Effects of Radishes, Apples, and Pears on the Lactic Acid Bacteria and Nutritional and Functional Qualities of Flavored Soy Sauce
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Flavored Soy Sauces at Laboratory Scale
2.3. Determination of the pH, Total Soluble Solids, and Browning Potential of the Flavored Soy Sauces
2.4. Sensory Evaluation
2.5. Preparation of the Flavored Soy Sauce at Plant Scale
2.6. Determination of Amino Nitrogen, Free Sugars, Organic Acids, and Amino Acids
2.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.8. Estimation of the Total Phenolics, Flavonoids, and In Vitro Antioxidative Activity
2.9. Cellular Antioxidative Potential
2.9.1. Cell Culture and Cell Viability Assay
2.9.2. Determination of Cellular Reactive Oxygen Species (ROS)
2.10. Enumeration, Identification, and Enzyme Profiling of the Lactic Acid Bacteria
2.11. Xanthine Oxidase Inhibitory Activity of the Lactic Acid Bacteria
2.12. Quantification of Biogenic Amines
2.13. Statistical Analysis
3. Results and Discussion
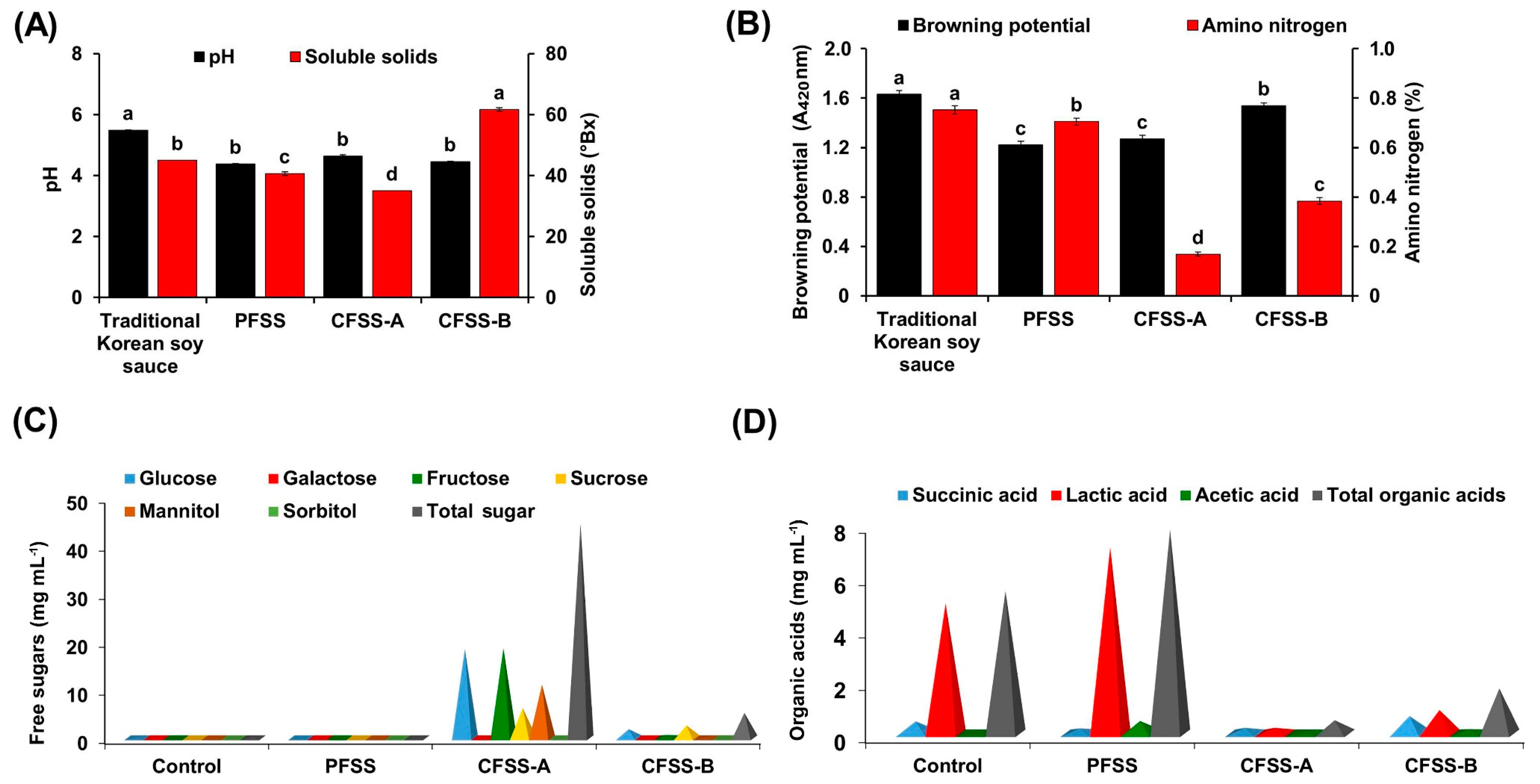
3.1. Analysis of the Physicochemical Properties of the Flavored Soy Sauces Prepared at Laboratory Scale
3.2. Sensory Evaluation
3.3. Comparative Study of Plant Scale Produced Flavored Soy Sauce with Commercial Flavored Soy Sauces
3.4. Analysis of the Physicochemical Properties
3.5. Quantification of Total Sugars and Organic Acids
3.6. Quantification of Free Amino Acids
3.7. GC-MS Profile of Flavored Soy Sauce
3.8. Total Phenolics, Flavonoids, and In Vitro Antioxidative Activity
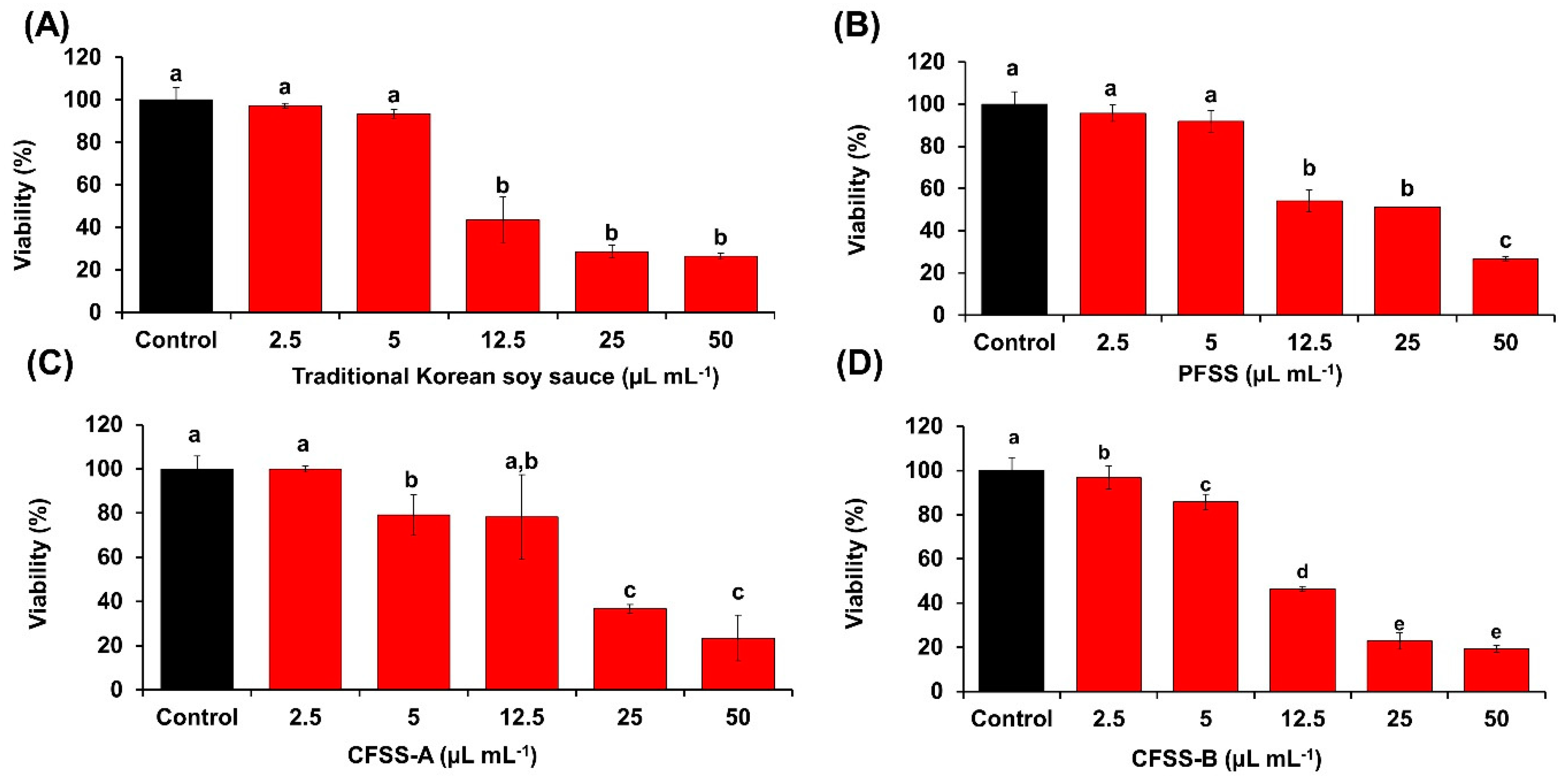
3.9. Cellular Antioxidative Potential
3.10. Enumeration, Identification, and Enzyme Profiling of Lactic Acid Bacteria
3.11. Xanthine Oxidase Inhibition
3.12. Quantification of Biogenic Amines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ly, D.; Mayrhofer, S.; Domig, K.J. Significance of traditional fermented foods in the lower Mekong subregion: A focus on lactic acid bacteria. Food Biosci. 2018, 26, 113–125. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.; Park, J.H.; Kim, M.K.; Park, S.; Dubey, A.; Jeon, J.; Khang, Y.; Kim, M. Evaluation of fungal microflora for aflatoxin producing possibility in novel quality Meju fermented with single and/or multiple additions of Nelumbo nucifera, Ginkgo biloba, and Allium sativum extracts. J. Food Saf. 2017, 37, e12368. [Google Scholar] [CrossRef]
