Tailoring the Functional Potential of Red Beet Purées by Inoculation with Lactic Acid Bacteria and Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Lactic Bacteria
2.3. Sample Preparation
2.3.1. Inoculum Preparation
2.3.2. Red Beet Pureés Preparation
2.4. Drying of the Samples
2.5. Extraction of Betalains
2.6. Determination of Betalain Compounds by Spectrophotometric Methods
2.7. Antioxidant Activity
2.8. Cells Viability
2.9. In Vitro Release of the Betalains
2.10. Color Parameters
2.11. Structural and Morphological Properties of the Dried Powders
2.12. FT-IR
2.13. Textural and Oscillatory Measurements
2.13.1. Texture Analysis
2.13.2. Rheological Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. The Content of Selected Phytochemicals in Fresh Red Beet Purées
3.2. The Content of Selected Phytochemicals in Dried Red Beet Purées
3.3. Cells Viability
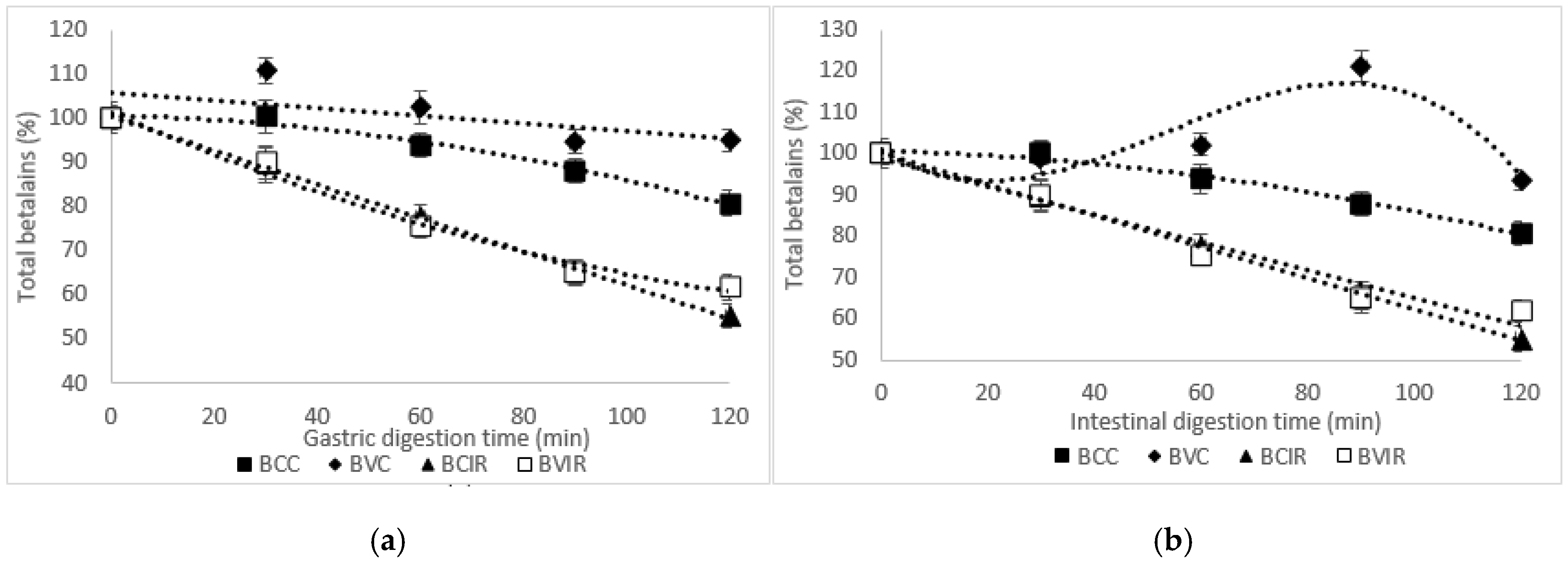
3.4. In Vitro Release of Betalains
3.5. Structural and Morphological Properties of the Dried Powders
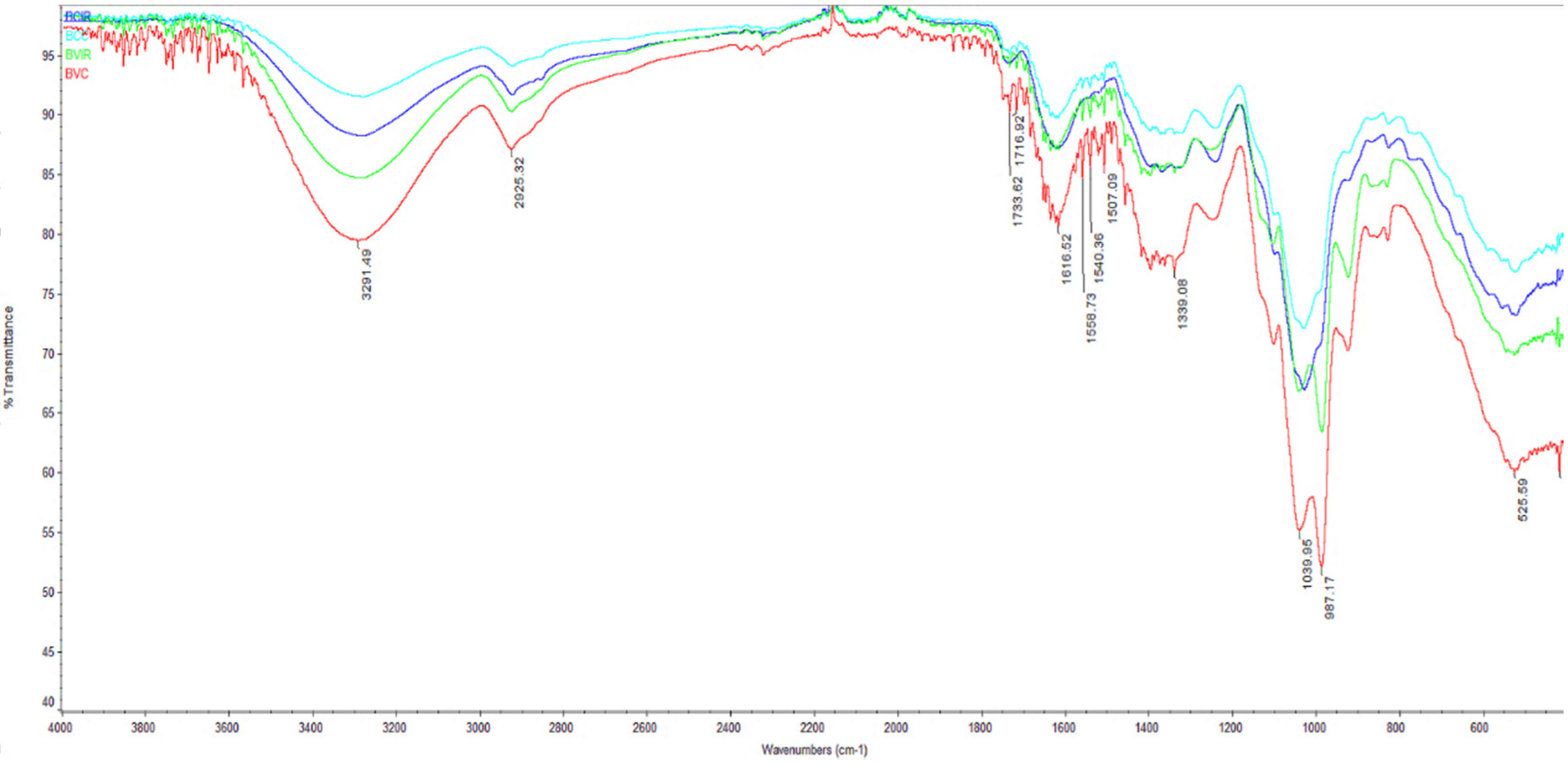
3.6. FTIR
3.7. Color Parameters
3.8. Textural and Oscillatory Measurements
3.8.1. Texture Analysis
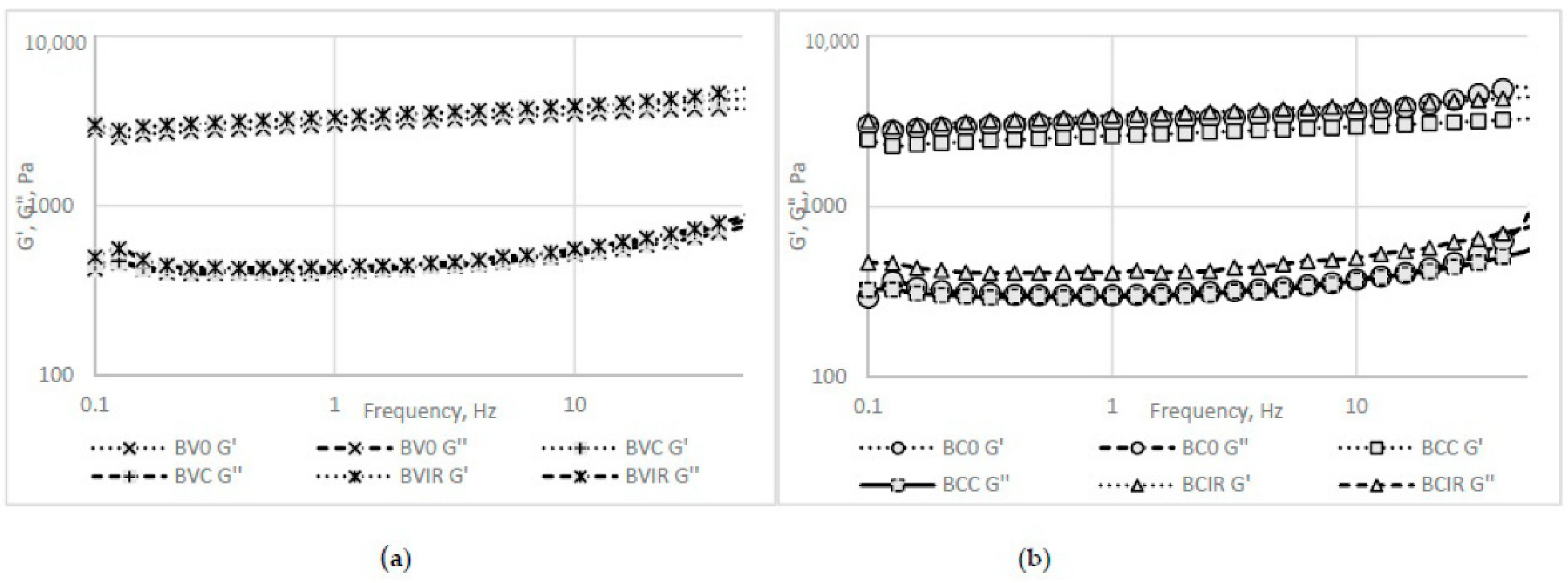
3.8.2. Rheological Analysis Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef]
- Rocchetti, G.; Tomas, M.; Zhang, L.; Zengin, G.; Lucini, L.; Capanoglu, E. Red beet (Beta vulgaris) and amaranth (Amaranthus sp.) microgreens: Effect of storage and in vitro gastrointestinal digestion on the untargeted metabolomic profile. Food Chem. 2020, 332, 127415. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Reddy, K.M.; Ruby, L.; Lindo, A.; Nair, G.M. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes and human tumor cells prolifieration by natural food color. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; Alessio, P.D. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef]
