Barley C-Hordein as the Calibrant for Wheat Gluten Quantification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gluten Composition Analysis by Reverse-Phase-High Performance Liquid Chromatography (RP-HPLC)
2.3. Isolation of Total Gluten and Their R5 Reactivity
2.4. Isolation of Gluten Subunits and R5 Sandwich Responses
2.5. Spiking Oat Flour and Oat Biscuits and Calibration with C-Hordein Standard
2.6. Statistical Analysis
3. Results
3.1. Protein Composition of 27 Common Wheat Cultivars
3.2. The R5 Reactivity of Total Gluten of 27 Cultivars
3.3. Reactivity of Gluten Types Against R5 Antibody
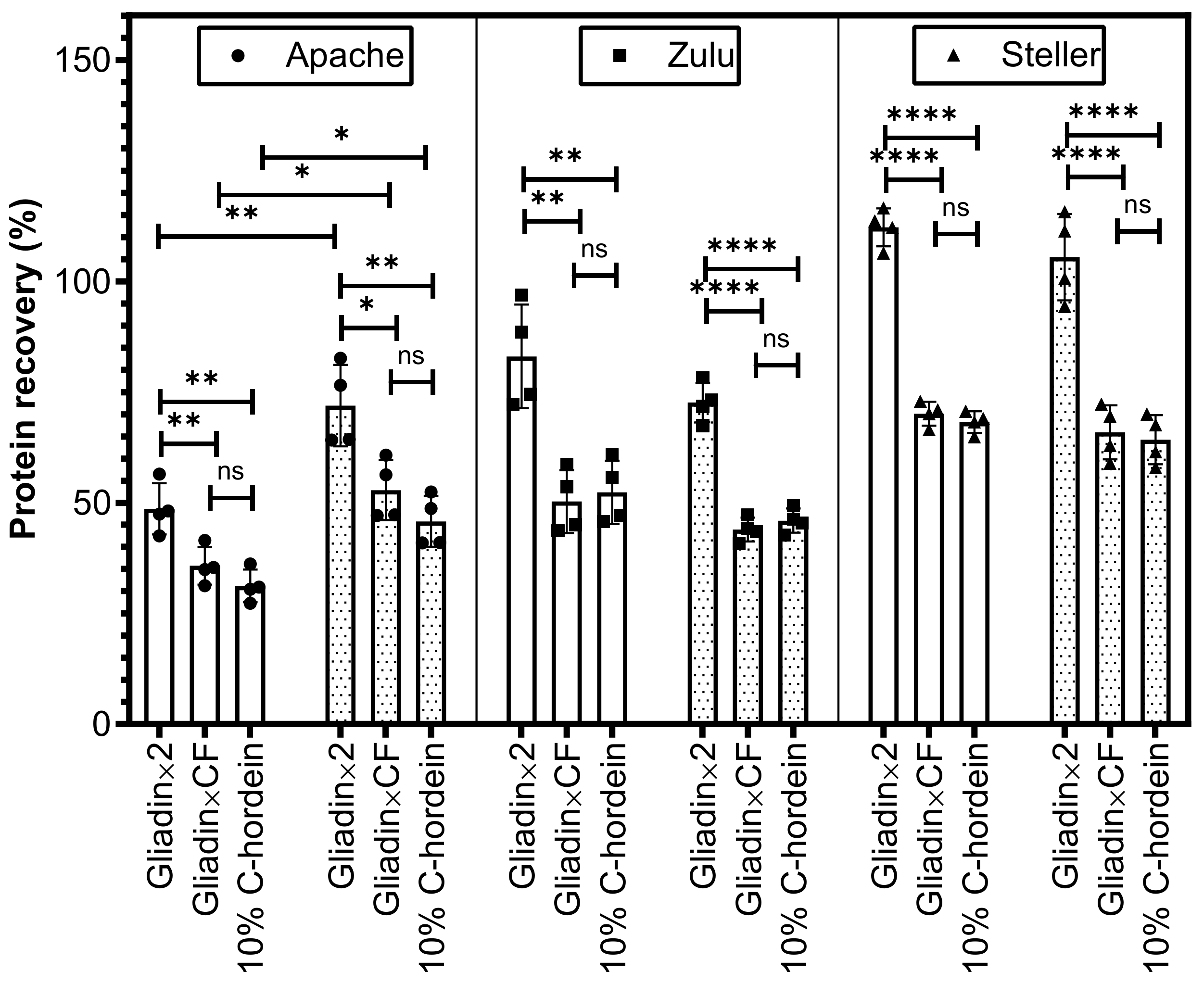
3.4. Calibration of Gluten Content in Spiked Oat Flour and Oat Biscuit Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lundin, K.E.A.; Sollid, L.M. Advances in coeliac disease. Curr. Opin. Gastroenterol. 2014, 30, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.S.; Shewry, P.R. The S-poor prolamins of wheat, barley and rye: Revisited. J. Cereal Sci. 2012, 55, 79–99. [Google Scholar] [CrossRef]
- Codex Alimentarius. Foods for Special Dietary Use for Persons Intolerant to Gluten; Codex Standard 118-1979 (rev. 2008); Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Vassiliou, A. Commission regulation (EC) No 41/2009 of 20 January 2009 concerning the composition and labelling of foodstuffs suitable for people intolerant to gluten (text with EEA relevance). Off. J. Eur. Union 2009, 21, L16. [Google Scholar]
- Food and Drug Administration, Health and Human Services. Food Labeling: Gluten-Free Labeling of Foods. Final rule; Federal Registry; Food and Drug Administration: Silver Spring, MD, USA, 2013; Volume 78, pp. 47154–47179.
- Health Canada. Health Canada’s Position on Gluten-Free Claims; Health Canada: Ottawa, ON, Canada, 2012.
- Sorell, L.; López, J.A.; Valdés, I.; Alfonso, P.; Camafeita, E.; Acevedo, B.; Chirdo, F.; Gavilondo, J.; Méndez, E. An innovative sandwich ELISA system based on an antibody cocktail for gluten analysis. FEBS Lett. 1998. [Google Scholar] [CrossRef]
- Kanerva, P.M.; Sontag-Strohm, T.S.; Ryöppy, P.H.; Alho-Lehto, P.; Salovaara, H.O. Analysis of barley contamination in oats using R5 and ω-gliadin antibodies. J. Cereal Sci. 2006, 44, 347–352. [Google Scholar] [CrossRef]