- Kim, H.G.; Hong, J.H.; Song, C.K.; Shin, H.W.; Kim, K.O. Sensory characteristics and consumer acceptability of fermented soybean paste (Doenjang). J. Food Sci. 2010, 75, S375. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Z.; Qian, Y.L.; Ji, F.D.; Chen, J.Y.; Han, B.Z. Microbial composition during Chinese soy sauce koji-making based on culture dependent and independent methods. Food Microbiol. 2013, 34, 189–195. [Google Scholar] [CrossRef]
- Kobayashi, M. Immunological functions of soy sauce: Hypoallergenicity and antiallergenic activity of soy sauce. J. Biosci. Bioeng. 2005, 100, 144–151. [Google Scholar] [CrossRef]
- Feng, Y.; Su, G.; Zhao, H.; Cai, Y.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Characterization of aroma profiles of commercial soy sauce by odor activity value and omission test. Food Chem. 2015, 167, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Jeong, D.Y.; Baik, S.H. Effects of indigenous yeasts on physicochemical and microbial properties of Korean soy sauce prepared by low-salt fermentation. Food Microbiol. 2015, 51, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Jeong, D. Korean traditional fermented soybean products: Jang. J. Ethn. Foods 2015, 2, 2–7. [Google Scholar] [CrossRef]
- Choi, U.K.; Jeong, Y.S.; Kwon, O.J.; Park, J.D.; Kim, Y.C. Comparative study of quality characteristics of Korean soy sauce made with soybeans germinated under dark and light conditions. Int. J. Mol. Sci. 2011, 12, 8105–8118. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Zhao, M.; Feng, Y.; Sun, L.; Wang, Y. Assessment on the improvement of soy sauce fermentation by Aspergillus oryzae HG76. Biocatal. Agric. Biotechnol. 2013, 2, 344–351. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Review: Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reiland, H.; Slavin, J. Systematic review of pears and health. Nutr. Today 2015, 50, 301–305. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 1st ed.; Academic Press: London, UK, 1985. [Google Scholar]
