In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Grape Pomace Treatment and Extract Preparation
2.2.2. Mass Spectrometry Analysis
2.2.3. Bioaccessibility of Bioactive Compounds
Release of Bioaccessible Compounds
Antioxidant Assays
- In vitro antioxidant capacity studies
- Intracellular ROS determination
Anti-Inflammatory Capacity
Inhibition of α-Glucosidase and α-Amylase Enzymatic Activity
Glucose Transport
2.3. Statistical Analysis
3. Results and Discussions
3.1. Tannat Grape Skin Polyphenolic Composition
3.2. Bioaccessibility of Bioactive Compounds with Potential for Reducing the Risk or Treating Diabetes
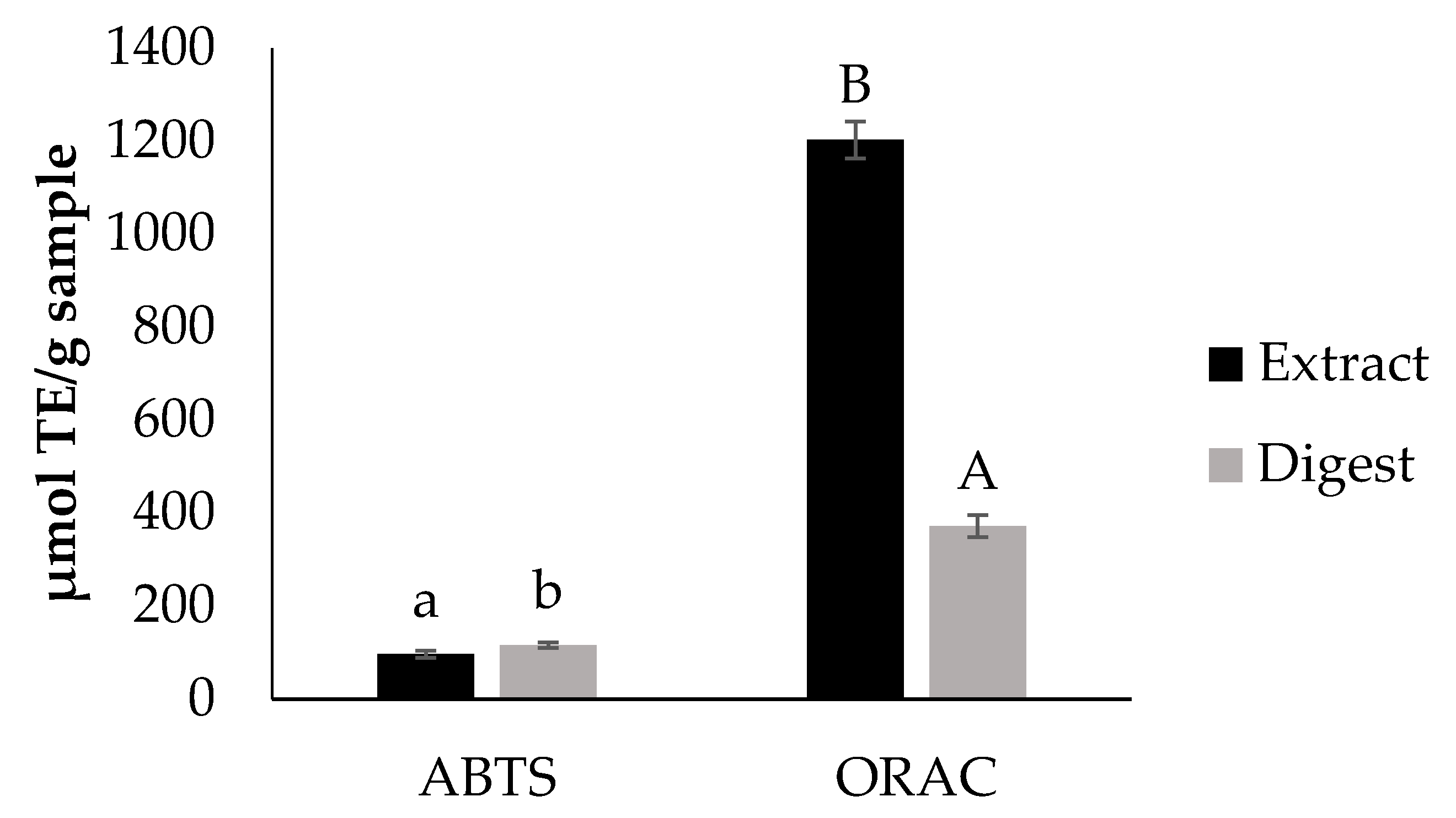
Bioaccessibility of Antioxidants
3.3. Bioaccessibility of Anti-Inflammatory Compounds
3.4. Bioaccessibility of Compounds with Potential to Modulate Glucose Metabolism
3.4.1. Inhibitors of Enzymatic Activity of α-Glucosidase and α-Amylase
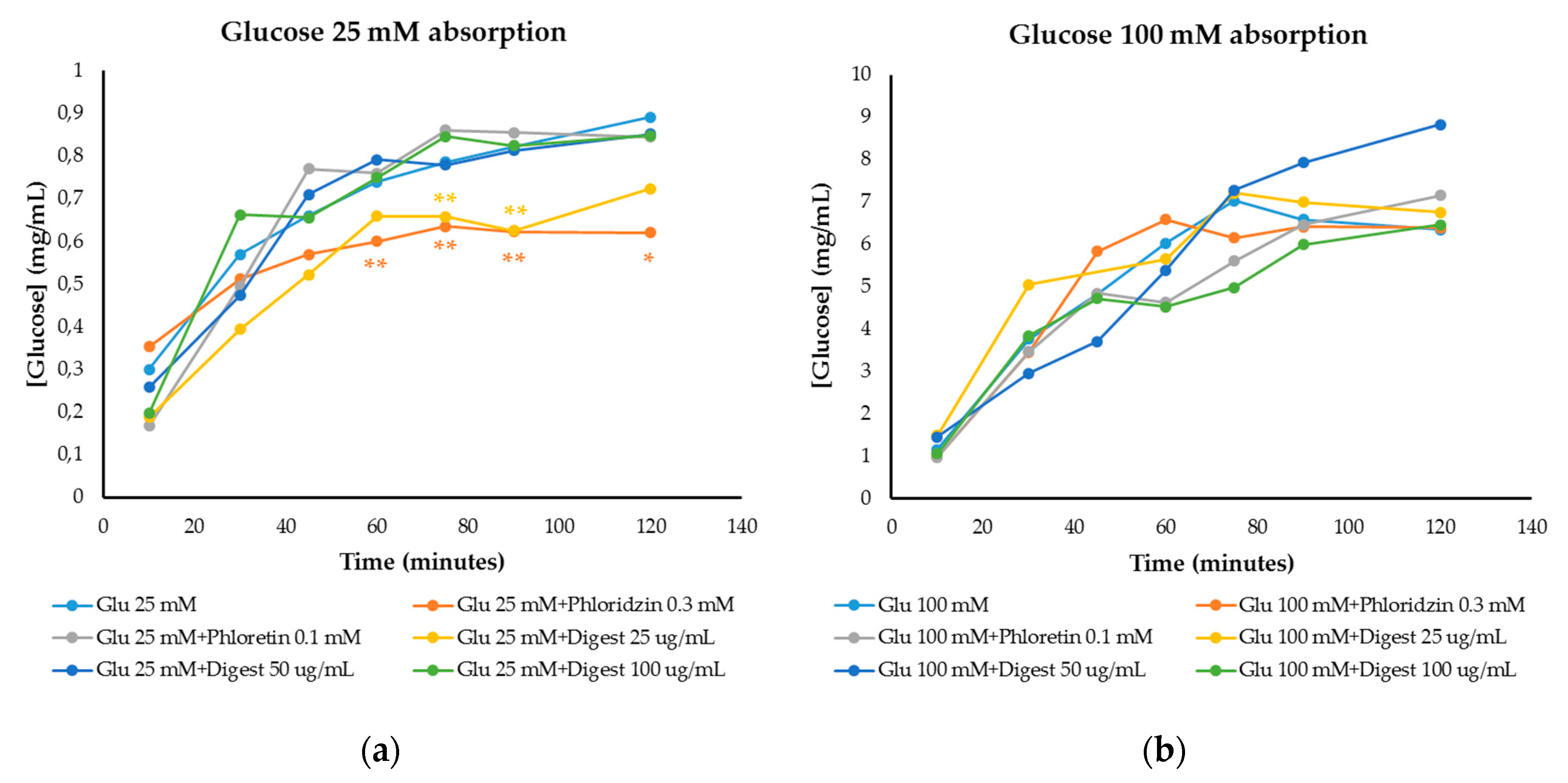
3.4.2. Modulation of Glucose Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boido, E.; García-Marino, M.; Dellacassa, E.; Carrau, F.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterisation and evolution of grape polyphenol profiles of Vitis vinifera L. cv. Tannat during ripening and vinification. Aust. J. Grape Wine Res. 2011, 17, 383–393. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of antioxidant, antidiabetic, antiobesity, and anti-inflammatory properties of a Tannat winemaking by-product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Arulselvan, P.; Ghofar, H.A.A.; Karthivashan, G.; Halim, M.F.A.; Ghafar, M.S.A.; Fakurazi, S. Antidiabetic therapeutics from natural source: A systematic review. Biomed. Prev. Nutr. 2014, 4, 607–617. [Google Scholar] [CrossRef]
- Li, B.; Terazono, Y.; Hirasaki, N.; Tatemichi, Y.; Kinoshita, E.; Obata, A.; Matsui, T. Inhibition of Glucose Transport by Tomatoside A, a Tomato Seed Steroidal Saponin, through the Suppression of GLUT2 Expression in Caco-2 Cells. J. Agric. Food Chem. 2018, 66, 1428–1434. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Serra, M.; Larcher, R. Non-targeted glycosidic profiling of international wines using neutral loss-high resolution mass spectrometry. J. Chromatogr. A 2018, 1557, 75–89. [Google Scholar] [CrossRef]