- Tanner, G.J.; Blundell, M.J.; Colgrave, M.L.; Howitt, C.A. Quantification of Hordeins by ELISA: The Correct Standard Makes a Magnitude of Difference. PLoS ONE 2013, 8, e56456. [Google Scholar] [CrossRef]
- Huang, X.; Kanerva, P.; Salovaara, H.; Stoddard, F.L.; Sontag-Strohm, T. Proposal for C-Hordein as Reference Material in Gluten Quantification. J. Agric. Food Chem. 2017, 65, 2155–2161. [Google Scholar] [CrossRef]
- Diaz-Amigo, C.; Popping, B. Accuracy of ELISA Detection Methods for Gluten and Reference Materials: A Realistic Assessment. J. Agric. Food Chem. 2013, 61, 5681–5688. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V. Immunological Methods in Gluten Risk Analysis: A Snapshot. Safety 2018, 4, 56. [Google Scholar] [CrossRef]
- Rzychon, M.; Brohée, M.; Cordeiro, F.; Haraszi, R.; Ulberth, F.; O’Connor, G. The feasibility of harmonizing gluten ELISA measurements. Food Chem. 2017, 234, 144–154. [Google Scholar] [CrossRef]
- Van Eckert, R.; Berghofer, E.; Ciclitira, P.J.; Chirdo, F.; Denery-Papini, S.; Ellis, H.J.; Ferranti, P.; Goodwin, P.; Immer, U.; Mamone, G.; et al. Towards a new gliadin reference material–isolation and characterisation. J. Cereal Sci. 2006, 43, 331–341. [Google Scholar] [CrossRef]
- Hajas, L.; Scherf, K.A.; Bugyi, Z.; Török, K.; Schall, E.; Köhler, P.; Tömösközi, S. ELISA response and gliadin composition of different wheat cultivars grown in multiple harvest years. Acta Aliment. 2017, 46, 187–195. [Google Scholar] [CrossRef]
- Hajas, L.; Scherf, K.A.; Török, K.; Bugyi, Z.; Schall, E.; Poms, R.E.; Koehler, P.; Tömösközi, S. Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis. Food Chem. 2018, 267, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Schall, E.; Scherf, K.A.; Bugyi, Z.; Hajas, L.; Török, K.; Koehler, P.; Poms, R.E.; D’Amico, S.; Schoenlechner, R.; Tömösközi, S. Characterisation and comparison of selected wheat (Triticum aestivum L.) cultivars and their blends to develop a gluten reference material. Food Chem. 2020. [Google Scholar] [CrossRef]
