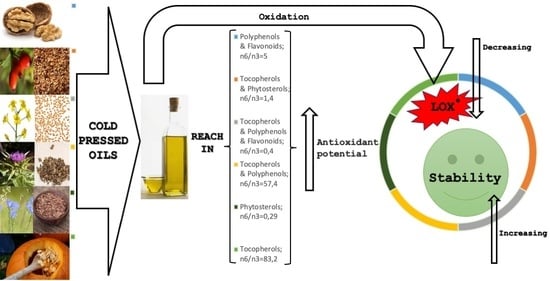
Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Oil Samples
2.2. Fatty Acid Composition
2.3. Phytosterol and Squalene Content
2.4. Tocopherol Content
2.5. Total Phenol and Flavonoid Content
2.6. Determination of Chlorophyll Content
2.7. Determination of Cu and Fe Content
2.8. Antiradical Scavenging Activity
2.9. Oxidative Stability Parameters of Oils
2.10. Statistical Analysis
3. Results
3.1. Fatty Acid and Phytosterol Composition
3.2. Antioxidants, Other Minor Components and Antiradical Scavenging Activity of Oils
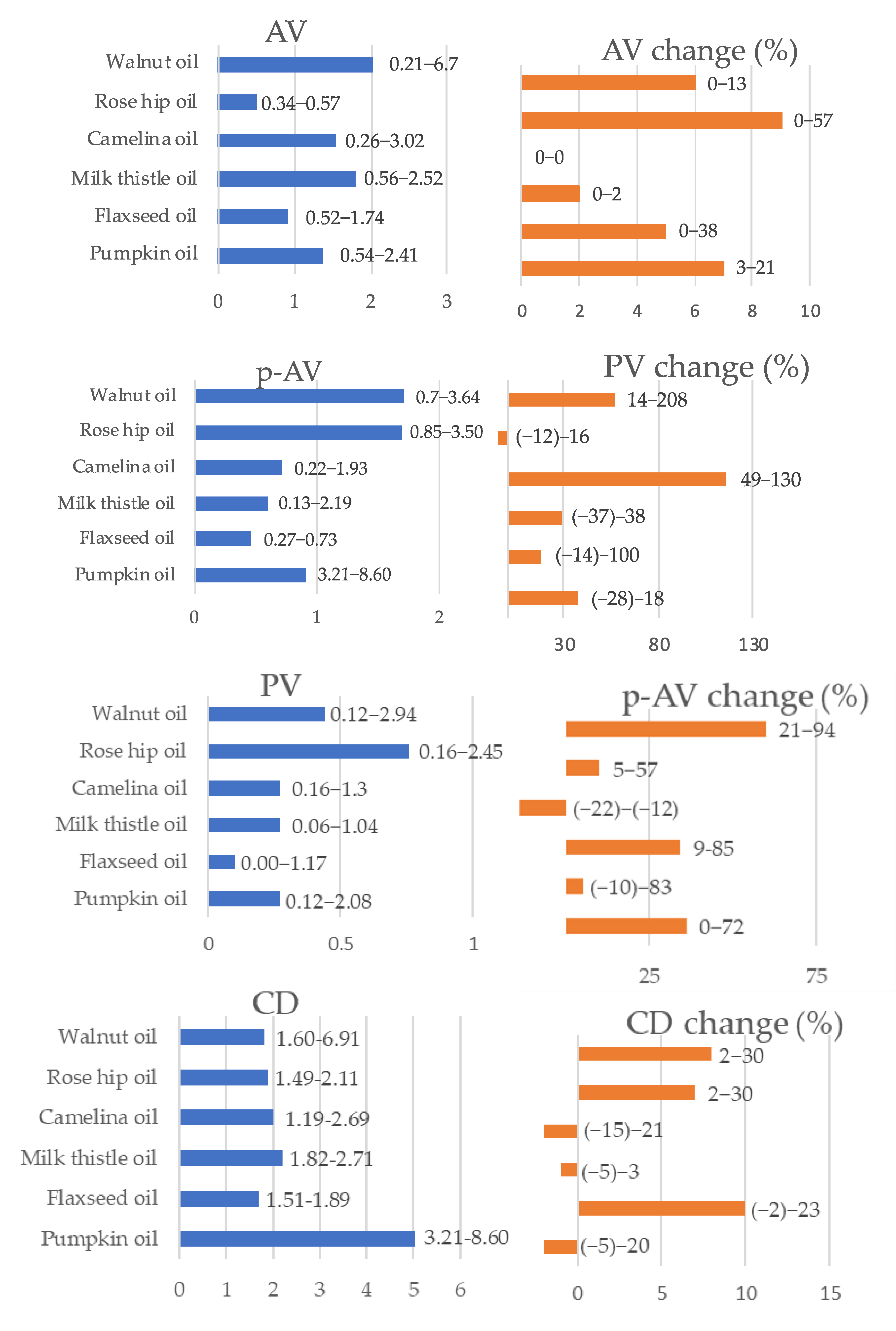
3.3. Oxidative Stability Parameters of Fresh and Stored Oils and Correlation with Studied Oil Characteristics
4. Discussion
4.1. Fatty Acid and Phytosterol Composition
4.2. Antioxidants, Minor Components, and Antiradical Scavenging Activity of Oils
4.3. Oxidative Stability Parameters of Fresh and Stored Oils and Correlation with Studied Oil Characteristics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to increase the oxidative stability of cold pressed oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Böhm, T.; Berger, H.; Nejabat, M.; Riegler, T.; Kellner, F.; Kuttke, M.; Sagmeister, S.; Bazanella, M.; Stolze, K.; Daryabeigi, A. Food-derived peroxidized fatty acids may trigger hepatic inflammation: A novel hypothesis to explain steatohepatitis. J. Hepatol. 2013, 59, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Sielicka, M.; Małecka, M.; Purłan, M. Comparison of the antioxidant capacity of lipid-soluble compounds in selected cold-pressed oils using photochemiluminescence assay (PCL) and DPPH method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Budilarto, E.S.; Kamal-Eldin, A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1095–1137. [Google Scholar] [CrossRef]
- Parry, J.; Hao, Z.; Luther, M.; Su, L.; Zhou, K.; Yu, L.L. Characterization of cold-pressed onion, parsley, cardamom, mullein, roasted pumpkin, and milk thistle seed oils. J. Am. Oil Chem. Soc. 2006, 83, 847–854. [Google Scholar] [CrossRef]
- Huang, S.-W.; Frankel, E.N.; German, J.B. Antioxidant activity of alpha -and gamma-tocopherols in bulk oils and in oil-in-water emulsions. J. Agric. Food Chem. 1994, 42, 2108–2114. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef]