- Cho, J.H.; Zhao, H.L.; Kim, J.S.; Kim, S.H.; Chung, C.H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Innov. Food Sci. Emerg. Technol. 2018, 45, 186–195. [Google Scholar] [CrossRef]
- Shukla, S.; Lee, J.S.; Park, H.K.; Kim, J.K.; Kim, M. Effect of novel starter culture on reduction of biogenic amines, quality improvement, and sensory properties of Doenjang, a traditional Korean soybean fermented sauce variety. J. Food Sci. 2015, 80, M1794–M1803. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ramalingam, S.; Ji, I.G.; Kwon, Y.S.; Bahuguna, A.; Oh, Y.S.; Kwon, O.-J.; Kim, M. Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars. Food Res. Int. 2018, 109, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Lamien-Meda, A.; Lamien, C.E.; Compaoré, M.M.; Meda, R.N.; Kiendrebeogo, M.; Zeba, B.; Millogo, J.F.; Nacoulma, O.G. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 2008, 13, 581–594. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Int. Food Res. J. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Battcock, M.; Azam-Ali, S. Fermented fruits and vegetables: A global perspective. In Food and Agriculture Organization Agricultural Services Bulletin; No.134; FAO: Rome, Italy, 1998. [Google Scholar]
- Ogueke, C.C.; Anosike, F.; Owuamanam, C.I. Prediction of amino nitrogen during ugba (Pentaclethra macrophylla) production under different fermentation variables: A response surface approach. Niger. Food J. 2015, 33, 61–66. [Google Scholar] [CrossRef][Green Version]
- Dibner, J.J.; Buttin, P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002, 11, 453–463. [Google Scholar] [CrossRef]
- Xu, Y.T.; Liu, L.I.; Long, S.F.; Pan, L.; Piao, X.S. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed Sci. Technol. 2018, 235, 110–119. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, T.; An, X.; Zhang, J.; Ma, X.; Cui, J. Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC-MS combined with HS-SPME and discrimination with electronic nose. J. Food Sci. Technol. 2017, 54, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Lee, Y.L.; Li, R.C.; Mau, J.L. Non-volatile flavor components of Ganoderma tsugae. Food Chem. 2005, 90, 409–415. [Google Scholar] [CrossRef]