- Schall, E.; Scherf, K.A.; Bugyi, Z.; Török, K.; Koehler, P.; Schoenlechner, R.; Tömösközi, S. Further Steps Toward the Development of Gluten Reference Materials—Wheat Flours or Protein Isolates? Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Antes, S.; Seilmeier, W. Quantitative Determination of Gluten Protein Types in Wheat Flour by Reversed-Phase High-Performance Liquid Chromatography. Cereal Chem. J. 1998, 75, 644–650. [Google Scholar] [CrossRef]
- Kanerva, P.M.; Sontag-Strohm, T.; Brinck, O.; Salovaara, H. Improved extraction of prolamins for gluten detection in processed foods. Agric. Food Sci. 2011, 20, 206–216. [Google Scholar] [CrossRef]
- Maclean, W.; Harnly, J.; Chen, J.; Chevassus-Agnes, S.; Gilani, G.; Livesey, G.; Warwick, P. Food energy—Methods of analysis and conversion factors. Food Agric. Organ. Food Nutr. Pap. 2003, 77, 8–9. [Google Scholar]
- Lexhaller, B.; Tompos, C.; Scherf, K.A. Fundamental study on reactivities of gluten protein types from wheat, rye and barley with five sandwich ELISA test kits. Food Chem. 2017, 237, 320–330. [Google Scholar] [CrossRef]
- Bromilow, S.N.L.; Gethings, L.A.; Langridge, J.I.; Shewry, P.R.; Buckley, M.; Bromley, M.J.; Mills, E.N.C. Comprehensive Proteomic Profiling of Wheat Gluten Using a Combination of Data-Independent and Data-Dependent Acquisition. Front. Plant Sci. 2017, 7, 2020. [Google Scholar] [CrossRef]
- Osman, A.A.; Uhlig, H.H.; Valdes, I.; Amin, M.; Méndez, E.; Mothes, T. A monoclonal antibody that recognizes a potential coeliac-toxic repetitive pentapeptide epitope in gliadins. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1189–1193. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P. Is the calculation of the gluten content by multiplying the prolamin content by a factor of 2 valid? Eur. Food Res. Technol. 2009, 229, 9–13. [Google Scholar] [CrossRef]
- Koenig, A.; Konitzer, K.; Wieser, H.; Koehler, P. Classification of spelt cultivars based on differences in storage protein compositions from wheat. Food Chem. 2015, 168, 176–182. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Lexhaller, B.; Colgrave, M.L.; Scherf, K.A. Characterization and Relative Quantitation of Wheat, Rye, and Barley Gluten Protein Types by Liquid Chromatography–Tandem Mass Spectrometry. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Huang, X.; Schuppan, D.; Rojas Tovar, L.E.; Zevallos, V.F.; Loponen, J.; Gänzle, M. Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity. Foods 2020, 9, 943. [Google Scholar] [CrossRef]