- Isnardy, B.; Wagner, K.-H.; Elmadfa, I. Effects of α-, γ-, and δ-Tocopherols on the Autoxidation of Purified Rapeseed Oil Triacylglycerols in a System Containing Low Oxygen. J. Agric. Food Chem. 2003, 51, 7775–7780. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Inhibition of methyl linoleate autoxidation by phenolics and other related compounds under mild oxidative conditions. J. Sci. Food Agric. 2004, 84, 631–638. [Google Scholar] [CrossRef]
- Karvonen, H.M.; Aro, A.; Tapola, N.S.; Salminen, I.; Uusitupa, M.I.; Sarkkinen, E.S. Effect of alpha-linolenic acid-rich Camelina Sativa oil on serum fatty acid composition and serum lipids in hypercholesterolemic subjects. Metabolism 2002, 51, 1253–1260. [Google Scholar] [CrossRef]
- Abascal, K.; Yarnell, E. The many faces of Sylibum marianum (Milk Thistle). Part 2 -Clinical uses, safety and types of preparations. Altern. Complement. Ther. 2003, 9, 170–175. [Google Scholar] [CrossRef]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Farhadi, P.; Attar, A.; Mosleh, A.; Amirmoezi, F.; Azimi, A. Effects of walnut oil on lipid profiles in hyperlipidemic type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Nutr. Diabetes 2017, 7, e259. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Tong, Y.C.; Cheng, J.T.; Lee, C.H.; Yang, F.S.; Lee, H.Y. Pumpkin seed oil and phytosterol-F can block testosterone/prazosin-induced prostate growth in rats. Urol. Int. 2006, 77, 269–274. [Google Scholar] [CrossRef]
- Prescha, A.; Swiedrych, A.; Biernat, J.; Szopa, J. Increase in lipid content in potato tubers modified by 14-3-3 gene overexpression. J. Agric. Food Chem. 2001, 49, 3638–3643. [Google Scholar] [CrossRef]
- Grajzer, M.; Wiatrak, B.; Gębarowski, T.; Matkowski, A.; Grajeta, H.; Rój, E.; Kulma, A.; Prescha, A. Chemistry, oxidative stability and bioactivity of oil extracted from Rosa rugosa (Thunb.) seeds by supercritical carbon dioxide. Food Chem. 2021, 335, 127649. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.K.S.; Dutta, P.C.; Artz, W.E. Camelina oil and its unusual cholesterol content. J. Am. Oil. Chem. Soc. 2002, 79, 965–969. [Google Scholar] [CrossRef]