- Bahuguna, A.; Shukla, S.; Lee, J.S.; Bajpai, V.K.; Kim, S.-Y.; Huh, Y.S.; Han, Y.-K.; Kim, M. Garlic augments the functional and nutritional behavior of Doenjang, a traditional Korean fermented soybean paste. Sci. Rep. 2019, 9, 5436. [Google Scholar] [CrossRef] [PubMed]
- Petroff, O.A.C. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Ohmori, T.; Tahara, M.; Ohshima, T. Mechanism of gamma-aminobutyric acid (GABA) production by a lactic acid bacterium in yogurt-sake. Process Biochem. 2018, 74, 21–27. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, H.; Du, M.; Song, L.; Xu, X. Dispersive liquid-liquid microextraction for rapid and inexpensive determination of tetramethylpyrazine in vinegar. Food Chem. 2019, 286, 141–145. [Google Scholar] [CrossRef]
- Peng, X.; Li, X.; Shi, X.; Guo, S. Evaluation of the aroma quality of Chinese traditional soy paste during storage based on principal component analysis. Food Chem. 2014, 151, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Harada, R.; Yuzuki, M.; Ito, K.; Shiga, K.; Bamba, T.; Fukusaki, E. Microbe participation in aroma production during soy sauce fermentation. J. Biosci. Bioeng. 2018, 125, 688–694. [Google Scholar] [CrossRef]
- Song, Y.R.; Jeong, D.Y.; Baik, S.H. Monitoring of yeast communities and volatile flavor changes during traditional Korean soy sauce fermentation. J. Food. Sci. 2015, 80, M2005–M2014. [Google Scholar] [CrossRef]
- Shukla, S.; Park, J.; Park, J.H.; Lee, J.S.; Kim, M. Development of novel Meju starter culture using plant extracts with reduced Bacillus cereus counts and enhanced functional properties. Sci. Rep. 2017, 7, 11409. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Kayashima, T.; Katayama, T. Oxalic acid is available as a natural antioxidant in some systems. Biochim. Biophys. Acta 2002, 1573, 1–3. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Jeong, J.H.; Noh, M.Y.; Choi, J.H.; Lee, H.; Kim, S.H. Neuroprotective and antioxidant activities of bamboo salt soy sauce against H2O2-induced oxidative stress in rat cortical neurons. Exp. Ther. Med. 2016, 11, 1201–1210. [Google Scholar] [CrossRef]