- García, E.; Llorente, M.; Hernando, A.; Kieffer, R.; Wieser, H.; Méndez, E. Development of a general procedure for complete extraction of gliadins for heat processed and unheated foods. Eur. J. Gastroenterol. Hepatol. 2005, 17, 529–539. [Google Scholar] [CrossRef]
- Gessendorfer, B.; Wieser, H.; Koehler, P. Optimisation of a solvent for the complete extraction of prolamins from heated foods. J. Cereal Sci. 2010, 52, 331–332. [Google Scholar] [CrossRef]
- Mena, M.C.; Lombardía, M.; Hernando, A.; Méndez, E.; Albar, J.P. Comprehensive analysis of gluten in processed foods using a new extraction method and a competitive ELISA based on the R5 antibody. Talanta 2012, 91, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Careri, M. The role of incurred materials in method development and validation to account for food processing effects in food allergen analysis. Anal. Bioanal. Chem. 2019, 411, 4465–4480. [Google Scholar] [CrossRef] [PubMed]
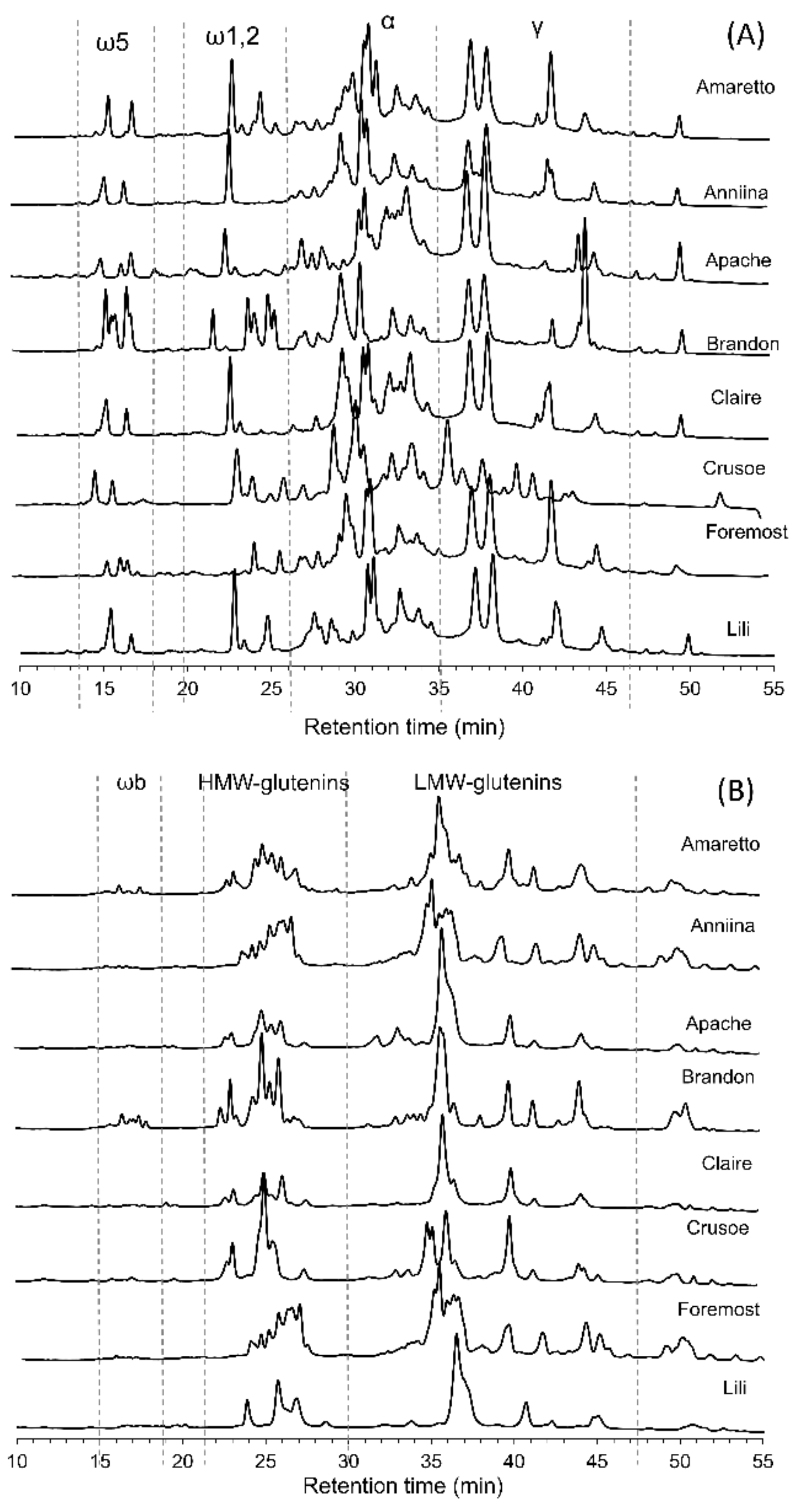
| Cultivar | Albumins + Globulins (%) | ω5-Gliadins (%) | ω1,2-Gliadins (%) | α-Gliadins (%) | γ-Gliadins (%) | ωb-Gliadins (%) | HMW-Glutenins (%) | LMW-Glutenins (%) | Total Gliadins (%) | Total Glutenins (%) | Gliadins: Glutenins | Conversion Factor (Gluten:Gliadins) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amaretto | 17.7 edf | 3.3 abcdef | 5.1 jk | 25.1 b | 21.0 abcd | 1.0 ijk | 9.6 j | 17.2 n | 55.4 b | 26.8 p | 2.07 | 1.48 |
| Anniina | 15.6 bcde | 4.1 ghij | 2.8 cd | 33.0 jk | 24.3 fghi | 0.7 efghij | 6.2 efgh | 13.2 fghi | 64.9 hij | 19.3 ghijkl | 3.35 | 1.30 |
| Apache | 28.7 i | 3.0 abcd | 2.1 ab | 21.9 a | 20.7 abc | 0.8 fghij | 6.0 defgh | 16.7 lmn | 48.5 a | 22.7 mno | 2.14 | 1.47 |
| Brandon | 11.9 ab | 8.9 m | 9.0 m | 28.4 cde | 26.6 jk | 0.4 abcd | 6.4 fgh | 8.3 ab | 73.2 m | 14.7 bc | 4.98 | 1.20 |