- Fromm, M.; Bayha, S.; Kammerer, D.R.; Carle, R. Identification and quantitation of carotenoids and tocopherols in seed oils recovered from different Rosaceae species. J. Agric. Food Chem. 2012, 60, 10733–10742. [Google Scholar] [CrossRef] [PubMed]
- Grajzer, M.; Prescha, A.; Korzonek, K.; Wojakowska, A.; Dziadas, M.; Kulma, A.; Grajeta, H. Characteristics of rose hip (Rosa canina L.) cold-pressed oil and its oxidative stability studied by the differential scanning calorimetry method. Food Chem. 2015, 188, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 505–512. [Google Scholar] [CrossRef]
- Choo, W.-S.; Birch, J.; Dufour, J.-P. Physicochemical and quality characteristics of cold-pressed flaxseed oils. J. Food Compos. Anal 2007, 20, 202–211. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and axidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Abramovič, H.; Butinar, B.; Nikolič, V. Changes occurring in phenolic content, tocopherol composition and oxidative stability of Camelina sativa oil during storage. Food Chem. 2007, 104, 903–909. [Google Scholar] [CrossRef]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A. Profile of fatty acids, tocopherols, phytosterols and polyphenols in mediterranean oils (argan oils, olive oils, milk thistle seed oils and nigella seed oil) and evaluation of their antioxidant and cytoprotective activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef]
- Wood, K.; Mantzioris, E.; Gibson, R.; Ramsden, C.; Muhlhausler, B. The effect of modifying dietary LA and ALA intakes on omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) status in human adults: A systematic review and commentary. Prostag. Leukotr. Ess. 2015, 95, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Münger, L.H.; Jutzi, S.; Lampi, A.-M.; Nyström, L. Comparison of Enzymatic Hydrolysis and Acid Hydrolysis of Sterol Glycosides from Foods Rich in Δ7-Sterols. Lipids 2015, 50, 735–748. [Google Scholar] [CrossRef]
- Parkash Kochhar, S.; Gertz, C. New theoretical and practical aspects of the frying process. Eur. J. Lipid Sci. Technol. 2004, 106, 722–727. [Google Scholar] [CrossRef]
