Risk Assessment of the Dietary Phosphate Exposure in Taiwan Population Using a Total Diet Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Classifying and Clustering Food Products
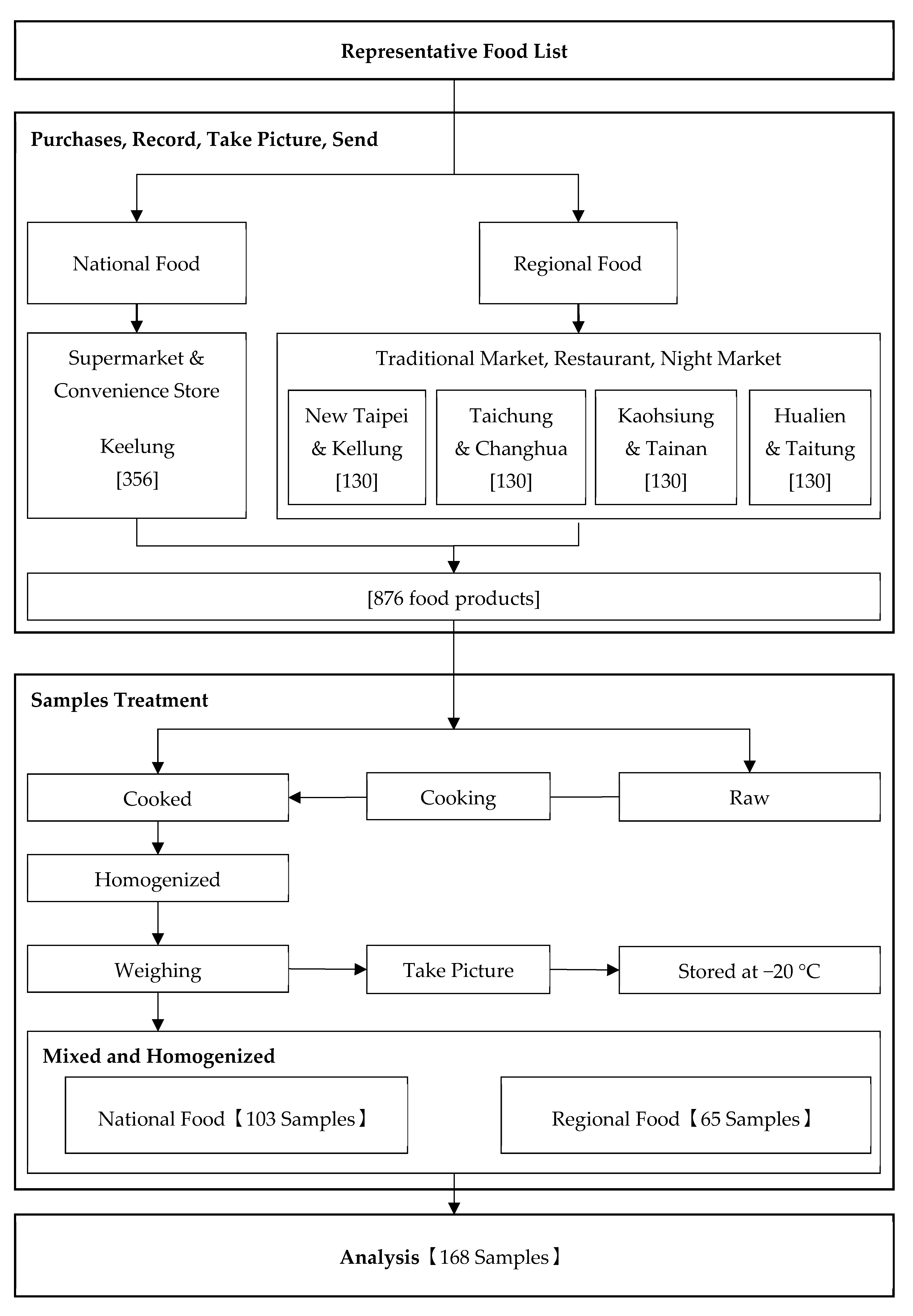
2.2. Representative Food Product List
- Scenario 1: If the total proportion of food consumption was ≥50% and if these items had well-known brand names, then they were listed as purchased items.