- Różyło, R. Recenttrends in methods used to obtain natural food colorants by freeze-dryin. Trends Food Sci. Technol. 2020, 102, 39–50. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability: A review. Int. J. Food Sci. Tech. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Dandamrongrak, R.; Young, G.; Mason, R. Evaluation of various pre-treatments for the dehydration of banana and selection of suitable drying models. J. Food Eng. 2002, 55, 136–146. [Google Scholar] [CrossRef]
- Vallespir, F.; Rodríguez, O.; Eim, V.S.; Rosselló, C.; Simal, S. Effects of freezing treatments before convective drying on quality parameters: Vegetables with different microstructures. J. Food Eng. 2019, 249, 15–24. [Google Scholar] [CrossRef]
- Kaleta, A.; Górnicki, K. Some remarks on evaluation of drying models of red beet particles. Energy Convers. Manag. 2010, 51, 2967–2978. [Google Scholar] [CrossRef]
- Torki-Harchegani, M.; Ghanbarian, D.; Maghsoodi, V.; Moheb, A. Infrared thin layer drying of saffron (Crocus sativus L.) stigmas: Mass transfer parameters and quality assessment. Chin. J. Chem. Eng. 2017, 25, 426–432. [Google Scholar] [CrossRef]
- Kipcak, A.S.; Doymaz, İ.; Derun, E.M. Infrared drying kinetics of blue mussels and physical properties. Chem. Ind. Chem. Eng. Q. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Jafari, F.; Movagharnejad, K.; Sadeghi, E. Infrared drying effects on the quality of eggplant slices and process optimization using response surface methodology. Food Chem. 2020, 333, 127423. [Google Scholar] [CrossRef]
- Begot, C.; Desnier, I.; Daudin, J.D.; Labadie, J.C.; Lebert, A. Recommendations for calculating growth parameters by optical density measurements. J. Microbiol. Methods 1996, 25, 225–232. [Google Scholar] [CrossRef]
- Wehr, H.M.; Frank, J.F. Standard Methods for the Examination of Dairy Products, 17th ed.; APHA Press-American Public Health Association: Washington, DC, USA, 2004. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors influencing the antioxidant activity determined by the ABTS∙+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Mahadi, H.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.E.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–135. [Google Scholar] [CrossRef]