- Wang, T.; Hicks, K.B.; Moreau, R. Antioxidant activity of phytosterols, oryzanol, and other phytosterol conjugates. J. Am. Oil Chem. Soc. 2002, 79, 1201–1206. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Zaunschirm, M.; Pignitter, M.; Kienesberger, J.; Hernler, N.; Riegger, C.; Eggersdorfer, M.; Somoza, V. Contribution of the ratio of tocopherol homologs to the oxidative stability of commercial vegetable oils. Molecules 2018, 23, 206. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Salta, F.N.; Efstathiou, P.; Andrikopoulos, N.K. Pan-frying of French fries in three different edible oils enriched with olive leaf extract: Oxidative stability and fate of microconstituents. LWT-Food Sci. Technol. 2009, 42, 1090–1097. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Van Camp, J.; Trawka, A.; Verhé, R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2010, 112, 208–217. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2019, 279, 279–287. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef] [PubMed]
- Kostadinović Veličkovska, S.; Brühl, L.; Mitrev, S.; Mirhosseini, H.; Matthäus, B. Quality evaluation of cold-pressed edible oils from Macedonia. Eur. J. Lipid Sci. Technol. 2015, 117, 2023–2035. [Google Scholar] [CrossRef]
- Teh, S.-S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Dessì, M.A.; Deiana, M.; Day, B.W.; Rosa, A.; Banni, S.; Corongiu, F.P. Oxidative stability of polyunsaturated fatty acids: Effect of squalene. Eur. J. Lipid Sci. Technol. 2002, 104, 506–512. [Google Scholar] [CrossRef]
- Gorjanović, S.Ž.; Rabrenović, B.B.; Novaković, M.M.; Dimić, E.B.; Basić, Z.N.; Sužnjević, D.Ž. Cold-Pressed Pumpkin Seed Oil Antioxidant Activity as Determined by a DC Polarographic Assay Based on Hydrogen Peroxide Scavenge. J. Am. Oil Chem. Soc. 2011, 88, 1875–1882. [Google Scholar] [CrossRef]
- Nakić, S.N.; Rade, D.; Škevin, D.; Štrucelj, D.; Mokrovčak, Ž.; Bartolić, M. Chemical characteristics of oils from naked and husk seeds of Cucurbita pepo L. Eur. J. Lipid Sci. Technol. 2006, 108, 936–943. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Antioxidant behavior in bulk oil: Limitations of polar paradox theory. J. Agric. Food Chem. 2011, 60, 4–6. [Google Scholar] [CrossRef]
- Codex Alimentarius Comission. Codex Alimentarius. In Codex Standards for Named Vagateble Oils; Joint FAO/WHO: London, UK, 2001; Volume Codex Stan 210-1999; pp. 11–25. [Google Scholar]