| Britannia | 23.7 gh | 3.7 defgh | 4.0 fgh | 30.9 fghi | 25.4 hijk | 0.3 ab | 4.3 a | 7.8 a | 64.1 ghi | 12.0 a | 5.34 | 1.19 |
| Brons | 25.0 hi | 3.8 efgh | 1.9 a | 29.6 efg | 20.2 ab | 0.9 hij | 4.8 abc | 13.5 ghij | 56.5 bc | 18.3 fghijk | 3.08 | 1.32 |
| Cellule | 13.7 abc | 7.8 l | 4.8 ij | 31.5 ghij | 19.0 a | 1.3 k | 6.7 ghi | 15.0 ijkl | 64.4 hij | 21.7 lmn | 2.97 | 1.34 |
| Claire | 25.2 hi | 3.2 abcde | 2.8 bcd | 30.8 fghi | 23.6 efgh | 0.1 a | 4.3 a | 10.0 bcd | 60.4 defg | 14.3 ab | 4.24 | 1.24 |
| Crusoe | 17.5 cdef | 3.0 abcd | 4.8 ij | 34.5 k | 21.0 abcd | 0.6 bcdefg | 7.1 hi | 11.5 def | 63.9 ghi | 18.5 fghijk | 3.45 | 1.29 |
| Foremost | 15.1 bcd | 2.8 abc | 2.9 cd | 31.8 hij | 29.2 l | 0.3 abc | 5.2 abcde | 12.5 efgh | 67.0 ijk | 17.7 efghi | 3.78 | 1.26 |
| Gregory | 18.1 def | 4.0 fghi | 3.3 def | 27.0 bc | 22.4 bcdef | 0.5 bcde | 7.8 i | 16.9 mn | 57.1 bcd | 24.7 op | 2.31 | 1.43 |
| Hereford | 25.2 hi | 3.2 abcde | 2.4 abc | 29.6 efg | 23.6 efgh | 0.7 defghi | 4.9 abcd | 10.3 cd | 59.4 cdef | 15.2 bcd | 3.90 | 1.26 |
| Julius DE | 19.2 ef | 4.0 fghi | 3.6 efg | 31.5 ghij | 23.8 efgh | 0.6 cdefgh | 5.3 abcdef | 11.8 defg | 63.6 ghi | 17.1 cdefg | 3.73 | 1.27 |
| Julius SE | 24.1 gh | 2.8 ab | 3.1 cde | 29.7 efg | 20.8 abcd | 0.5 bcdef | 5.8 bcdefg | 13.2 fghi | 56.8 bcd | 19.0 fghijk | 2.98 | 1.34 |
| Kerubino | 16.7 cde | 4.1 ghij | 5.5 k | 28.1 cde | 23.9 efgh | 0.8 fghij | 5.6 bcdefg | 15.2 jklm | 62.4 fgh | 20.8 klm | 3.01 | 1.33 |
| Lancer | 13.8 abc | 3.6 cdefg | 4.5 hij | 29.0 def | 24.6 ghij | 0.4 abcde | 7.7 i | 16.2 klmn | 62.1 efgh | 23.9 no | 2.60 | 1.38 |
| Lili | 21.2 fg | 3.5 bcdefg | 3.8 fgh | 27.3 cd | 23.5 efgh | 0.6 cdefgh | 6.0defgh | 13.9 hij | 58.8 bcdef | 19.8 hijkl | 2.96 | 1.34 |
| Mace | 15.2 bcd | 6.5 k | 2.8 cd | 29.8 efgh | 27.2 kl | 0.5 bcdef | 7.6 i | 10.2 cd | 66.8 ij | 17.8 efghi | 3.76 | 1.27 |
| Patras | 18.9 def | 4.9 j | 4.2 ghi | 30.5 fghi | 23.7 efgh | 0.8 fghij | 5.3 abcdef | 11.5 def | 64.1 ghij | 16.8 cdef | 3.82 | 1.26 |
| Penhold | 11.1 a | 4.0 fghi | 7.0 l | 32.3 ij | 27.2 kl | 0.3 abc | 5.6 bcdef | 12.3 efgh | 70.8 lm | 17.9 fghij | 3.96 | 1.25 |