- [CIE LEAD, EN ISO/CIE 11664-4:2019] Colorimetry—Part 4: CIE 1976 L * a * b * Colour Space; ISO/CIE 11664-4:2019; ISO Copyright Office: Geneva, Switzerland, 2019.
- Bolin, H.R.; Huxsoll, C.C. Control of minimally processed carrot (Daucus carota) surface discoloration caused by abrasion peeling. J. Food Sci. 1991, 46, 416–418. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Miguel, M.C. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Topolska, J.; Honke, J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by genotype and vegetation period. J. Funct. Foods 2014, 7, 201–211. [Google Scholar] [CrossRef]
- Sawicki, T.; Baczek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Antinelli, A.; Biagiotti, E.; Di Gioacchino, A.M.; Piccoli, G.; Stocchi, V.; Brandi, G. Characterization and biological activity of the main flavonoids from Swiss chard (Beta vulgaris subspecies cycla). Phytomed. Int. J. Phytother. Phytopharm. 2007, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Stampar, F.; Vebaric, R.; Jakopic, J. HPLC-MSn identification of betalains profile of different beetroot (Beta vulgaris L. ssp. vulgaris) parts and cultivars. J. Food Sci. 2015, 80, 1952–1958. [Google Scholar] [CrossRef]
- Seremet, L.; Nistor, O.V.; Andronoiu, D.G.; Mocanu, G.D.; Barbu, V.V.; Maidan, A.; Rudi, L.; Botez, E. Development of several hybrid drying methods used to obtain red beetroot powder. Food Chem. 2020, 310, 125637. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Toydemir, T.; Memis, K.N.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef]
- Sawicki, T.; Martinez-Villaluenga, C.; Frias, J.; Wiczkowski, W.; Peñas, E.; Baczek, N.; Zieliñski, H. The effect of processing and in vitro digestion on the betalain profile and ACE inhibition activity of red beetroot products. J. Funct. Foods 2019, 55, 229–237. [Google Scholar] [CrossRef]
- Sawicki, T.; Wiczkowski, W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef]