- Yeo, J.; Jeong, M.K.; Lee, J. Correlation of antioxidant content and absorbance changes of DPPH during lipid oxidation. Food Sci. Biotechnol 2012, 21, 199–203. [Google Scholar] [CrossRef]
- Dachtler, M.; van de Put, F.H.M.; Stijn, F.v.; Beindorff, C.M.; Fritsche, J. On-line LC-NMR-MS characterization of sesame oil extracts and assessment of their antioxidant activity. Eur. J. Lipid Sci. Technol. 2003, 105, 488–496. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Rennick, K.A.; Palmquist, D.A.; Berhow, M.A.; Vaughn, S.F. Comparison of the impact of γ-oryzanol and corn steryl ferulates on the polymerization of soybean oil during frying. J. Am. Oil Chem. Soc. 2012, 89, 243–252. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Budilarto, E. Antioxidant activities and interactions of α-and γ-tocopherols within canola and soybean emulsions. Eur. J. Lipid Sci. Technol. 2014, 116, 781–782. [Google Scholar] [CrossRef]
- Tsimogiannis, D.I.; Oreopoulou, V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′,4′-hydroxy substituted members. Innov. Food Sci. Emerg. Technol. 2006, 7, 140–146. [Google Scholar] [CrossRef]
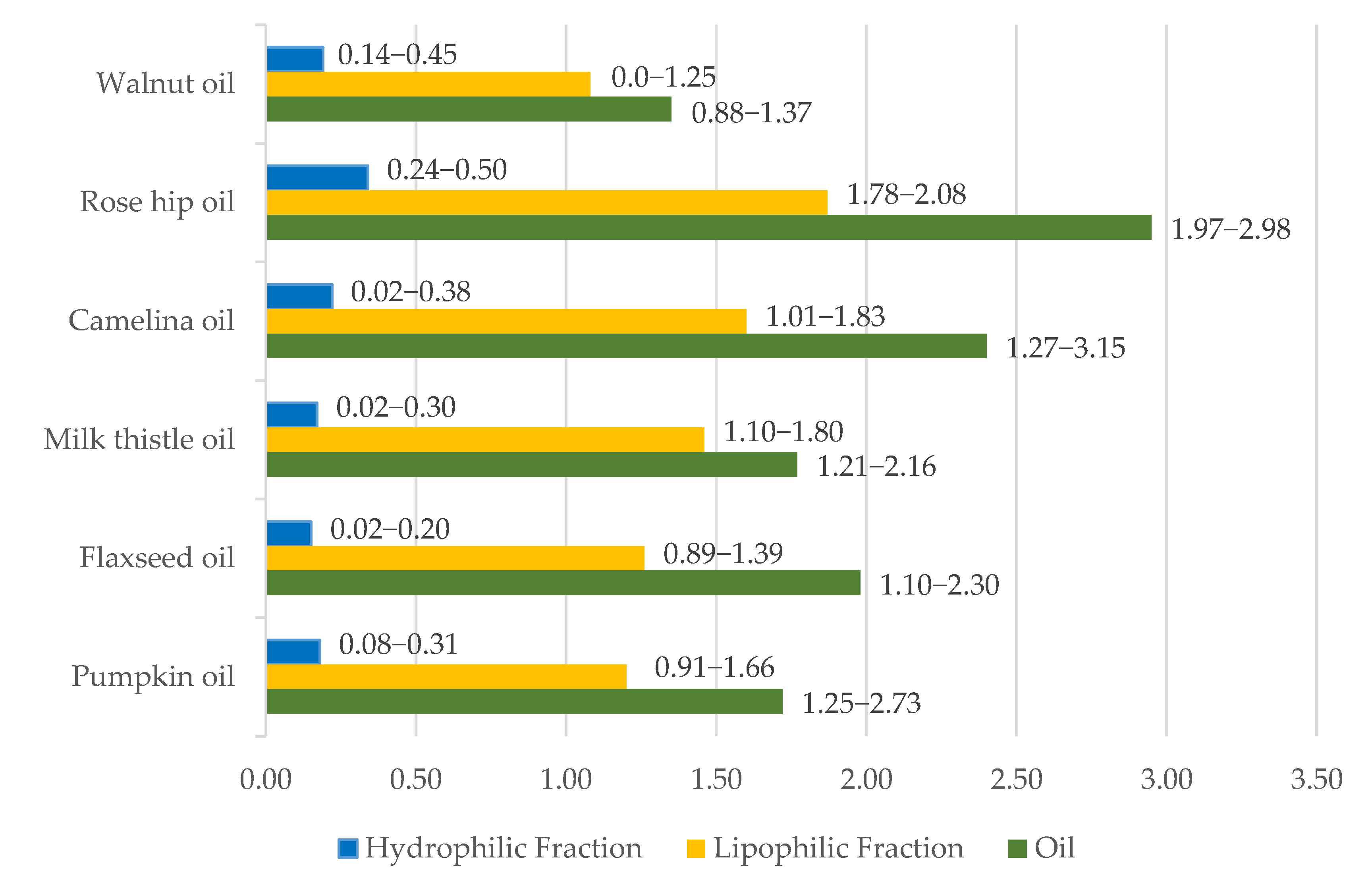

| Fatty Acids | Walnut Oil | Rosehip Oil | Camelina Oil | Milk Thistle Oil | Flaxseed Oil | Pumpkin Oil |
|---|---|---|---|---|---|---|
| C16:0 | 7.5 | 4.4 | 5.7 | 8.6 | 6.2 | 12.2 |
| (6.6–8.2) | (4.3–4.5) | (5.6–7.0) | (7.4–9.1) | (5.1–6.7) | (10.6–13.4) | |
| C18:0 | 2.6 | 2.1 | 2.6 | 4.9 | 4.4 | 5.8 |
| (2.0–3.1) | (2.1–2.1) | (2.4–3.3) | (4.6–5.4) | (4.0–5.6) | (4.7–6.2) | |
| C18:1n-9 | 18.9 | 16.9 | 15.8 | 22.4 | 20.5 | 30.8 |
| (17.2–20.4) | (16.1–17.0) | (12.8–20.3) | (20.4–23.5) | (15.9–23.2) | (24.0–42.6) | |
| C18:2n-6 | 58.5 | 43.7 | 17.4 | 57.4 | 15.2 | 49.9 |
| (55.3–60.7) | (43.5–43.7) | (16.1–21.6) | (55.4–61.1) | (12.5–18.2) | (39.6–54.7) | |
| C18:3n-3 | 11.7 | 30.9 | 49.9 | 1.0 | 53.1 | 0.6 |
| (10.1–12.5) | (30.7–31.2) | (41.2–53.6) | (0.9–1.0) | (49.3–59.3) | (0.4–1.1) | |
| C20:0 | tr. | 0.7 | 1.5 | 2.3 | 0.2 | 0.4 |
| (0.7–0.8) | (1.0–1.6) | (0.0–3.0) | (0.0–0.2) | (0.4–0.8) | ||
| C20:1n-9 | tr. | 0.4 | 1.6 | 0.6 | tr. | tr. |