- Scenario 2: If the total proportion of food consumption was ≥50%, but they were from unknown brand names, then the food products were ignored. Eight food products with brand names were selected and sorted out in descending order based on the food consumption proportions.
- Scenario 3: If the items were all unknown brand names, then eight sets of food products were randomly purchased in the market.
- Scenario 1: If the total proportion of food consumption amount for a whole group was ≥50% and it had multiple items of different types, then those having similar total phosphorus content were listed as purchased items.
- Scenario 2: If the total proportion of food consumption amount for a whole group was ≥50% and it had multiple items with different or unknown total phosphate, then they were listed as individually purchased.
2.3. Sampling and Preparing Samples
2.4. Analyzing the Total Phosphorus and Calcium Concentration in Samples
2.5. Total Phosphorus and Calcium Intake and Risk Assessment
3. Results and Discussion
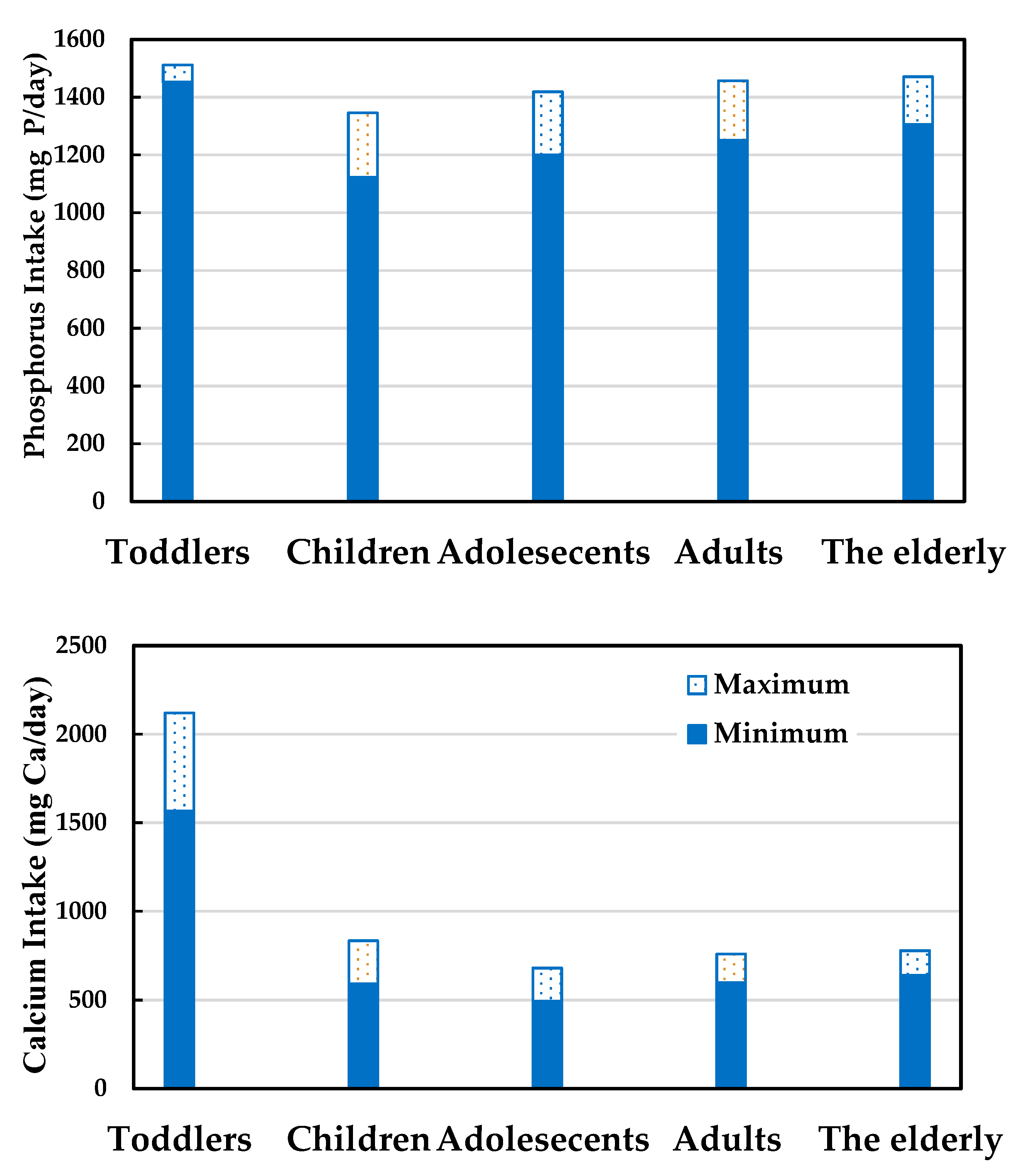
3.1. Calcium and Phosphorus Intake and Potential Health Risks