- Paraschiv, D.; Vasile, A.; Constantin, M.; Ciobanu, A.; Bahrim, G. Study of physiological properties of some probiotics in multiple cultures with mesophilic lactic acid bacteria by Flora Danica Ch. Hansen commercial starter. Ann. Univ. Dunarea Jos Galati Fasc. VI Food Technol. 2011, 35, 56–65. [Google Scholar]
- Trisnawita, Y.; Silalahi, J.; Sinaga, S.M. The effect of storage condition on viability of lactic acid bacteria in probiotic product. Asian J. Pharm. Clin. Res. 2018, 11, 84–86. [Google Scholar] [CrossRef][Green Version]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation. Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In Vitro Digestion of Betalainic Foods. Stability and Bioaccessibility of Betaxanthins and Betacyanins and Antioxidative Potential of Food Digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Barbu, V.; Cotârleț, M.; Bolea, C.A.; Cantaragiu, A.; Andronoiu, D.G.; Bahrim, G.E.; Enachi, E. Three Types of Beetroot Products Enriched with Lactic Acid Bacteria. Foods 2020, 9, 786. [Google Scholar] [CrossRef]
- Nesakumar, N.; Baskar, C.; Kesavan, S.; Rayappan, J.B.B.; Alwarappan, S. Analysis of Moisture Content in Beetroot using Fourier Transform Infrared Spectroscopy and by Principal Component Analysis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ganesapillai, M.; Gnanasundaram, N. Optimizaton of extraction of betalain pigments from beta vulgaris peels by microwave pretreatment. Mater. Sci. Eng. 2017, 263, 1–10. [Google Scholar] [CrossRef]
- Henao-Ardila, A.; Quintanilla-Carvajal, M.X.; Moreno, F.L. Combination of freeze concentration and spray drying for the production of feijoa (Acca sellowiana b.) pulp powder. Powder Technol. 2019, 344, 190–198. [Google Scholar] [CrossRef]
- Ng, M.L.; Sulaiman, R. Development of beetroot (Beta vulgaris) powder using foam mat drying. LWT 2018, 88, 80–86. [Google Scholar] [CrossRef]
- Ochoa-Martinez, L.A.; Garza-Juarez, S.E.; Rocha-Guzman, N.E.; Morales-Castro, J.; Gonzalez-Herrera, S.M. Functional Properties, Color and Betalain Content in Beetroot-Orange Juice Powder Obtained by Spray Drying. Res. Rev. J. Food Dairy Technol. 2015, 3, 30–36. [Google Scholar]