| Quarna | 17.4 cdef | 4.4 hij | 4.7 ij | 30.8 fghi | 21.7 bcde | 0.6 cdefgh | 9.2 j | 11.1 cde | 62.2 fgh | 20.2 jklm | 3.08 | 1.32 |
| Revelation | 18.6 def | 3.5 bcdefg | 3.4 def | 30.7 fghi | 22.7 cdefg | 1.0 jk | 5.1 abcde | 14.9 ijk | 61.3 efgh | 20.0 ijkl | 3.07 | 1.33 |
| Siskin | 18.0 def | 4.7 ij | 2.8 cd | 32.0 ij | 26.4 ijk | 0.7 defghi | 5.9 cdefg | 9.5 abc | 66.5 ij | 15.4 bcde | 4.32 | 1.23 |
| Spitfire | 17.2 cde | 4.2 ghij | 3.4 def | 27.5 cd | 22.4 bcdef | 0.9 ghij | 7.7 i | 16.5 klmn | 58.3 bcde | 24.2 o | 2.41 | 1.41 |
| Steller | 11.9 ab | 6.3 k | 7.5 l | 27.4 cd | 28.9 l | 0.6 bcdefg | 5.8 bcdefg | 11.6 def | 70.6 klm | 17.4 defgh | 4.06 | 1.25 |
| Suntop | 15.6 bcde | 2.6 a | 4.3 hi | 31.3 ghij | 25.3 hijk | 0.4 abcd | 7.6 i | 12.9 efgh | 63.8 ghi | 20.4 klm | 3.13 | 1.32 |
| Zulu | 17.8 edf | 3.7 defgh | 3.6 efg | 37.1 l | 23.0 defg | 0.5 bcdef | 4.7 ab | 9.5 abc | 67.9 jkl | 14.2 ab | 4.79 | 1.21 |
| Min | 11.1 | 2.6 | 1.9 | 21.9 | 19.0 | 0.1 | 4.3 | 7.8 | 48.5 | 12.0 | 2.07 | 1.19 |
| Max | 28.7 | 8.9 | 9.0 | 37.1 | 29.2 | 1.3 | 9.6 | 17.2 | 73.262.6 | 26.8 | 5.34 | 1.48 |
| Mean | 18.3 | 4.2 | 4.1 | 30.0 | 23.8 | 0.6 | 6.2 | 12.7 | 18.9 | 3.46 | 1.31 |
| Cultivar | EC50, ng/mL | Cultivar | EC50, ng/mL | Calibrant/Reference Material | EC50, ng/mL |
|---|---|---|---|---|---|
| Amaretto | 38.1 | Kerubino | 74.8 | Average of all cultivars | 62.1 |
| Anniina | 85.9 | Lancer | 31.2 | 10% C-hordein | 61.5 |
| Apache | 376.6 | Liili | 61.2 | 20% C-hordein | 37.5 |
| Brandon | 35.0 | Mace | 140 | 30% C-hordein | 27.5 |
| Britannia | 664.3 | Patras | 47.9 | ||
| Brons | 324.6 | Penhold | 57.7 | ||
| Cellule | 57.9 | Quarna | 51.4 | ||
| Claire | 38.6 | Revelation | 53.4 | ||
| Crusoe | 30.4 | Siskin | 66.9 | ||
| Foremost | 142.7 | Spitfire | 55.0 | ||
| Gregory | 103.8 | Steller | 30.7 | ||
| Hereford | 147.9 | Suntop | 32.6 | ||
| Julius DE | 80.9 | Zulu | 66.8 | ||
| Julius SE | 40.0 |
| Gluten Type | EC50 (ng/mL) |
|---|---|
| ω1.2-gliadin | 13.7 |
| γ-gliadin | 44.3 |
| α-gliadin | 35.9 |
| HMW glutenin | 91.9 |
| LMW glutenin | 473.3 |
| ω5-gliadin | -- |
| Cultivar | ω1.2-Gliadins (EC50, ng/mL) | Cultivar | α-Gliadins (EC50, ng/mL) | γ-Gliadins (EC50, ng/mL) |