- Jeong, J.H.; Om, A.S. Specially-treated soy sauces regulate antioxidant activity and ROS in human astrocyte U373MG cells. Cancer Prev. Res. 2007, 12, 296–302. [Google Scholar]
- Abushelaibi, A.; Al-Mahadin, S.; El-Tarabily, K.; Shah, N.P.; Ayyash, M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT Food Sci. Technol. 2017, 79, 316–325. [Google Scholar] [CrossRef]
- Savino, M.J.; Sánchez, L.A.; Saguir, F.M.; de Nadra, M.C.M. Lactic acid bacteria isolated from apples are able to catabolise arginine. World J. Microbiol. Biotechnol. 2012, 28, 1003–1012. [Google Scholar] [CrossRef]
- Jeong, D.W.; Heo, S.; Lee, J.H. Safety assessment of Tetragenococcus halophilus isolates from doenjang, a Korean high-salt-fermented soybean paste. Food Microbiol. 2017, 62, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Jeon, H.L.; Yang, S.J.; Lee, N.K.; Paik, H.D. In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb. Pathog. 2017, 112, 135–141. [Google Scholar] [CrossRef]
- Muzychka, O.V.; Kobzar, O.L.; Popova, A.V.; Frasinyuk, M.S.; Vovk, A.I. Carboxylated aurone derivatives as potent inhibitors of xanthine oxidase. Bioorg. Med. Chem. 2017, 25, 3606–3613. [Google Scholar] [CrossRef]
- Naila, A.; Finlt, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food-existing and emerging approaches. J. Food Sci. 2010, 75, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.H.; Park, Y.K.; Jin, Y.H.; Lee, J.H.; Hwang, H.J. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods 2019, 8, 85. [Google Scholar] [CrossRef] [PubMed]
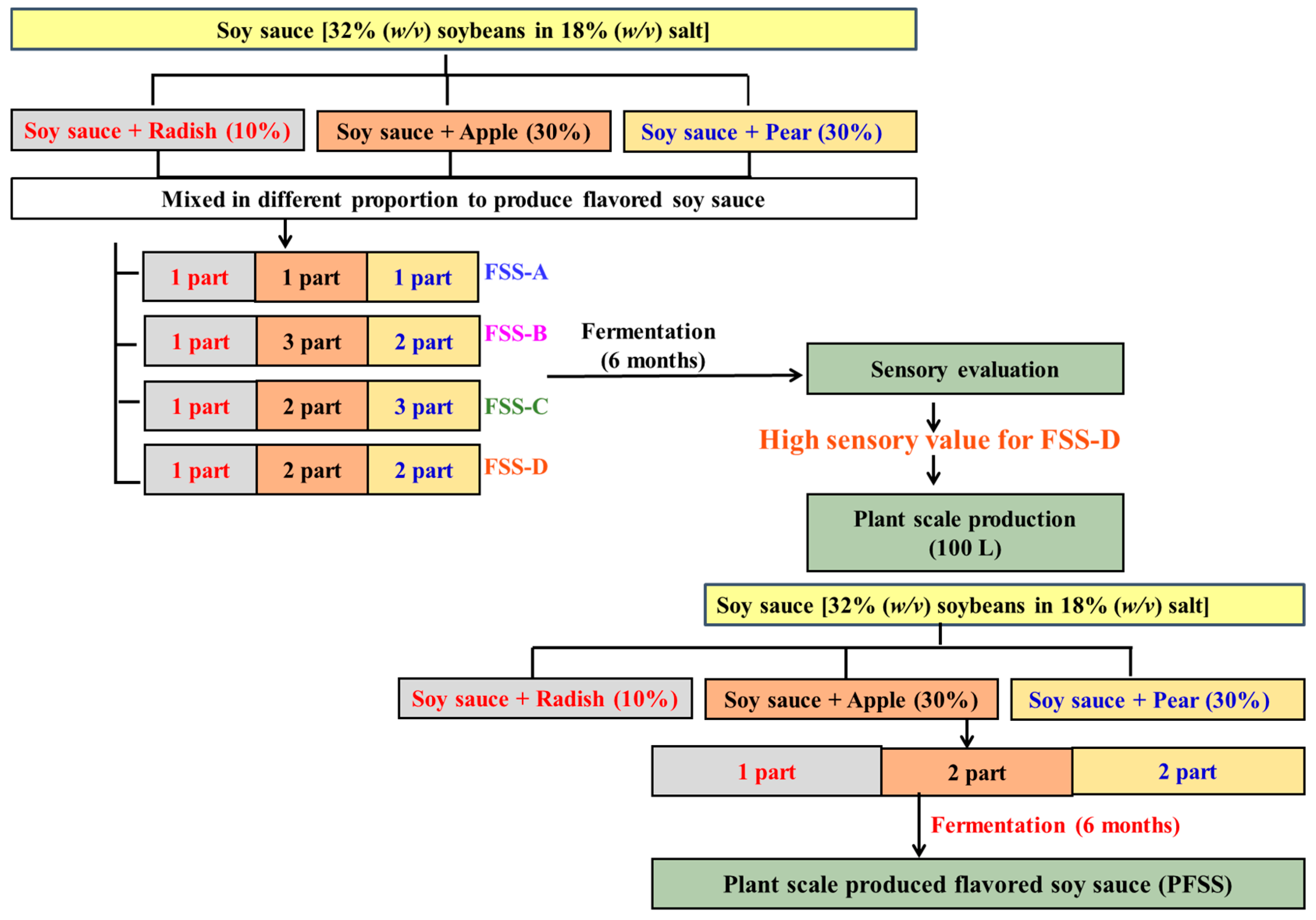




| Flavored Soy Sauce (Sample Code) | Flavored Soy Sauce Was Prepared by Combining Various Traditional Korean Soy Sauce that Were Supplemented with Pulverized Radish (10%; w/w), Apple (30%; w/w), and Pear (30%; w/w) in Different Proportions |
|---|---|
| Control | Traditional Korean soy sauce (without any pulverized vegetable/fruit) |
| FSS-A | 1:1:1 (Radish added soy sauce:pear added soy sauce:apple added soy sauce) |
| FSS-B | 1:3:2 (Radish added soy sauce:pear added soy sauce:apple added soy sauce) |