- Hollebeeck, S.; Borlon, F.; Schneider, Y.-J.; Larondelle, Y.; Rogez, H. Development of a standardised human in vitro digestion protocol based on macronutrient digestion using response surface methodology. Food Chem. 2013, 138, 1936–1944. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Suberviola, J.; Gómez-Cordovés, C. ORAC-fluorescein as a model for evaluating antioxidant activity of wines. Polish J. Food Nutr. Sci. 2003, 12, 133–136. [Google Scholar]
- Iriondo-DeHond, A.; Ramírez, B.; Escobar, F.V.; del Castillo, M.D. Antioxidant properties of high molecular weight compounds from coffee roasting and brewing byproducts. Bioact. Compd. Health Dis. 2019, 2, 48. [Google Scholar] [CrossRef]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crops Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Du, J.; Deng, X.; Li, C. Persimmon Tannin Decreased the Glycemic Response through Decreasing the Digestibility of Starch and Inhibiting α-Amylase, α-Glucosidase, and Intestinal Glucose Uptake. J. Agric. Food Chem. 2018, 66, 1629–1637. [Google Scholar] [CrossRef]
- Favre, G.; Hermosín-Gutiérrez, I.; Piccardo, D.; Gómez-Alonso, S.; González-Neves, G. Selectivity of pigments extraction from grapes and their partial retention in the pomace during red-winemaking. Food Chem. 2019, 277, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.; Dörnyei, Á.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Kilár, F.; Márk, L. Rapid MALDI-TOF-MS Detection of Anthocyanins in Wine and Grape Using Different Matrices. Food Anal. Methods 2011, 4, 108–115. [Google Scholar] [CrossRef]
