41, 903–908. [Google Scholar] [CrossRef]
- Jia, C.; Huang, W.; Wu, C.; Lv, X.; Rayas-Duarte, P.; Zhang, L. Characterization and yeast cryoprotective performance for thermostable ice-structuring proteins from Chinese Privet (Ligustrum vulgare) leaves. Food Res. Int. 2012, 49, 280–284. [Google Scholar] [CrossRef]
- Shi, K.; Yu, H.; Lee, T.-C. A novel approach for improving yeast viability and baking quality of frozen dough by adding biogenic ice nucleators from Erwinia herbicola. J. Cereal Sci. 2013, 57, 237–243. [Google Scholar] [CrossRef]
- Jia, C.; Huang, W.; Rayas-Duarte, P.; Zou, Q.; Zhang, L.; Li, Y. Hydration, polymerization and rheological properties of frozen gluten-water dough as influenced by thermostable ice structuring protein extract from Chinese privet (Ligustrum vulgare) leaves. J. Cereal Sci. 2014, 59, 132–136. [Google Scholar] [CrossRef]
- DeLuca, C.I.; Comley, R.; Davies, P.L. Antifreeze proteins bind independently to ice. Biophys. J. 1998, 74, 1502–1508. [Google Scholar] [CrossRef][Green Version]
- Barrett, J. Thermal hysteresis proteins. Int. J. Biochem. Cell Biol. 2001, 33, 105–117. [Google Scholar] [CrossRef]
- Strom, C.S.; Liu, X.; Jia, Z. Antifreeze Protein-induced Morphological Modification Mechanisms Linked to Ice Binding Surface. J. Biol. Chem. 2004, 279, 32407–32417. [Google Scholar] [CrossRef]
- Venketesh, S.; Dayananda, C. Properties, potentials, and prospects of antifreeze proteins. Crit. Rev. Biotechnol. 2008, 28, 57–82. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Xiao, J. Effect of barley antifreeze protein on thermal properties and water state of dough during freezing and freeze-thaw cycles. Food Hydrocoll. 2015, 47, 32–40. [Google Scholar] [CrossRef]
- Arav, A.; Rubinsky, B.; Seren, E.; Roche, J.F.; Boland, M.P. The role of thermal hysteresis proteins during cryopreservation of oocytes and embryos. Theriogenology 1994, 41, 107–112. [Google Scholar] [CrossRef]
- Urrutia, M.E.; Duman, J.G.; Knight, C.A. Plant thermal hysteresis proteins. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1992, 1121, 199–206. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, H.; Chen, H.; Wang, L.; Qian, H.; Qi, X. Extraction, purification and identification of antifreeze proteins from cold acclimated malting barley (Hordeum vulgare L.). Food Chem. 2015, 175, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Doring, C.; Jekle, M.; Becker, T. Mechanisms behind Distiller’s Grains Impact on Wheat Dough and Bread Quality. Food Bioprocess Technol. 2016, 9, 274–284. [Google Scholar] [CrossRef]
- Gao, J.; Wong, J.X.; Lim, J.C.-S.; Henry, J.; Zhou, W. Influence of bread structure on human oral processing. J. Food Eng. 2015, 167, 147–155. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Dynamic rheological properties of wheat flour dough and proteins. Trends Food Sci. Technol. 2007, 18, 132–138. [Google Scholar] [CrossRef]
- Zaidel, D.A.; Chin, N.L.; Yusof, Y.A. A Review on Rheological Properties and Measurements of Dough and Gluten. J. Appl. Sci. 2010, 10, 2478–2490. [Google Scholar]