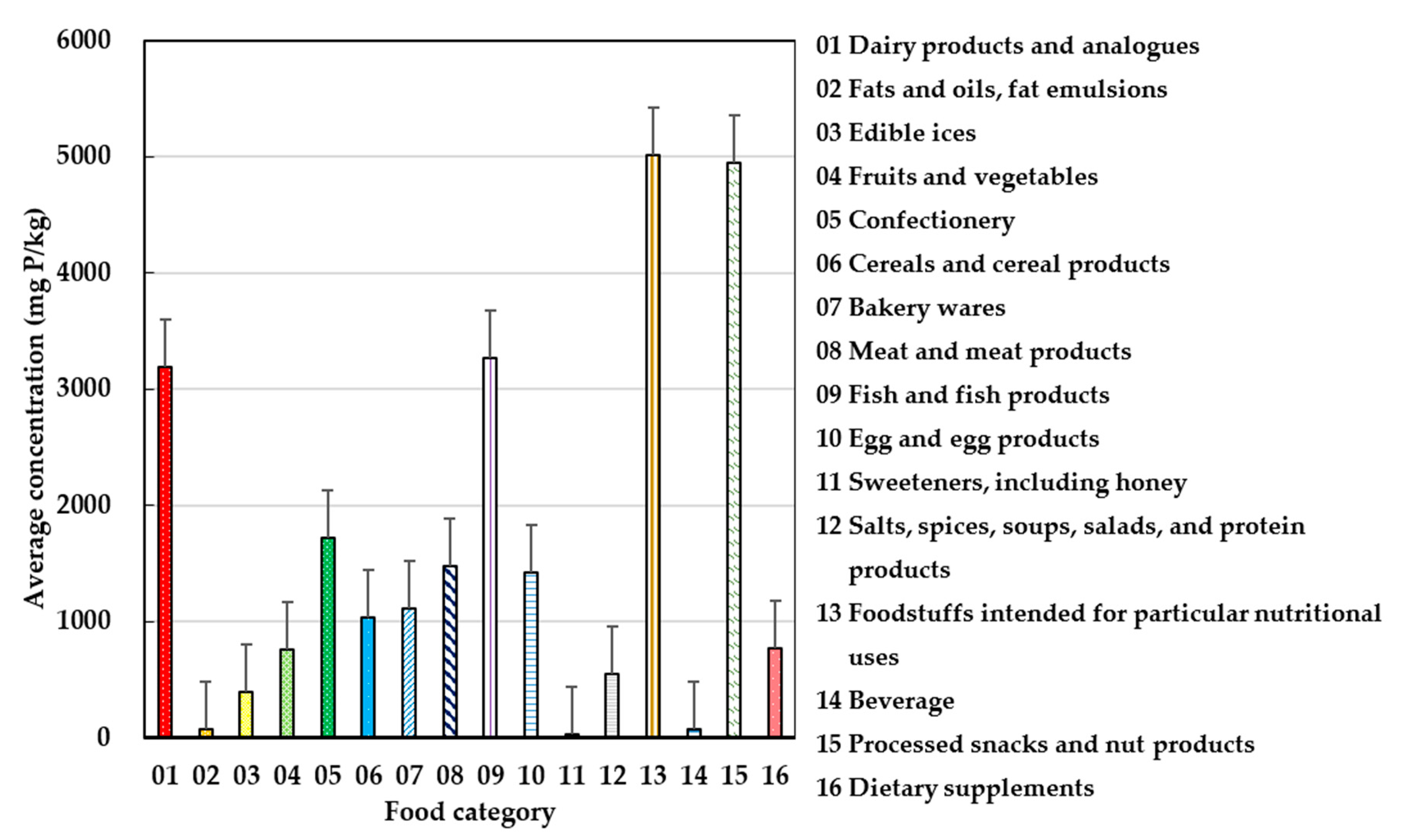
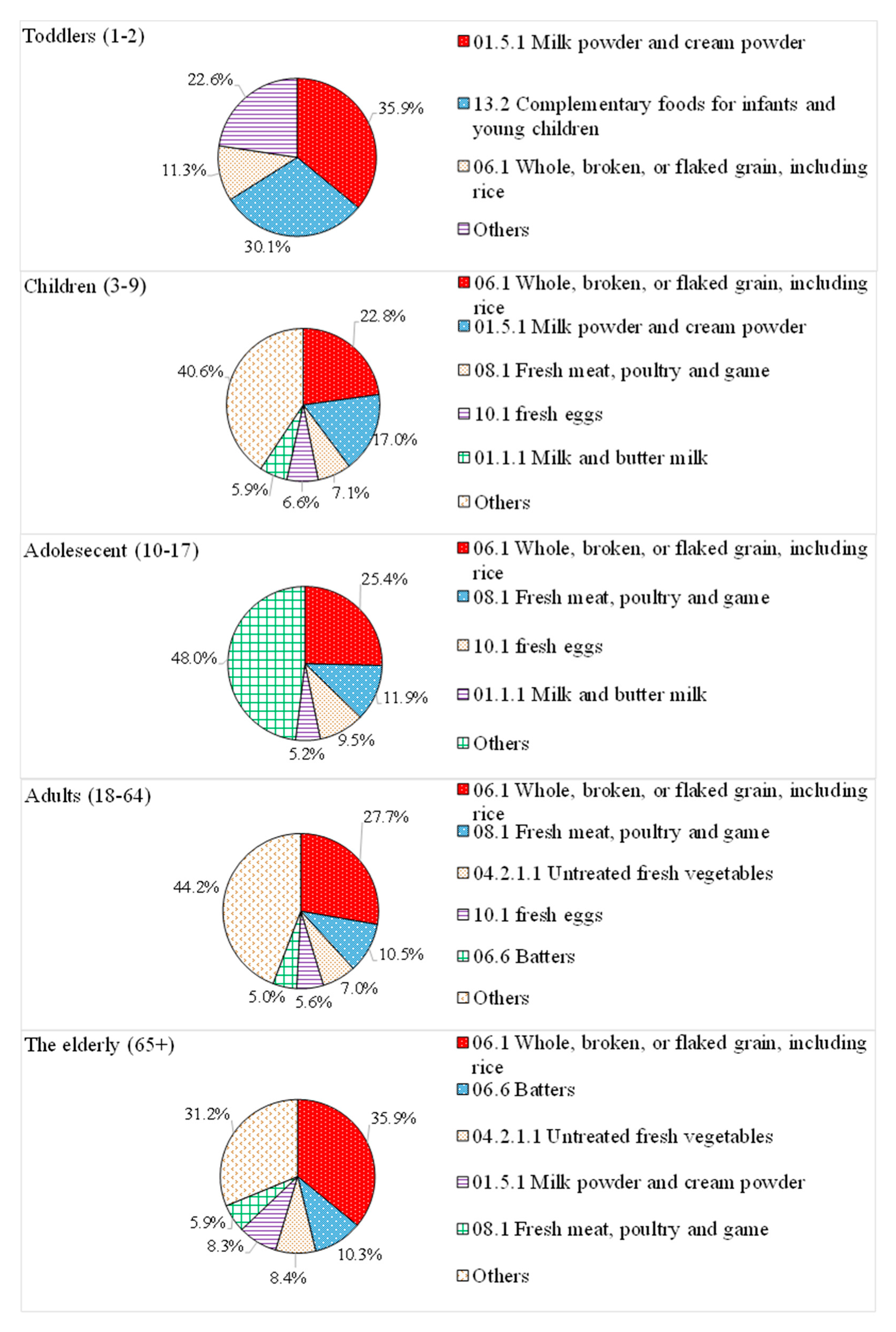
3.2. Main Contributing Food Products of Phosphate Exposure
3.3. Safety Risk Assessment of Toddler Food Products
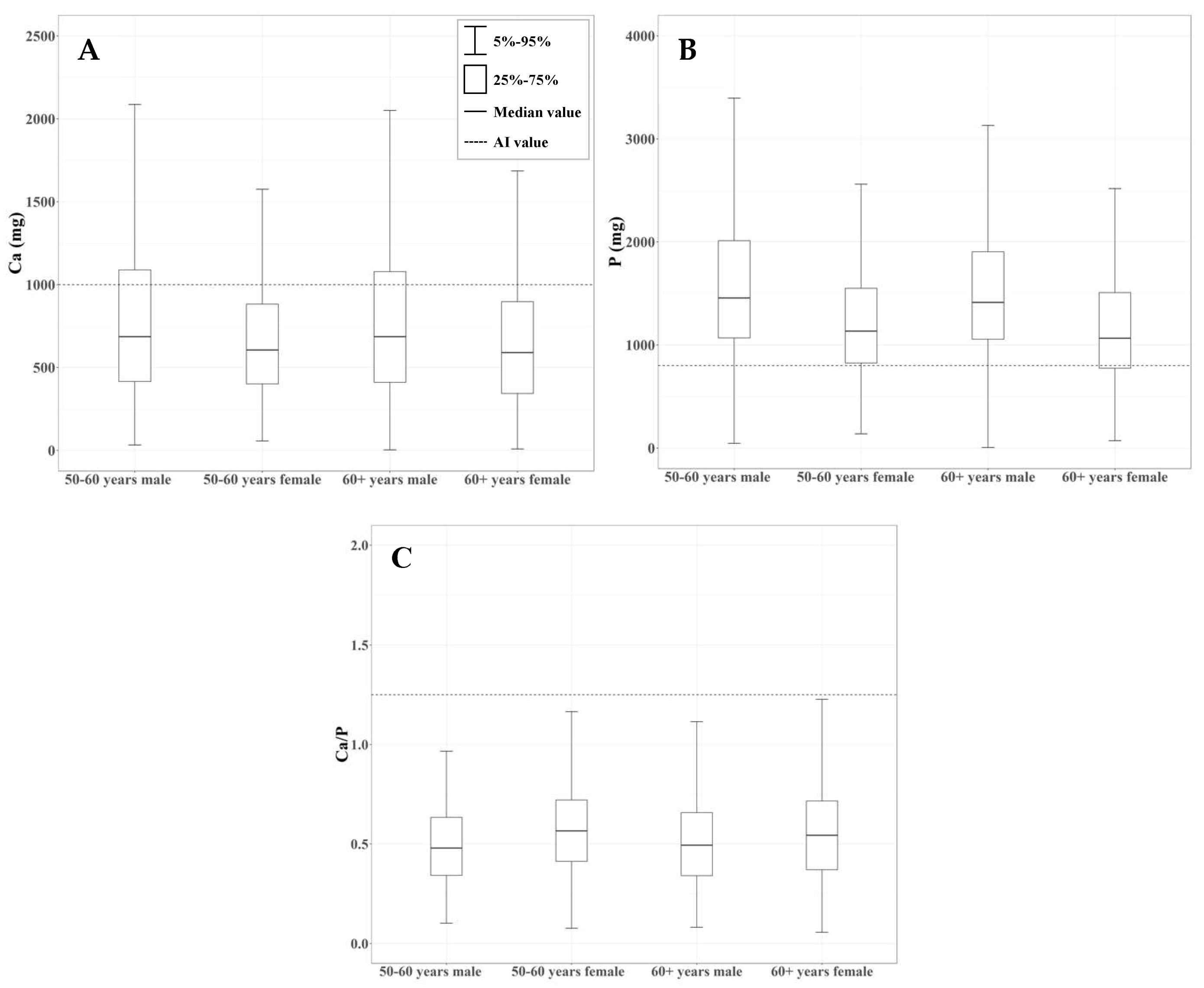
3.4. Survey of Calcium and Phosphorus Intake in the Elderly Population
3.5. Uncertainty Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noori, N.; Sims, J.J.; Kopple, J.D.; Shah, A.; Colman, S.; Shinaberger, C.S.; Kalantar-Zadeh, K. Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran. J. Kidney Dis. 2010, 4, 89. [Google Scholar] [PubMed]
- Wu, S.J.; Chang, Y.H.; Chang, H.Y.; Pan, W.H. Food sources of dietary calcium, phosphorus, iron, and sodium, nutrition and health survey in Taiwan (NAHSIT) 1993–1996. Mag. Nutr. Soc. Taiwan 2001, 26, 142–158. [Google Scholar]
- Foley, R.N.; Collins, A.J.; Herzog, C.A.; Ishani, A.; Kalra, P.A. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J. Am. Soc. Nephrol. 