| (0.4–0.4) | (1.1–3.7) | (0.0–0.9) | ||||
| C22:0 | n.d. | n.d. | n.d. | 2.1 | n.d. | n.d. |
| (1.9–2.3) | ||||||
| C24:0 | n.d. | n.d. | n.d. | 0.4 | n.d. | n.d. |
| (0.0–0.6) | ||||||
| ∑ SFA | 10.2 | 7.1 | 9.9 | 17.4 | 10.7 | 18.2 |
| (9.1–11.6) | (7.1–7.4) | (9.7–11.1) | (15.7–20.2) | (9.3–12.5) | (16.8–20.2) | |
| ∑ MUFA | 19.1 | 17.3 | 17.1 | 22.7 | 20.5 | 30.8 |
| (17.2–20.4) | (16.5–17.4) | (15.9–22.8) | (21.2–24.2) | (16.0–23.4) | (24.0–42.6) | |
| ∑ PUFA | 70.3 | 74.6 | 66.6 | 58.4 | 68.8 | 50.7 |
| (67.8–72.9) | (74.2–74.9) | (62.8–71.6) | (56.4–62.1) | (64.4–73.2) | (40.2–55.6) | |
| n-6/n-3 ratio | 5.1 | 1.4 | 0.4 | 57.6 | 0.3 | 80.0 |
| (4.4–5.7) | (1.4–1.4) | (0.3–0.5) | (56.0–62.4) | (0.2–0.3) | (48.4–110.9) |
| Compound | Product Ion Mas Spectra Data (m/z) | Walnut Oil | Rosehip Oil | Camelina Oil | Milk Thistle Oil | Flaxseed Oil | Pumpkin Oil | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesterol | 458 | 443 | 368 | 353 | 329 | 64.2 | n.d. | 165.6 | 197.2 | 38.3 | n.d. |
| (41.7–83.5) | (131.4–187.5) | (166.5–302.1) | (12.6–61.2) | ||||||||
| Δ5-Campesterol | 472 | 457 | 382 | 367 | 343 | 113.7 | 178.0 | 595.6 | 212.6 | 888.4 | 43.8 |
| (85.2–197.1) | (166.1–208.3) | (515.1–627.4) | (176.3–249.9) | (434.4–1266.7) | (13.5–202.4) | ||||||
| Δ5-Stigmasterol | 484 | 469 | 394 | 379 | 355 | 69.3 | 83.1 | 19.4 | 268.2 | 273.8 | 27.5 |
| (5.19–94.8) | (67.1–157.4) | (16.6–26.1) | (216.8–460.3) | (104.3–413.9) | (8.1–122.3) | ||||||
| Brassicasterol | 470 | 457 | 382 | 367 | 343 | n.d. | n.d. | 123.9 | n.d. | n.d. | n.d. |
| (109.4–145.9) | |||||||||||
| Δ7-Campesterol | 482 | 467 | 392 | 377 | n.d. | n.d. | n.d. | 111.7 | n.d. | n.d. | |
| (100.3–114.7) | |||||||||||
| Δ7,22,25-Stigmastatrienol | 482 | 467 | 392 | 377 | n.d. | n.d. | n.d. | n.d. | n.d. | 1355.8 | |
| (427.8–2121.2) | |||||||||||
| β-Sitosterol | 486 | 471 | 396 | 381 | 357 | 1091.9 | 4314.2 | 1440.2 | 1479.7 | 1839.0 | 172.3 |
| (715.1–2251.4) | (3856.9–4934.1) | (1231.4–1484.9) | (1120.1–2377) | (912.6–2326.1) | (39.2–1214.7) | ||||||
| α-Spinasterol | 484 | 469 | 394 | 379 | n.d. | n.d. | n.d. | n.d. | n.d. | 1412.4 | |
| (817.6–2192.5) | |||||||||||
| Δ5-Avenasterol | 484 | 469 | 394 | 379 | 355 | 101.1 | 282.2 | 141.7 | 43.6 | 441.5 | n.d. |