- Beres, C.; Freitas, S.P.; de Oliveira Godoy, R.L.; de Oliveira, D.C.R.; Deliza, R.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M.C. Antioxidant dietary fibre from grape pomace flour or extract: Does it make any difference on the nutritional and functional value? J. Funct. Foods 2019, 56, 276–285. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Heredia, J.B. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Haminiuk, C.W.I.; Barros, L.; Dias, M.I.; Calhelha, R.C.; Kato, C.G.; Correa, V.G.; Peralta, R.M.; Ferreira, I.C.F.R. Stability and biological activity of Merlot (Vitis vinifera) grape pomace phytochemicals after simulated in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2017, 36, 410–417. [Google Scholar] [CrossRef]
- Ohanna, K.; Inada, P.; Barcellos, T.; Silva, R.; Lobo, L.A.; Cavalcante, R.M.; Domingues, P.; Perrone, D.; Monteiro, M. Bioaccessibility of phenolic compounds of jaboticaba (Plinia jaboticaba) peel and seed after simulated gastrointestinal digestion and gut microbiota fermentation. J. Funct. Foods 2020, 67. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Álvarez, J.A.P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Fuentes, J.A.N. Comprehensive Study of the Grape (Vitis vinifera L.) as a Source of Bioavailable Phenolic Compounds with Biological Activity. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2015. [Google Scholar]
- Rebelo, M.J.; Sousa, C.; Valentão, P.; Rego, R.; Andrade, P.B. Phenolic profile of Douro wines and evaluation of their NO scavenging capacity in LPS-stimulated RAW 264.7 macrophages. Food Chem. 2014, 163, 16–22. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Lee, W.S.; Shin, S.C.; Kim, G.-Y.; Choi, B.T.; Choi, Y.H. Anthocyanins Downregulate Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglial Cells by Suppressing the NF-κB and Akt/MAPKs Signaling Pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Mukai, R.; Ichiyanagi, T.; Ashida, H. Suppression of Lipopolysaccharide and Galactosamine-Induced Hepatic Inflammation by Red Grape Pomace. J. Agric. Food Chem. 2012, 60, 9315–9320. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Denny, C.; Lazarini, J.G.; Franchin, M.; Melo, P.S.; Pereira, G.E.; Massarioli, A.P.; Moreno, I.A.M.; Paschoal, J.A.R.; Alencar, S.M.; Rosalen, P.L. Bioprospection of Petit Verdot grape pomace as a source of anti-inflammatory compounds. J. Funct. Foods 2014, 8, 292–300. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef]