2009, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Mehta, O. Dietary phosphorus restriction in dialysis patients: Potential impact of processed meat, poultry, and fish products as protein sources. Am. J. Kidney Dis. 2009, 54, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Sacks, F.; Pfeffer, M.; Gao, Z.; Curhan, G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005, 112, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.M.; Leon, J.B.; Sehgal, A.R. Phosphorus-containing food additives and the accuracy of nutrient databases: Implications for renal patients. J. Ren. Nutr. 2007, 17, 350–354. [Google Scholar] [CrossRef]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med. 2007, 167, 879–885. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Contributions to total phosphorus intake: All sources considered. Semin. Dial. 2013, 26, 54–61. [Google Scholar] [CrossRef]
- Parpia, A.S.; L’Abbé, M.; Goldstein, M.; Arcand, J.; Magnuson, B.; Darling, P.B. The impact of additives on the phosphorus, potassium, and sodium content of commonly consumed meat, poultry, and fish products among patients with chronic kidney disease. J. Ren. Nutr. 2018, 28, 83–90. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am. J. Clin. Nutr. 2013, 98, 6–15. [Google Scholar] [CrossRef]
- Borgi, L. Inclusion of phosphorus in the nutrition facts label. Clin. J. Am. Soc. Nephrol. 2019, 14, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Benini, O.; D’Alessandro, C.; Gianfaldoni, D.; Cupisti, A. Extra-phosphate load from food additives in commonly eaten foods: A real and insidious danger for renal patients. J. Ren. Nutr. 2011, 21, 303–308. [Google Scholar] [CrossRef]
- Larsson, T.E.; Olauson, H.; Hagström, E.; Ingelsson, E.; Ärnlöv, J.; Lind, L.; Sundström, J. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kemi, V.E.; Kärkkäinen, M.U.; Rita, H.J.; Laaksonen, M.M.; Outila, T.A.; Lamberg-Allardt, C.J. Low calcium: Phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Br. J. Nutr. 2010, 103, 561–568. [Google Scholar] [CrossRef]
- Pan, W.H.; Wu, H.J.; Yeh, C.J.; Chuang, S.Y.; Chang, H.Y.; Yeh, N.H.; Hsieh, Y.T. Diet and health trends in Taiwan: Comparison of two nutrition and health surveys from 1993–1996 and 2005–2008. Asia Pac. J. Clin. Nutr. 2011, 20, 238–250. [Google Scholar]