- Antigo, J.L.D.; Bergamasco, R.; Scaramal Madrona, G. Effect of ph on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. Camp. 2018, 38, 72–77. [Google Scholar] [CrossRef]
- Leverrier, C.; Almeida, G.; Espinosa-Munoz, L.; Cuvelier, G. Influence of Particle Size and Concentration on Rheological Behaviour of Reconstituted Apple Purees. Food Biophys. 2016, 11, 235–247. [Google Scholar] [CrossRef]


| Samples Code | Betalains | Total Polyphenols, mg GAE/g DW | Total Flavonoids, mg EC/g DW | ABTS Radical Scavenging Activity, mMol Trolox/g DW | |
|---|---|---|---|---|---|
| β-cyanin, mg/g DW | β-xanthin, mg/g DW | ||||
| BV0 | 7.03 ± 0.02 C | 4.06 ± 0.01 C | 32.88 ± 0.34 B | 23.31 ± 1.34 B,C | 53.94 ± 2.87 A |
| BC0 | 9.23 ± 0.07 C | 4.71 ± 0.06 C | 71.94 ± 2.21 B | 36.00 ± 1.78 B,C | 93.46 ± 2.51 A |
| BVC | 4.25 ± 0.02 C | 2.53 ± 0.02 C | 29.01 ± 0.97 B | 16.22 ± 0.81 B,C | 26.19 ± 0.32 A |
| BVIR | 4.49 ± 0.03 C | 2.49 ± 0.02 C | 27.76 ± 1.23 B | 26.19 ± 1.50 B,C | 92.21 ± 0.68 A |
| BCC | 4.19 ± 0.02 C | 2.21 ± 0.01 C | 26.05 ± 0.24 B | 14.02 ± 0.49 B,C | 55.95 ± 0.57 A |
| BCIR | 4.29 ± 0.02 C | 2.60 ± 0,08 C | 26.47 ± 1.18 B | 27.06 ± 1.57 B,C | 94.21 ± 0.38 A |
| Color Parameters | Fresh Samples | Dried Samples | ||||
|---|---|---|---|---|---|---|
| BV0 | BC0 | BVC | BVIR | BCC | BCIR | |
| L* | 21.16 ± 0.3 B | 21.19 ± 0.15 B | 25.78 ± 0.15 B | 30.67 ± 0.02 B | 29.68 ± 0.12 B | 32.15 ± 0.12 B |
| a* | 5.00 ± 0.2 B,C | 8.32 ± 0.03 B,C | 10.29 ± 0.22 B,C | 14.19 ± 0.02 B,C | 25.66 ± 0.26 B,C | 29.14 ± 0.04 B,C |
| b* | 1.45 ± 0.04 C | 1.41 ± 0.02 C | 5.07 ± 0.21 C | 6.56 ± 0.12 C | 6.02 ± 0.03 C | 7.78 ± 0.04 C |
| ΔE | - | - | 8.02 ± 0.27 B,C | 14.84 ± 0.28 B,C | 19.84 ± 0.28 B,C | 24.37 ± 0.21 B,C |
| C* | 5.21 ± 0.20 B,C | 8.44 ± 0.03 B,C | 11.47 ± 0.24 B,C | 15.64 ± 0.03 B,C | 26.35 ± 0.04 B,C | 30.16 ± 0.25 B,C |
| h* | 16.17 ± 0.31 B,C | 9.62 ± 0.11 B,C | 26.23 ± 0.18 B,C | 24.81 ± 0.47 B,C | 13.20 ± 0.06 B,C | 14.95 ± 0.10 B,C |