- Ostberg-Potthoff, J.J.; Berger, K.; Richling, E.; Winterhalter, P. Activity-Guided Fractionation of Red Fruit Extracts for the Identification of Compounds Influencing Glucose Metabolism. Nutrients 2019, 11, 1166. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.; Li, C.M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of Glucose Transporter Expression in Human Intestinal Caco-2 Cells following Exposure to an Anthocyanin-Rich Berry Extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed]
| Negative ESI | ||||
| Compound 1 | RT [min] | [M-H]− (m/z) | Fragments (m/z) | Extract 2 |
| 3-Phenyllactic acid | 10.6 | 165.0559 | 147.0455, 119.0504 | 0.0775 |
| 7-Hydroxy-2-(4-hydroxyphenyl)-4-oxo-3,4-dihydro-2H-chromen-5-yl β-D-glucopyranoside | 11.8 | 433.1154 | 271.0607, 151.0041 | 0.0044 |
| Astragalin isomer 1 | 11.1 | 447.0944 | 284.0334, 227.0356 | 0.0175 |
| Astragalin isomer 2 | 11.2 | 447.0949 | 284.0334, 227.0356 | 0.0234 |
| Caffeic acid | 9.5 | 179.0354 | 135.0455 | 0.0172 |
| cis-Aconitic acid | 2.8 | 173.0094 | 129.0197, 85.0297 | 0.0575 |
| Eriodictyol | 13.6 | 287.0570 | 151.0041, 135.0456 | 0.0046 |
| Gallic acid | 3.7 | 169.0145 | 125.0247 | 0.2303 |
| Isorhamnetin | 14.9 | 315.0519 | 300.0283, 151.0037 | 0.1849 |
| Myricetin | 12.3 | 317.0310 | 178.9989, 151.0040 | 0.1094 |
| Quercetin-3-galacturonide | 10.7 | 477.0685 | 301.0361, 151.0039 | 0.4236 |
| Quercetin | 13.5 | 301.0361 | 151.0040, 107.0141 | 0.3677 |
| Quercetin-3β-D-glucoside | 10.7 | 463.0900 | 300.0282, 271.0254 | 0.1575 |
| Syringic acid | 11.2 | 197.0459 | 182.0225, 123.0091 | 0.0595 |
| Vanillic acid | 10.8 | 167.0352 | 152.0118, 123.0091 | 0.0450 |
| Vanillyl alcohol | 5.2 | 153.0561 | 138.0325, 123.0091 | 0.0336 |