| (55.9–145.7) | (155.9–337) | (118.5–173.9) | (17.8–196.5) | (245.1–584.2) | |||||||
| Δ7,25-Stigmastadienol | 484 | 469 | 394 | 379 | n.d. | n.d. | n.d. | n.d. | n.d. | 1228.8 | |
| (1017.4–1611.6) | |||||||||||
| Δ5,24-Stigmastadienol | 484 | 469 | 394 | 379 | n.d. | n.d. | n.d. | 101.1 | n.d. | n.d. | |
| (50.8–138.5) | |||||||||||
| Δ7-Stigmastenol | 486 | 471 | 396 | 381 | n.d. | n.d. | n.d. | 1003.1 | n.d. | 223.1 | |
| 605.7–1374.3 | (148.6–561.5) | ||||||||||
| Δ7-Avenasterol | 484 | 469 | 394 | 379 | 227.3 | 76.6 | n.d. | 151.2 | n.d. | 829.7 | |
| (163.3–368.6) | (55.3–90.2) | (89.3–347.9) | (729.7–1468.9) | ||||||||
| Cycloartenol | 427 | 409 | 320 | 257 | 191 | n.d. | 288.8 | 24.6 | n.d. | 1562.3 | n.d. |
| (200.3–358.3) | (23.6–41.1) | (825.1–2260.6) | |||||||||
| Total | 1421.7 | 5358.2 | 2533.1 | 3421.1 | 5171.7 | 5459.9 | |||||
| (973.7–2880.3) | (4835.1–5837.3) | (2137.7–2755.7) | (2048.1–5501.3) | (2615.8–5979.4) | (3964.9–7977.7) | ||||||
| Walnut Oil | Rosehip Oil | Camelina Oil | Milk Thistle Oil | Flaxseed Oil | Pumpkin Oil | |
|---|---|---|---|---|---|---|
| The antioxidants content [mg/kg] | ||||||
| Tocopherols | ||||||
| α-Tocopherol | 48.6 | 123.8 | n.d. | 204.1 | 63.5 | 51.2 |
| (40.3–82.0) | (81.0–164.6) | (200.2–301.6) | (22.0–225.3) | (24.8–65.3) | ||
| γ-Tocopherol | 335.6 | 674.8 | 817.7 | 55.5 | 540.3 | 201.4 |
| (207.1–380.3) | (533.0–683.4) | (658.5–888.0) | (49.7–84.2) | (454.8–619.3) | (165.0–360.6) | |
| δ-Tocopherol | 45.6 | 252.9 | 126 | 14.6 | n.d. | 21.9 |
| (42.4–54.4) | (237.4–311.9) | (37.8–222.6) | (9.0–15.3) | (17.2–27.8) | ||
| Total tocopherols | 423.1 | 1036.0 | 972.3 | 262.0 | 588.7 | 290.8 |
| (300.7–476.9) | (866.9–1159.9) | (692.5–1026.7) | (253.8–354.6) | (476.8–490.0) | (206.9–426.4) | |
| Total phenols (CAE) | 83.6 | 86.8 | 117.3 | 78.8 | 55.8 | 106.6 |
| (59.6–252.1) | (74.71–117.1) | (34.12–138.9) | (71.7–124.7) | (37.57–84.9) | (53.67–184.6) | |
| Total flavonoids (LE) | 7.6 | 11.6 | 16.7 | 4.53 | 15.4 | 64.2 |
| (1.1–13.9) | (4.8–14.9) | (11.6–46.7) | (4.23–20.9) | (8.3–20.5) | (31.6–135.5) | |
| Squalene | 58.1 | 203.8 | 22.2 | 65.4 | n.d. | 1324.3 |