| BI | 28.57 ± 0.00 A | 35.29 ± 0.00 A | 47.05 ± 0.00 A | 52.94 ± 0.00 A | 76.47 ± 0.00 A | 88.23 ± 0.00 A |
| WI | 20.98 ± 0.29 B,C | 20.74 ± 0.15 B,C | 24.89 ± 0.11 B,C | 28.93 ± 0.03 B,C | 24.90 ± 0.11 B,C | 25.75 ± 0.04 B,C |
| YI | 9.78 ± 0.28 B,C | 9.51 ± 0.09 B,C | 28.09 ± 0.28 B,C | 30.56 ± 0.60 B,C | 28.97 ± 0.13 B,C | 34.57 ± 0.22 B,C |
| Parameter | BV0 | BVC | BVIR | BC0 | BCC | BCIR |
|---|---|---|---|---|---|---|
| Firmness, N | 0.76 ± 0.03 B | 1.31 ± 0.15 B | 0.97 ± 0.04 B | 0.72 ± 0.05 B | 1.26 ± 0.01 B | 1.02 ± 0.04 B |
| Adhesiveness, mJ | 3.38 ± 0.29 B | 0.69 ± 0.02 B | 0.35 ± 0.01 B | 4.58 ± 0.31 B | 0.44 ± 0.02 B | 0.25 ± 0.02 B |
| Cohesiveness, - | 0.72 ± 0.10 B | 0.47 ± 0.01 B | 0.46 ± 0.09 B | 0.67 ± 0.02 B | 0.45 ± 0.01 B | 0.42 ± 0.01 B |
| Springiness, mm | 9.18 ± 0.19 A | 5.77 ± 0.07 A | 6.23 ± 0.10 A | 8.72 ± 0.10 A | 5.29 ± 0.08 A | 6.06 ± 0.07 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, G.-D.; Chirilă, A.C.; Vasile, A.M.; Andronoiu, D.G.; Nistor, O.-V.; Barbu, V.; Stănciuc, N. Tailoring the Functional Potential of Red Beet Purées by Inoculation with Lactic Acid Bacteria and Drying. Foods 2020, 9, 1611. https://doi.org/10.3390/foods9111611
Mocanu G-D, Chirilă AC, Vasile AM, Andronoiu DG, Nistor O-V, Barbu V, Stănciuc N. Tailoring the Functional Potential of Red Beet Purées by Inoculation with Lactic Acid Bacteria and Drying. Foods. 2020; 9(11):1611. https://doi.org/10.3390/foods9111611
Chicago/Turabian StyleMocanu, Gabriel-Dănuț, Ana Cosmina Chirilă, Aida Mihaela Vasile, Doina Georgeta Andronoiu, Oana-Viorela Nistor, Vasilica Barbu, and Nicoleta Stănciuc. 2020. "Tailoring the Functional Potential of Red Beet Purées by Inoculation with Lactic Acid Bacteria and Drying" Foods 9, no. 11: 1611. https://doi.org/10.3390/foods9111611
APA StyleMocanu, G.-D., Chirilă, A. C., Vasile, A. M., Andronoiu, D. G., Nistor, O.-V., Barbu, V., & Stănciuc, N. (2020). Tailoring the Functional Potential of Red Beet Purées by Inoculation with Lactic Acid Bacteria and Drying. Foods, 9(11), 1611. https://doi.org/10.3390/foods9111611