- Sivam, A.S.; Sunwaterhouse, D.; Quek, S.Y.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Dhaka, V.; Khatkar, B.S. Effects of Gliadin/Glutenin and HMW-GS/LMW-GS Ratio on Dough Rheological Properties and Bread-Making Potential of Wheat Varieties. J. Food Qual. 2015, 38, 71–82. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Fido, R.J.; Tatham, A.S.; Schofield, J.D. Functional Properties of Wheat Gliadins. II. Effects on Dynamic Rheological Properties of Wheat Gluten. J. Cereal Sci. 2002, 35, 307–313. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Mohanad, B.; Xu, L.; Ning, Y.; Xu, J.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of frozen storage on physico-chemistry of wheat gluten proteins: Studies on gluten-, glutenin- and gliadin-rich fractions. Food Hydrocoll. 2014, 39, 187–194. [Google Scholar] [CrossRef]
- Wrigley, C.; Bekes, F.; Bushuk, W. Chapter 1 Gluten: A Balance of Gliadin and Glutenin. In Gliadin and Glutenin: The Unique Balance of Wheat Quality; American Association of Cereal Chemists International: Eagan, MN, USA, 2006; pp. 3–32. [Google Scholar]
- Goff, H.D. Low-temperature stability and the glassy state in frozen foods. Food Res. Int. 1992, 25, 317–325. [Google Scholar] [CrossRef]
- Amornwittawat, N.; Wang, S.; Duman, J.G.; Wen, X. Polycarboxylates enhance beetle antifreeze protein activity. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1942–1948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, K.; Keil, M.; Naldrett, M.J. A leucine-rich repeat protein of carrot that exhibits antifreeze activity. FEBS Lett. 1999, 447, 171–178. [Google Scholar] [CrossRef]
- Jia, C.; Huang, W.; Wu, C.; Zhong, J.; Rayas-Duarte, P.; Guo, C. Frozen bread dough properties modified by thermostable ice structuring proteins extract from Chinese Privet (Ligustrum vulgare) leaves. Cereal Chem. 2012, 89, 162–167. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, S.-S.; Jiang, L.-L.; Ye, X.-J.; Ng, T.B.; Wu, Z.-J. Plant antifungal proteins and their applications in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 4961–4981. [Google Scholar] [CrossRef] [PubMed]
- Meziani, S.; Ioannou, I.; Jasniewski, J.; Belhaj, N.; Muller, J.-M.; Ghoul, M.; Desobry, S. Effects of freezing treatments on the fermentative activity and gluten network integrity of sweet dough. LWT Food Sci. Technol. 2012, 46, 118–126. [Google Scholar] [CrossRef]
- Loveday, S.M.; Huang, V.T.; Reid, D.S.; Winger, R.J. Water dynamics in fresh and frozen yeasted dough. Crit. Rev. Food Sci. Nutr. 2012, 52, 390–409. [Google Scholar] [CrossRef]
- Wolt, M.; D’appolonia, B. Factors involved in the stability of frozen dough. I. the influence of yeast reducing compounds on frozen-dough stability. Cereal Chem. 1984, 61, 209–212. [Google Scholar]
- Xu, H.-N.; Huang, W.; Jia, C.; Kim, Y.; Liu, H. Evaluation of water holding capacity and breadmaking properties for frozen dough containing ice structuring proteins from winter wheat. J. Cereal Sci. 2009, 49, 250–253. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.-H.; Li, L.; Wang, S.-Y. The cryoprotective effects of antifreeze peptides from pigskin collagen on texture properties and water mobility of frozen dough subjected to freeze–thaw cycles. Eur. Food Res. Technol. 2017, 243, 1149–1156. [Google Scholar] [CrossRef]
- Guarda, A.; Rosell, C.M.; Benedito, C.; Galotto, M.J. Different hydrocolloids as bread improvers and antistaling agents. Food Hydrocoll. 2004, 18, 241–247. [Google Scholar] [CrossRef]