|---|---|---|---|---|
| Amaretto | 10.6 | Amaretto | 31.9 | 21.3 |
| Anniina | 5.3 | Apache | 26.7 | 17.0 |
| Brandon | 11.4 | Brandon | 41.6 | 33.7 |
| Claire | 14.0 | Foremost | 30.7 | 22.0 |
| Lili | 8.9 |
| Wheat Cultivar Used in Spiking | Level of Flour Spiked in Oat Flour (mg/kg) | Protein Content of the Wheat Flour | Proportion of Gluten in Total Protein (From Table 1) | Theoretical Gluten Spiked Level in Oat Flour (mg/kg) | ELISA Calibration Result: Gliadin × 2 (mg/kg) | ELISA Calibration Result: Gliadin × CF (mg/kg) | ELISA Calibration Result: 10% C-Hordein (mg/kg) |
|---|---|---|---|---|---|---|---|
| Apache | 1000 | 10.2% | 71.3% | 72.7 | 35 ± 2 | 26 ± 2 | 23 ± 1 |
| Zulu | 1000 | 11.7% | 82.2% | 96.2 | 80 ± 5 | 48 ± 3 | 50 ± 3 |
| Steller | 1000 | 17.8% | 88.1% | 156.8 | 176 ± 3 | 110 ± 2 | 107 ± 2 |
| Wheat Cultivar Used in Spiking | Gluten Content in the Spiked Oat Biscuits Flour (mg/kg) | Gluten Content in the Biscuit Recipe (mg/kg) | Theoretical Gluten Content After Baking Moisture Loss, (mg/kg) | ELISA Calibration Result: Gliadin × 2 (mg/kg) | ELISA Calibration Result: Gliadin × CF (mg/kg) | ELISA Calibration 10% C-Hordein (mg/kg) |
|---|---|---|---|---|---|---|
| Apache | 72.7 | 33.0 | 37.7 | 27 ± 2 | 20 ± 1 | 17 ± 1 |
| Zulu | 96.2 | 43.7 | 49.5 | 36 ± 1 | 22 ± 1 | 23 ± 1 |
| Steller | 156.8 | 71.3 | 80.4 | 85 ± 4 | 53 ± 2 | 52 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Ma, K.; Leinonen, S.; Sontag-Strohm, T. Barley C-Hordein as the Calibrant for Wheat Gluten Quantification. Foods 2020, 9, 1637. https://doi.org/10.3390/foods9111637
Huang X, Ma K, Leinonen S, Sontag-Strohm T. Barley C-Hordein as the Calibrant for Wheat Gluten Quantification. Foods. 2020; 9(11):1637. https://doi.org/10.3390/foods9111637
Chicago/Turabian StyleHuang, Xin, Kaiyue Ma, Sara Leinonen, and Tuula Sontag-Strohm. 2020. "Barley C-Hordein as the Calibrant for Wheat Gluten Quantification" Foods 9, no. 11: 1637. https://doi.org/10.3390/foods9111637
APA StyleHuang, X., Ma, K., Leinonen, S., & Sontag-Strohm, T. (2020). Barley C-Hordein as the Calibrant for Wheat Gluten Quantification. Foods, 9(11), 1637. https://doi.org/10.3390/foods9111637