- Pan, W.H. Nutrition and Health Survey in Taiwan 2004–2008. Project Report. DOH94-FS-6-4; Food Drug Adm. Dep. Health. Taiwan (R.O.C.): Taipei City, Taiwan, 2008. [Google Scholar]
- Tu, S.H.; Chen, C.; Hsieh, Y.T.; Chang, H.Y.; Yeh, C.J.; Lin, Y.C.; Pan, W.H. Design and sample characteristics of the 2005‒2008 Nutrition and Health Survey in Taiwan. Asia Pac. J. Clin. Nutr. 2011, 20, 225–237. [Google Scholar]
- European Food Safety Authority (EFSA). Use of the EFSA comprehensive European food consumption database in exposure assessment. Efsa J. 2011, 9, 2097. [Google Scholar] [CrossRef]
- Taiwan Food and Drug Administration (TFDA). General Method of Test for Heavy Metals. Available online: https://www.fda.gov.tw/tc/includes/GetFile.ashx?mid=133&id=22608&t=s (accessed on 30 June 2020).
- JECFA. Evaluation of Certain Food Additives and Contaminants: Thirteenth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 1970. [Google Scholar]
- Health Promotion Administration (HPA). Dietary Reference Intakes of Taiwanese Seventh Edition. Available online: https://www.hpa.gov.tw/File/Attach/725/File_1674.pdf (accessed on 30 June 2020).