| Naringenin | 14.8 | 271.0620 | 151.0041, 119.0505 | 0.0071 |
| Positive ESI | ||||
| Compound 3 | RT [min] | [M]+ (m/z) | Fragments (m/z) | Extract 2 |
| Cyanidin 3-(6-O-acetylglucoside) | 9.8 | 491.1184 | 287.0550 | 0.00001 |
| Cyanidin-3-O-(6-p-coumaroyl) glucoside | 10.9 | 595.1446 | 287.0550 | 0.00004 |
| Cyanidin-3-pyranoside | 8.5 | 449.1078 | 287.0550 | 0.00003 |
| Delphinidin-3-(6-O-acetylglucoside) | 9.2 | 507.1133 | 303.0500 | 0.00003 |
| Delphinidin-3-O-(6-p-coumaroyl) glucoside | 10.5 | 611.1395 | 303.0500 | 0.00015 |
| Delphinidin-3-pyranoside | 7.8 | 465.1027 | 303.0500 | 0.00010 |
| Malvidin-3-(6-O-acetylglucoside) | 10.4 | 535.1446 | 331.0800 | 0.00444 |
| Malvidin-3-O-(6-p-coumaroyl) glucoside | 11.5 | 639.1708 | 331.0800 | 0.00757 |
| Malvidin-3-pyranoside | 9.2 | 493.1340 | 331.0800 | 0.00886 |
| Peonidin-3-(6-O-acetylglucoside) | 10.4 | 505.1341 | 301.0700 | 0.00019 |
| Peonidin-3-O-(6-p-coumaroyl) glucoside | 11.5 | 609.1603 | 301.0700 | 0.00037 |
| Peonidin-3-pyranoside | 9.2 | 463.1235 | 301.0700 | 0.00033 |
| Petunidin-3-(6-O-acetylglucoside) | 9.9 | 521.1290 | 317.0700 | 0.00037 |
| Petunidin-3-O-(6-p-coumaroyl) glucoside | 11.0 | 625.1552 | 317.0700 | 0.00072 |
| Petunidin-3-pyranoside | 11.2 | 479.1184 | 317.0700 | 0.00051 |
| Digest | Assays | ||
|---|---|---|---|
| (µg/mL) | Antioxidant Properties | ||
| Intracellular ROS Formation in CCD-18Co Cells (%) | |||
| Physiological Conditions | Induced by t-BOOH (1 mM) | ||
| Prevention | Prevention with Co-Administration | ||
| 0 | 100.0 ± 6.3 a,A | 151.0 ± 12.7 a,b,B | 178.0 ± 7.0 b,B |
| 100 | 152.5 ± 39.2 b,A | 146.4 ± 30.5 a,b,A | 117.9 ± 30.0 a,A |
| 250 | 125.8 ± 13.7 a,b,A | 174.7 ± 57.2 b,A | 128.6 ± 21.2 a,A |
| 500 | 144.1 ± 10.4 b,A | 122.8 ± 13.8 b,A | 138.8 ± 32.0 a,A |
| 1000 | 135.1 ± 24.8 a,b,A | 192.4 ± 19.6 b,B | 185.3 ± 19.0 b,B |
| Intracellular ROS Formation in RAW 264.7 Cells (%) | |||
| Physiological Conditions | Induced by t-BOOH (1 mM) | ||
| Prevention | Prevention with Co-Administration | ||