| (9.1–251.8) | (151.5.0–214.9) | (15.2–68.9) | (41.8–185.6) | (1050.8–1787.1) | ||
| Chlorophyll and transient metal contents [mg/kg] | ||||||
| Chlorophyll | 0.87 | 0.86 | 3.80 | 1.36 | 0.79 | 6.63 |
| (0.00–1.64) | (0.07–0.91) | (0.03–13.44) | (0.39–2.62) | (0.32–3.37) | (1.28–13.4) | |
| Cu | 0.013 | 0.014 | 0.009 | 0.037 | 0.013 | 0.03 |
| (0.008–0.017) | (0.011–0.016) | (0.008–0.018) | (0.01–0.051) | (0.01–0.017) | (0.006–0.190) | |
| Fe | 0.23 | 0.08 | 0.14 | 0.19 | 0.16 | 0.40 |
| (0.17–0.34) | (0.04–0.25) | (0.12–0.43) | (0.16–0.44) | (0.1–1.72) | (0.19–0.84) | |
| DPPH Oil | DPPH Hydrophilic Fraction | DPPH Lipophilic Fraction | ||
|---|---|---|---|---|
| Polyphenols | b | 0.480657 | ||
| B | 0.146997 | |||
| p | 0.002703 | |||
| Phytosterols | b | 0.630663 | ||
| B | 0.139384 | |||
| p | 0.000083 | |||
| Tocopherols | b | 0.655721 | 0.443316 | |
| B | 0.135603 | 0.146997 | ||
| p | 0.000034 | 0.005180 | ||
| PV after 3 months | b | −0.480145 | ||
| B | 0.671066 | |||
| p | 0.027654 |
| DPPH | Walnut Oils | Rosehip Oils | Camelina Oils | Milk Thistle Oils | Flaxseed Oils | Pumpkin Oils | ||
|---|---|---|---|---|---|---|---|---|
| Tocopherols | b | 0.013669 | ||||||
| B | 0.003224 | ◉ | ◉ | ◉ | ◉ | |||
| p | 0.000493 | |||||||
| Polyphenols | b | 0.101689 | ||||||
| B | 0.056526 | ◉ | ◉ | ◉ | ||||
| p | 0.043608 | |||||||
| Flavonoids | b | 0.427534 | ||||||
| B | 0.136117 | ◉ | ◉ | |||||
| p | 0.009393 | |||||||
| Phytosterols | b | 0.005055 | ||||||
| B | 0.001433 | ◉ | ◉ | |||||
| p | 0.009617 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. https://doi.org/10.3390/foods9111630
Grajzer M, Szmalcel K, Kuźmiński Ł, Witkowski M, Kulma A, Prescha A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods. 2020; 9(11):1630. https://doi.org/10.3390/foods9111630
Chicago/Turabian StyleGrajzer, Magdalena, Karolina Szmalcel, Łukasz Kuźmiński, Mateusz Witkowski, Anna Kulma, and Anna Prescha. 2020. "Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability" Foods 9, no. 11: 1630. https://doi.org/10.3390/foods9111630
APA StyleGrajzer, M., Szmalcel, K., Kuźmiński, Ł., Witkowski, M., Kulma, A., & Prescha, A. (2020). Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods, 9(11), 1630. https://doi.org/10.3390/foods9111630