- Pan, L.B.; Wang, Z.; Huang, W.; Zhang, J.Z.; Cao, B.; Zhang, Z.H. Investigation on the dietary intake and nutritional status of residents in Shenzhen, Guangdong. Chin. Prev. Med. 2011, 12, 953–955. [Google Scholar]
- Leblanc, J.C.; Guérin, T.; Noël, L.; Calamassi-Tran, G.; Volatier, J.L.; Verger, P. Dietary exposure estimates of 18 elements from the 1st French Total Diet Study. Food Addit. Contam. 2005, 22, 624–641. [Google Scholar] [CrossRef]
- Gimou, M.M.; Charrondière, U.R.; Leblanc, J.C.; Noël, L.; Guérin, T.; Pouillot, R. Dietary exposure and health risk assessment for 11 minerals and trace elements in Yaoundé: The Cameroonian Total Diet Study. Food Addit. Contam. Part A 2013, 30, 1556–1572. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Ministry of Health and Welfare (MOHW), Executive Yuan. Nutritional Reference Manual for Toddlers (Draft). Available online: https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/7365/File_6856.pdf (accessed on 30 June 2017).
- Pettifor, J.M.; Marie, P.J.; Sly, M.R.; Ross, F.; Isdale, J.M.; de Klerk, W.A.; van der Walt, W.H. The effect of differing dietary calcium and phosphorus contents on mineral metabolism and bone histomorphometry in young vitamin D-replete baboons. Calcif. Tissue Int. 1984, 36, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Han, Y.C.; Wu, Y.T.; Tsauo, J.Y. National health insurance data in hospitalized patients with osteoporosis-related fractures. Formos. J. Med. 2007, 11, 22–28. [Google Scholar] [CrossRef]
- Reuben, A.; Kathy, R.; Anderson, J.J.B. Intakes of calcium and phosphorus and calculated calcium: Phosphorus ratios of older adults: NHANES 2005–2006 data. Nutrients 2015, 7, 9633–9639. [Google Scholar] [CrossRef]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES iii. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
| Exposure Groups (Years) | EDI (mg P/kg/day) | %MTDI | |
|---|---|---|---|
| Toddlers (1–2) | Whole | 118.1–122.9 | 168.7–175.6 |
| Children (3–9) | Male | 49.4–59.0 | 70.54–84.3 |
| Female | 44.2–53.2 | 63.2–76.0 | |
| Whole | 46.8–56.0 | 66.8–80.0 | |
| Adolescents (10–17) | Male | 24.0–28.3 | 34.3–40.4 |
| Female | 20.5–24.4 | 29.3–34.9 | |
| Whole | 22.1–26.2 | 31.6–37.4 | |
| Adults (18–64) | Male | 21.3–24.7 | 30.4–35.3 |
| Female | 18.4–21.5 | 26.3–30.7 | |
| Whole | 19.9–23.1 | 28.4–33.0 | |
| The elderly (65+) | Male | 22.7–25.8 | 32.4–36.9 |
| Female | 20.4–22.7 | 29.1–32.4 | |
| Whole | 21.6–24.3 | 30.8–34.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, M.-P.; Huang, J.-D.; Hsiao, H.-A.; Chang, Y.-W.; Kao, Y.-T. Risk Assessment of the Dietary Phosphate Exposure in Taiwan Population Using a Total Diet Study. Foods 2020, 9, 1574. https://doi.org/10.3390/foods9111574
Ling M-P, Huang J-D, Hsiao H-A, Chang Y-W, Kao Y-T. Risk Assessment of the Dietary Phosphate Exposure in Taiwan Population Using a Total Diet Study. Foods. 2020; 9(11):1574. https://doi.org/10.3390/foods9111574
Chicago/Turabian StyleLing, Min-Pei, Jun-Da Huang, Huai-An Hsiao, Yu-Wei Chang, and Yi-Ting Kao. 2020. "Risk Assessment of the Dietary Phosphate Exposure in Taiwan Population Using a Total Diet Study" Foods 9, no. 11: 1574. https://doi.org/10.3390/foods9111574
APA StyleLing, M.-P., Huang, J.-D., Hsiao, H.-A., Chang, Y.-W., & Kao, Y.-T. (2020). Risk Assessment of the Dietary Phosphate Exposure in Taiwan Population Using a Total Diet Study. Foods, 9(11), 1574. https://doi.org/10.3390/foods9111574