| FSS-C | 1:2:3 (Radish added soy sauce:pear added soy sauce:apple added soy sauce) |
| FSS-D | 1:2:2 (Radish added soy sauce:pear added soy sauce:apple added soy sauce) |
| Physicochemical Parameter | Sample Code | Fermentation Time (Months) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 5 | 6 | ||
| pH | Control | 5.37 ± 0.00 NS | 5.29 ± 0.01 NS | 5.39 ± 0.00 NS | 5.54 ± 0.00 NS | 5.37 ± 0.00 NS | 5.47 ± 0.00 NS |
| FSS-A | 5.34 ± 0.02 NS | 5.10 ± 0.00 NS | 4.78 ± 0.00 NS | 4.94 ± 0.0 NS | 4.81 ± 0.01 NS | 4.94 ± 0.01 NS | |
| FSS-B | 5.34 ± 0.01 NS | 5.06 ± 0.00 NS | 4.65 ± 0.00 NS | 4.84 ± 0.01 NS | 4.71 ± 0.01 NS | 4.85 ± 0.00 NS | |
| FSS-C | 5.31 ± 0.02 NS | 5.09 ± 0.01 NS | 4.86 ± 0.0 NS | 5.20 ± 0.01 NS | 4.99 ± 0.00 NS | 5.12 ± 0.01 NS | |
| FSS-D | 5.35 ± 0.03 NS | 5.07 ± 0.00 NS | 4.95 ± 0.01 NS | 5.33 ± 0.00 NS | 5.14 ± 0.01 NS | 5.25 ± 0.00 NS | |
| Soluble solids (°Bx) | Control | 38.07 ± 0.06 a | 38.33 ± 0.57 a | 38.77 ± 0.20 a | 39.40 ± 0.00 a | 39.83 ± 0.06 a | 40.60 ± 0.00 a |
| FSS-A | 32.67 ± 0.06 b | 32.33 ± 0.57 b | 32.03 ± 0.06 b | 32.40 ± 0.00 b | 32.93 ± 0.06 b | 33.53 ± 0.15 b | |
| FSS-B | 32.53 ± 0.06 b | 31.00 ± 0.00 b | 30.73 ± 0.00 b | 30.63 ± 0.06 b | 31.43 ± 0.12 b | 31.83 ± 0.06 c | |
| FSS-C | 32.20 ± 0.10 b | 31.33 ± 0.58 b | 30.23 ± 0.06 b | 30.23 ± 0.06 b | 30.90 ± 0.10 b | 31.60 ± 0.00 c | |
| FSS-D | 31.40 ± 0.10 b | 32.67 ± 0.57 b | 31.83 ± 0.06 b | 31.90 ± 0.00 b | 32.27 ± 0.06 b | 32.63 ± 0.16 b,c | |
| Browning potential (A420 nm) | Control | 1.58 ± 0.01 a | 1.35 ± 0.03 a | 1.37 ± 0.03 a | 1.82 ± 0.00 a | 2.03 ± 0.08 a | 2.63 ± 0.06 a |
| FSS-A | 1.38 ± 0.01 b | 1.04 ± 0.03 b | 1.12 ± 0.02 b | 1.38 ± 0.00 b | 2.00 ± 0.03 a | 2.62 ± 0.05 a | |
| FSS-B | 1.35 ± 0.01 b | 1.05 ± 0.04 b | 0.99 ± 0.02 b | 1.17 ± 0.00 c | 1.64 ± 0.03 b | 2.14 ± 0.05 c | |
| FSS-C | 1.34 ± 0.01 b | 0.99 ± 0.03 b | 1.05 ± 0.03 b | 1.40 ± 0.10 b | 1.80 ± 0.03 a | 2.24 ± 0.04 b | |
| FSS-D | 1.37 ± 0.01 b | 0.97 ± 0.02 b | 1.08 ± 0.03 b | 1.30 ± 0.00 b | 1.82 ± 0.06 a | 2.11 ± 0.05 c | |
| Amino Acid (mg mL−1) | Sample | ||||
|---|---|---|---|---|---|
| Traditional Korean Soy Sauce (Control) | PFSS (a) | CFSS-A (b) | CFSS-B (c) | ||