- de la Hera, E.; Rosell, C.M.; Gomez, M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014, 151, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, H.; Wang, L.; Yao, H. Validation of antifreeze properties of glutathione based on its thermodynamic characteristics and protection of baker’s yeast during cryopreservation. J. Agric. Food Chem. 2007, 55, 4698–4703. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, Y.; Huang, W.; Jia, C.; Xu, B. Effects of ice structuring proteins on freeze-thaw stability of corn and wheat starch gels. Cereal Chem. 2010, 87, 497–503. [Google Scholar] [CrossRef]
- Cauvain, S.P.; Young, L.S. Technology of Breadmaking; Springer: London, UK, 2007; pp. 299–332. [Google Scholar]

| Hm | T1 | Hm’ | Total Volume | Retention Volume | R | ||
|---|---|---|---|---|---|---|---|
| Control | Fresh | 33.2 ± 1.13 a | 57 ± 1.41 a | 52.7 ± 0.42 a | 1233 ± 2.83 a | 1111 ± 0.71 a | 90.1 ± 0.28 a |
| C1 | 27.1 ± 0.28 b | 73 ± 0.71 b | 45.1 ± 1.27 c | 1078 ± 2.83 c | 992 ± 2.12 c | 92.0 ± 0.71 c | |
| C2 | 13.1 ± 0.28 e | 107 ± 0.00 e | 38.4 ± 0.57 d | 930 ± 1.41 e | 846 ± 4.95 f | 90.9 ± 0.28 b | |
| C3 | 12.5 ± 0.35 e | 125 ± 1.41 f | 30.5 ± 0.78 f | 751 ± 0.00 g | 717 ± 0.71 i | 95.4 ± 0.28 ef | |
| C4 | 7.0 ± 0.14 g | 180 ± 2.12 h | 29.1 ± 0.28 f | 721 ± 1.41 h | 692 ± 0.71 j | 95.9 ± 0.42 f | |
| BaAFP-1 | Fresh | 34.2 ± 0.21 a | 58 ± 0.71 a | 53.2 ± 1.41 a | 1148 ± 3.54 b | 1092 ± 3.54 b | 95.1 ± 0.71 e |
| C1 | 28.1 ± 0.07 b | 70 ± 2.83 b | 47.4 ± 0.92 b | 1004 ± 0.00 d | 959 ± 0.71 d | 95.4 ± 0.28 ef | |
| C2 | 20.2 ± 0.21 c | 86 ± 2.12 c | 39.0 ± 1.41 d | 934 ± 0.71 e | 867 ± 0.71 e | 92.8 ± 0.71 d | |
| C3 | 15.3 ± 0.42 d | 102 ± 2.12 d | 35.7 ± 0.21 e | 817 ± 1.41 f | 735 ± 2.12 g | 89.9 ± 0.42 a | |
| C4 | 11.2 ± 0.07 f | 136 ± 2.12 g | 29.6 ± 0.28 f | 752 ± 0.71 g | 729 ± 0.71 h | 96.9 ± 0.42 g |
| Number | Average Area (mm2) | ||
|---|---|---|---|
| Control | Fresh | 508.46 ± 8.32 b | 125 ± 0.71 b |
| C1 | 551.15 ± 8.70 c | 113 ± 1.41 c | |
| C2 | 699.42 ± 12.03 d | 106 ± 2.12 d | |
| C3 | 881.46 ± 7.70 f | 84 ± 1.41 f | |
| C4 | 1232.10 ± 7.94 h | 60 ± 1.41 h | |
| BaAFP-1 | Fresh | 469.17 ± 12.99 a | 134 ± 2.83 a |
| C1 | 567.87 ± 14.62 c | 112 ± 2.83 c | |
| C2 | 691.60 ± 5.97 d | 93 ± 2.12 e | |
| C3 | 809.66 ± 8.22 e | 90 ± 1.41 e | |
| C4 | 989.93 ± 3.10 g | 78 ± 2.12 g | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Li, T.; Zhang, H.; Guan, C.; Qian, J.; Zhou, X. Effect of Barley Antifreeze Protein on Dough and Bread during Freezing and Freeze-Thaw Cycles. Foods 2020, 9, 1698. https://doi.org/10.3390/foods9111698
Ding X, Li T, Zhang H, Guan C, Qian J, Zhou X. Effect of Barley Antifreeze Protein on Dough and Bread during Freezing and Freeze-Thaw Cycles. Foods. 2020; 9(11):1698. https://doi.org/10.3390/foods9111698
Chicago/Turabian StyleDing, Xiangli, Tingting Li, Hui Zhang, Chengran Guan, Jianya Qian, and Xiaoyan Zhou. 2020. "Effect of Barley Antifreeze Protein on Dough and Bread during Freezing and Freeze-Thaw Cycles" Foods 9, no. 11: 1698. https://doi.org/10.3390/foods9111698
APA StyleDing, X., Li, T., Zhang, H., Guan, C., Qian, J., & Zhou, X. (2020). Effect of Barley Antifreeze Protein on Dough and Bread during Freezing and Freeze-Thaw Cycles. Foods, 9(11), 1698. https://doi.org/10.3390/foods9111698