| 0 | 100.0 ± 8.3 a,A | 211.4 ± 44.4 b,B | 211.4 ± 44.4 b,B |
| 100 | 98.8 ± 20.0 a,A | 136.6 ± 15.8 a,B | 150.1 ± 39.3 a,B |
| 250 | 143.2 ± 39.1 b,A | 132.2 ± 10.9 a,A | 110.8 ± 21.4 a,A |
| 500 | 99.3 ± 24.3 a,A | 150.2 ± 21.1 a,B | 124.8 ± 16.6 a,A,B |
| 1000 | 116.3 ± 44.8 a,b,A | 99.4 ± 27.9 a,A | 129.9 ± 46.4 a,A |
| Anti-inflammatory Properties | |||
| (µg/mL of NO Formation in RAW 264.7 Cells Induced by LPS 1 µg/mL) | |||
| Prevention | Prevention with Co-Administration | ||
| 0 | 9.9 ± 0.8 c,A | 9.9 ± 0.8 c,A | |
| 100 | 5.3 ± 0.7 b,A | 5.6 ± 0.9 b,A | |
| 250 | 4.1 ± 0.9 a,A | 5.4 ± 1.0 b,B | |
| 500 | 3.3 ± 0.6 a,A | 4.9 ± 0.7 a,b,B | |
| 1000 | 3.1 ± 1.1 a,A | 4.0 ± 1.2 a,A | |
| Sample | α-Glucosidase Activity (IC50, μg/mL) | α-Amylase Activity (IC50, μg/mL) |
|---|---|---|
| Acarbose | 4.0 ± 0.3 a | 34.1 ± 0.8 a |
| Extract | 888.5 ± 79.3 b | 1855.8 ± 21.3 b |
| Digest | 2945.7 ± 288.7 c | 55,068.0 ± 1227.9 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Nardin, T.; Larcher, R.; Dellacassa, E.; Medrano-Fernandez, A.; Castillo, M.D.d. In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods 2020, 9, 1575. https://doi.org/10.3390/foods9111575
Fernández-Fernández AM, Iriondo-DeHond A, Nardin T, Larcher R, Dellacassa E, Medrano-Fernandez A, Castillo MDd. In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods. 2020; 9(11):1575. https://doi.org/10.3390/foods9111575
Chicago/Turabian StyleFernández-Fernández, Adriana Maite, Amaia Iriondo-DeHond, Tiziana Nardin, Roberto Larcher, Eduardo Dellacassa, Alejandra Medrano-Fernandez, and María Dolores del Castillo. 2020. "In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes" Foods 9, no. 11: 1575. https://doi.org/10.3390/foods9111575
APA StyleFernández-Fernández, A. M., Iriondo-DeHond, A., Nardin, T., Larcher, R., Dellacassa, E., Medrano-Fernandez, A., & Castillo, M. D. d. (2020). In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods, 9(11), 1575. https://doi.org/10.3390/foods9111575