| Sweet taste | Threonine | 2.28 | 0.31 | 1.04 | 1.17 |
| Serine | 3.40 | ND (d) | 1.49 | 2.33 | |
| Glycine | 2.49 | 2.69 | 0.84 | 1.81 | |
| Alanine | 6.80 | 8.38 | 1.58 | 2.06 | |
| Total | 15.51 | 11.38 | 4.95 | 7.37 | |
| Umami taste | Aspartic acid | 1.33 | 0.83 | 3.55 | 1.53 |
| Glutamic acid | 14.53 | 0.87 | 3.09 | 15.17 | |
| Cystine | 0.12 | ND | ND | ND | |
| Total | 15.98 | 1.70 | 6.64 | 16.70 | |
| Bitter taste | Methionine | 1.32 | 0.87 | 0.33 | 0.66 |
| Isoleucine | 4.45 | 3.46 | 1.52 | 1.26 | |
| Leucine | 6.92 | 5.42 | 2.32 | 3.07 | |
| Lysine | 67.58 | 5.83 | 1.25 | 1.17 | |
| Total | 80.27 | 15.58 | 5.42 | 6.16 | |
| Non-taste | Tyrosine | 0.85 | 0.19 | 0.28 | 1.02 |
| Phenylalanine | 4.53 | 2.97 | 1.60 | 2.21 | |
| Valine | 4.68 | 4.55 | 1.65 | 1.4 | |
| Histidine | 0.36 | 0.02 | 0.28 | 0.54 | |
| Proline | 54.88 | 2.41 | 1.40 | 5.34 | |
| Arginine | 0.68 | 0.01 | 1.14 | 1.82 | |
| Total | 65.97 | 10.16 | 6.34 | 12.36 | |
| Non-proteinogenic amino acids | Ornithine | 2.61 | 1.51 | 0.13 | 0.25 |
| β-Amino isobutyric acid | 0.36 | 0.23 | 0.10 | 0.32 | |
| α-Amino adipic acid | 0.88 | 0.32 | 0.06 | 0.13 | |
| Citrulline | 1.65 | ND | 0.23 | ND | |
| γ-Amino-n-butyric acid | 0.44 | 7.71 | 0.25 | 0.10 | |
| Ethanol amine | 0.16 | 0.06 | 0.03 | 0.03 | |
| Hydroxylysine | 0.09 | 0.04 | 0.02 | 0.03 | |
| Total | 6.19 | 9.86 | 0.82 | 0.86 | |
| Grand total | 83.93 | 48.68 | 24.18 | 43.46 | |
| Enzyme | Substrate | Enzyme Activity (nmol) |
|---|---|---|
| Alkaline phosphatase | 2-naphthyl phosphate | >40 |
| Esterase | 2-naphthyl butyrate | 20 |
| Esterase lipase | 2-naphthyl caprylate | 20 |
| Lipase | 2-naphthyl myristate | 0 |
| Leucine arylamidase | L-leucyl-2-naphthylamide | 0 |
| Valine arylamidase | L-valyl-2-naphthylamide | 0 |
| Cystine arylamidase | L-cystyl-2-naphthylamide | 0 |
| Trypsin | N-benzoyl-DL-arginine-2-naphthylamide | 0 |
| α-Chymotrypsin | N-glutaryl-phenylanine-2-naphthylamide | 0 |
| Acid phosphatase | 2-naphtyl phosphate | >40 |
| Naphthol-AS-BI-phosphohydrolase | naphthol-AS-BI-phosphate | 5 |
| α-Galactosidase | 6-Br-2-naphthyl-αD-galactopyranoside | 0 |
| β-Galactosidase | 2-naphthyl-βD-galactopyranoside | 0 |
| β-Glucuronidase | naphthol-AS-BI-βD-glucuronide | 0 |
| α-Glucosidase | 2-naphthyl-αD-glucopyranoside | 0 |
| β-Glucosidase | 6-Br-2-naphthyl-βD-glucopyranoside | 0 |
| N-Acetyl-β-glucosaminidase | 1-naphthyl-N-acetyl-βD-glucosaminide | 0 |
| α-Mannosidase | 6-Br-2-naphthyl-αD-mannopyranoside | 0 |
| α-Fucosidase | 2-naphthyl-αL-fucopyranoside | 0 |
| Biogenic Amine | Traditional Korean Soy Sauce (Control) | PFSS | CFSS-A | CFSS-B |
|---|---|---|---|---|
| Tryptamine | 18.19 ± 0.00 | 13.92 ± 1.19 | 6.13 ± 0.07 | 0.96 ± 0.01 |
| 2-Phenylethylamine | 20.69 ± 0.02 | 24.68 ± 0.77 | 2.16 ± 0.03 | 1.68 ± 0.02 |
| Putrescine | 348.28 ± 7.54 | 438.20 ± 0.42 | 3.74 ± 0.01 | 1.73 ± 0.03 |
| Cadaverine | 11.07 ± 0.46 | 13.97 ± 0.53 | 1.11 ± 0.02 | 0.57 ± 0.01 |
| Histamine | 468.65 ± 3.43 | 461.99 ± 0.99 | 18.68 ± 0.9 | 9.32 ± 0.54 |
| Tyramine | 23.49 ± 0.66 | 27.85 ± 0.16 | 3.10 ± 0.33 | 0.73 ± 0.02 |
| Spermidine | 0.67 ± 0.04 | 0.24 ± 0.09 | 2.35 ± 0.32 | 0.62 ± 0.13 |
| Spermine | 0.06 ± 0.02 | 0.00 ± 0.00 | 0.56 ± 0.06 | 0.18 ± 0.05 |
| Total | 891.11 ± 12.17 b | 980.85 ± 4.15 a | 37.27 ± 1.74 c | 15.79 ± 0.81 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahuguna, A.; Jo, I.G.; Lee, J.S.; Kim, M. Effects of Radishes, Apples, and Pears on the Lactic Acid Bacteria and Nutritional and Functional Qualities of Flavored Soy Sauce. Foods 2020, 9, 1562. https://doi.org/10.3390/foods9111562
Bahuguna A, Jo IG, Lee JS, Kim M. Effects of Radishes, Apples, and Pears on the Lactic Acid Bacteria and Nutritional and Functional Qualities of Flavored Soy Sauce. Foods. 2020; 9(11):1562. https://doi.org/10.3390/foods9111562
Chicago/Turabian StyleBahuguna, Ashutosh, Il Guk Jo, Jong Suk Lee, and Myunghee Kim. 2020. "Effects of Radishes, Apples, and Pears on the Lactic Acid Bacteria and Nutritional and Functional Qualities of Flavored Soy Sauce" Foods 9, no. 11: 1562. https://doi.org/10.3390/foods9111562
APA StyleBahuguna, A., Jo, I. G., Lee, J. S., & Kim, M. (2020). Effects of Radishes, Apples, and Pears on the Lactic Acid Bacteria and Nutritional and Functional Qualities of Flavored Soy Sauce. Foods, 9(11), 1562. https://doi.org/10.3390/foods9111562





